Nodal staging (original) (raw)
Abstract
Lymph node metastases are a poor prognostic indicator in many tumours and therefore accurate identification during staging is important prior to commencing treatment. The presence of lymph node metastases can significantly alter patient management and therefore accurate diagnosis of the presence and extent of nodal disease can help optimise patient management. In this review, the radiologic features that aid in the differentiation of malignant and benign lymph nodes are discussed. The keys to successful interpretation on cross-sectional computed tomography (CT) and magnetic resonance imaging of nodal metastases are highlighted. The clinical role of positron emission tomography-CT imaging for nodal staging is discussed and emerging imaging techniques that may further improve nodal staging accuracy are surveyed.
Keywords: Lymph node, computed tomography, magnetic resonance imaging
Introduction
Nodal disease is most frequently staged using the TNM staging system. This classifies tumours according to Tumour extent, Nodal involvement and the presence or absence of Metastases. The nodal stage is of prognostic value and therefore influences the choice of therapy. Nodal stage in some tumours (e.g. colorectal, gastric, breast and renal) is determined by the number of regional lymph nodes. In other tumours, such as lung, oesophagus and prostate, it is the site of nodal involvement that determines nodal stage.
Newer imaging techniques such as positron emission tomography (PET) and PET-computed tomography (CT) are utilised with increasing frequency to diagnose nodal involvement.
Imaging lymph nodes in oncology
Historically, contrast lymphography was used to assess lymph nodes but this has been superseded by ultrasound, CT and magnetic resonance imaging (MRI). CT is the principle imaging technique for initial nodal assessment and tumour staging although newer techniques such as PET-CT are being used with increasing frequency. Imaging by conventional techniques is used to discriminate between malignant and benign lymph nodes in the following manner.
Ultrasound
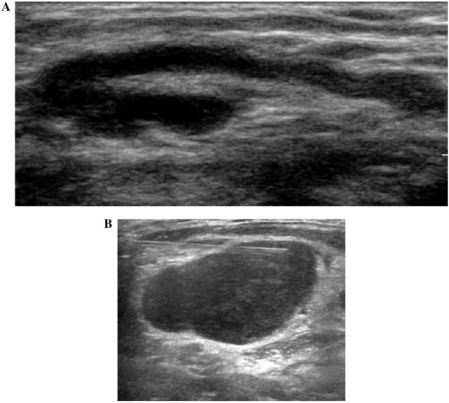
Superficial lymph nodes, particularly in the head and neck, axilla and inguinal regions are amenable to ultrasound (US) evaluation. A normal lymph node is ovoid in shape, hypoechoic to the adjacent muscle and frequently contains an echogenic fatty hilum (Fig. 1a). The hilum is a linear, echogenic, non-shadowing structure that contains the nodal vessels and it appears continuous with the fat around the node. The key advantage of US is the ability to perform an image-guided cytologic sample (Fig. 1b). The drawbacks of ultrasound include significant intra- and inter-operator variability and it is unreliable for the evaluation of deep metastatic lymph nodes. Deep seated lymph nodes in the body are also difficult to visualise.
Figure 1.
(A) An ultrasound demonstrating a normal lymph node in a 24-year-old man. (B) An ultrasound demonstrating fine-needle aspiration of an irregular right supraclavicular lymph node in a 46-year-old woman with breast cancer.
The following criteria have been applied to discriminate between normal and malignant nodes on US evaluation.
Nodal size
Lymph nodes measuring more than 1 cm in the short axis diameter are considered malignant. However, the size threshold does vary with anatomic site and underlying tumour type; e.g. in rectal cancer, lymph nodes larger than 5 mm are regarded as pathological.
Nodal shapes
Benign nodes are more likely to be ovoid and they become more rounded as a result of malignant infiltration. If the ratio of the long axis to short axis diameter is less than 2 the lymph node is more likely to be malignant[1].
Nodal appearance
The sonographic features that are encountered in malignancy include loss of echogenic nodal hilum, irregular nodal contour and internal nodal heterogeneity.
Vascularity on Doppler ultrasound
Normal and benign nodes tend to show central hilar vascularity and central symmetric vascularity. Malignant nodes tend to demonstrate eccentric or absent hilar vascularity, multifocal aberrant vascularity, peripheral perfusion, focal perfusion defects or peripheral subcapsular vascularity[2,3]. Malignant nodes have higher resistive index (>1.0) and pulsatility index (>1.5)[4,5]. Sonographic contrast medium increases the perception of nodal blood vessels, but this does not necessarily improve the accuracy in the detection of malignant nodes[6,7].
CT and MR imaging
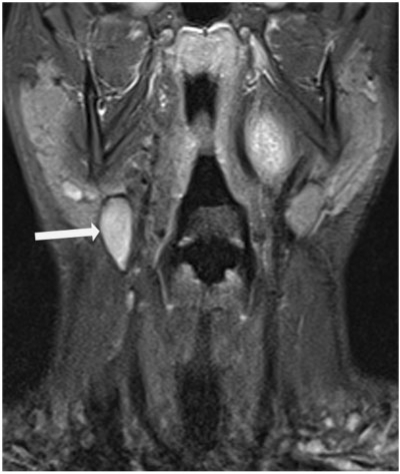
On CT imaging, normal lymph nodes are well demonstrated on CT. They are ovoid in shape and are of soft tissue density. MR imaging must cover the entire pathway of locoregional spread of the tumour being evaluated and the sequences used depend on the anatomic region assessed. Lymph nodes are best demonstrated on T1-weighted images and normal lymph nodes are typically isointense to muscle on T1-weighted imaging, isointense or mildly hyperintense on T2-weighted imaging. Short tau inversion time (STIR) sequences are useful as malignant nodes can be of high signal intensity (Fig. 2). However, this is not specific for malignancy and therefore cannot always be used to differentiate benign from malignant lymph nodes. Furthermore, the signal change of the lymph node during therapy may be helpful for estimating therapy response.
Figure 2.
A coronal STIR image demonstrating a high signal intensity node(arrow) at right level II region in a 67-year-male patient with squamous cell cancer of the oropharynx.
The most widely used CT and MR criteria to determine if a node is benign or malignant is nodal size. Nodal enlargement can result from reactive nodal hyperplasia or coincidental diseases. The following parameters should be taken into consideration when evaluating nodal disease.
Size
Currently, the only widely accepted method for discriminating between normal and pathologic nodes is by size. There is considerable interobserver variation in lymph node assessment and therefore nodes must be measured using a reproducible method. The short axis diameter of a lymph node should be measured as it has been demonstrated that this is constant despite orientation because it is likely to become rounder before it elongates. The short axis diameter is measured perpendicular to the longest diameter of the lymph node.
In the abdomen, the upper limit of the short axis diameter of normal nodes varies from 6 to 10 mm[8,9]. For example the upper limit of a normal retrocrural node is 6 mm, a retroperitoneal node is 10 mm[10] and 8–10 mm for nodes in the pelvis[10,11].
Unfortunately 10–20% of normal-sized locoregional nodes will contain tumour deposits and 30% of enlarged nodes will demonstrate only inflammatory hyperplasia[12–14]. In some tumours, the incidence of metastatic disease within normal-sized nodes is greater than others. For example, in patients with colorectal cancer, 90% of nodal metastases occur in nodes <1 cm[13,15].
Shape and contour
The usefulness of nodal shape on CT or MR imaging is less certain compared with reports in the ultrasound literature. However, the nodal contour on CT and MR imaging can have a greater discriminatory value. Malignant nodes demonstrate irregular borders due to extracapsular extension of disease. This has been shown to be more accurate than nodal size in determining involvement of mesorectal nodes using MR imaging in patients with rectal cancer[16].
Number of nodes
A group of otherwise normal-appearing nodes on CT or MRI is of concern and can suggest malignancy (e.g. at the root of the small bowel mesentery in patients with lymphoma). However, the specificity of this sign is low and can lead to false-positive interpretation[17].
Nodal morphology
A number of features can help to determine metastatic involvement on CT and MR imaging:
- Fat density. A normal node tends to have a uniform appearance and the presence of fat often but not invariably indicates benignity.
- Calcifications. Granulomatous disease can cause non-malignant calcifications in mediastinal and mesenteric lymph nodes. It can also be observed on CT within metastatic nodes arising from colorectal, breast, bladder and ovarian cancers. Malignant nodes can show calcifications following successful treatment, such as in lymphoma and seminomatous germ cell tumours but it is not a reliable indicator of complete tumour response to treatment.
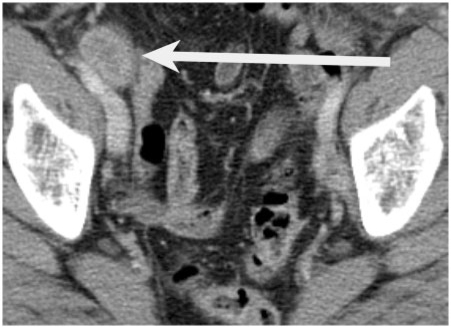
- Heterogeneous appearance. Large metastatic nodes frequently appear heterogeneous on contrast-enhanced CT (Fig. 3) and MRI. A lower density nodal centre on CT can be as a result of necrosis and this is particularly common in primary squamous cell cancers of the head and neck and even normal-sized necrotic nodes should be considered malignant in these patients. On T2-weighted MRI central necrosis demonstrates high signal, and this has a very high positive predicative value in patients with cervical cancer[18]. In patients with rectal cancer, nodal signal heterogeneity is a feature of malignant mesorectal lymph nodes on high-resolution T2-weighted MR imaging[16].
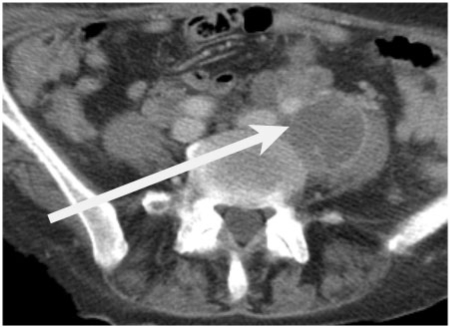
- Low density cystic appearance. Metastatic nodes arising from non-seminomatous germ cell tumour of the testes frequently demonstrate a central low density on CT (Fig. 4)[19]. A non-enlarged cystic lymph node in this tumour group is likely to be involved. They are typically high signal on T2-weighted images. Solid to cystic change within a lymph node after chemotherapy in patients with non-seminomatomous germ cell tumour represents mature differentiation of teratoma. Low attenuation nodes are not pathognomic of malignant infiltration as they are found in tuberculosis and fungal infections.
- Contrast enhancement. Heterogeneous enhancement of an enlarged node is likely to represent malignant infiltration[20,21]. Metastatic nodes can demonstrate an enhancement pattern similar to the primary tumour[22].
- Nodal signal characteristics on MR imaging. Generally it is not possible to distinguish between malignant (Fig. 5a,b) and benign nodes on MR imaging based on nodal signal characteristics alone as normal nodes return a range of signal intensities on T1- and T2-weighted imaging.
Figure 3.
An axial contrast-enhanced CT demonstrating an enlarged heterogeneous right external iliac lymph node (arrow) in a 64-year-old patient with endometrial cancer.
Figure 4.
An axial contrast-enhanced CT demonstrating a large left common iliac cystic lymph node (arrow) in a 34-year-old man with a germ cell tumour.
Figure 5.
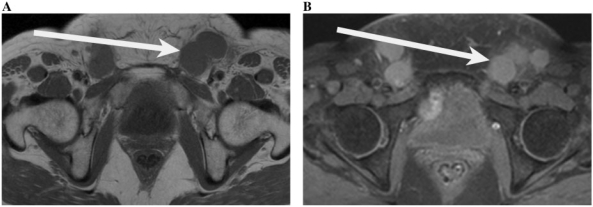
(A) T1-weighted axial image of the pelvis demonstrating multiple enlarged inguinal lymph nodes (arrow) which are low signal in a 61-year-old woman with cervical cancer. (B) Fat-saturated post-contrast images of the pelvis demonstrating multiple enlarged inguinal lymph nodes (arrow) which are of high signal in a 61-year-old woman with cervical cancer.
Potential pitfalls in nodal assessment on CT
Using multi-planar reformats makes errors in interpretation less likely. Both normal structures and other pathologic processes can mimic nodal disease. Common pitfalls include (a) small bowel loops in close proximity to the retroperitoneum that can mimic nodal disease; (b) normal ovaries can simulate external iliac nodal enlargement; (c) blood vessels and especially aberrant vessels can be mistaken for a lymph node especially on non–contrast-enhanced CT; normal anatomic variants such as a left-sided inferior vena cava (IVC) or duplicated IVC may simulate nodal disease; a prominent cistern chyli can also simulate retrocrural nodal enlargement[23]; (d) peritoneal nodules can mimic mesenteric or pelvic lymph nodes; (e) post-operative haematoma and abscesses can simulate nodal disease; (f) following surgery, lymphocoeles can mimic a low attenuation lymph node.
PET and PET-CT
PET performed with fluoro-2-deoxy-D-glucose (FDG) has proved valuable in providing important tumour-related qualitative and quantitative metabolic information that is critical to diagnosis and follow-up. PET-CT is a unique combination of the cross-sectional anatomic information provided by CT and the metabolic information provided by PET, which are acquired during a single examination and fused. The uptake of FDG is used to discriminate between benign and malignant nodes. PET-CT can detect malignancy in non-enlarged nodes, which can lead to a change in patient management. There is increasing evidence for the use of PET-CT for the evaluation of nodal disease in a range of tumour types, including oesophageal, cervical (Fig. 6), head and neck, and melanoma. For example, in oesophageal cancer, a recent study demonstrated that using PET-CT images improved nodal staging in 30% over reading of PET and CT images side by side[24].
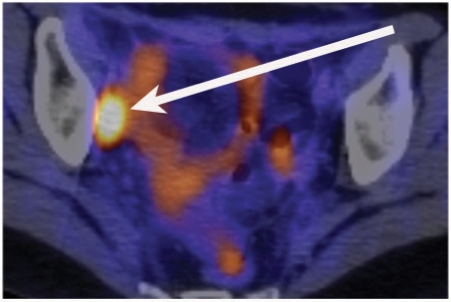
Figure 6.
A PET-CT image demonstrating a right obturator lymph node (arrow) in a 29-year-old woman with small cell carcinoma of the cervix.
Some tumours are FDG negative and they can be examined by other tracers, such as [11C]acetate. Prostate cancer shows marked uptake of [11C]acetate in not only the primary prostate cancer but also its metastatic sites (including lymph nodes). [11C]Acetate has a higher sensitivity than FDG-PET in evaluating patients with prostatic cancer.
However, there are some potential pitfalls using PET-CT for nodal staging. These include (a) nodes smaller than 1 cm may be beyond the ability of the PET camera to detect the tracer activity; (b) tumours with low FDG metabolism (e.g. bronchoalveolar cell carcinoma, prostrate carcinoma, low grade lymphoma) can lead to false-negative results; (c) inflammatory processes may cause false-positive findings.
Optimisation of nodal assessment by imaging
Metastases are frequently found in nodes that are not enlarged by conventional criteria[25]. The key to successful interpretation of imaging for nodal diseases requires a thorough understanding of the normal nodal anatomy, pathways of dissemination, clinical and pathological features of the disease.
Patterns of tumour spread
An understanding of the pathway of tumour spread allows close scrutiny of the most likely sites of nodal involvement. For example, prostate carcinoma spreads via the lymphatics in the neurovascular bundles to the obturator, presacral, hypogastric and external iliac lymph nodes. Further spread is to the common iliac and paraaortic nodes. The obtutaror and external iliac nodes are commonly involved in up to 50–60% of cases[26]. Another example where knowledge of the pathway of dissemination aids in diagnosis is in patients with testicular cancer. Lymphatic spread occurs along lymphatic channels that accompany the spermatic cord. These lymphatic vessels drain into the nodes within the retroperitoneum. Typically, right-sided testicular tumours disseminate to the retroperitoneal nodes on the right (precaval, paracaval, aortocaval and retrocaval nodes) and left-sided tumours disseminate to the left-sided pre-aortic and para-aortic nodes.
Clinical and pathologic features
The incidence of nodal disease increases with the stage of the primary tumour in most abdominal and pelvic tumours. The grade and other histologic characteristics of tumours have a bearing on the likelihood of nodal metastases; e.g. in early gastric cancer the presence of submucosal and vascular invasion predicts for the likelihood of nodal disease[27]. Other biologic indices can help to alert the radiologist to the likelihood of nodal metastases. For example, in patients with prostate cancer, a high prostate-specific antigen (PSA) level or high Gleeson score on biopsy have a higher likelihood of nodal involvement and extracapsular prostatic disease and nodal disease.
Details of previous treatment
Knowledge of previous therapy is vital as it modifies the pattern of nodal disease. For example, in patients with prostate cancer, nodal relapse after radiotherapy or radical prostatectomy is usually outside the pelvis[28]. Following total mesorectal excision surgery for rectal cancer, nodal recurrence can occur within the obturator chain along the pelvic sidewall or more cranially within the retroperitoneum.
Diagnostic accuracy of nodal staging
The diagnostic accuracy of CT and MR imaging for nodal staging of cancers in the abdomen and pelvis varies widely in the literature. For pelvic malignancies, the accuracy of CT and MR imaging is similar[29–31]. The reported sensitivities range from 40 to 87% and the specificities from 64 to 100%. In a study of pancreatic cancer, a sensitivity of 14%, specificity of 85% and accuracy of 73% was achieved for nodal staging[29–33]. Conventional CT and MR imaging are limited by their ability to detect metastases in normal or minimally enlarged lymph nodes.
In lymphoma patients PET-CT has been found to be superior to 67Ga imaging, and equal or superior to CT for the detection of nodal and extranodal lymphoma at initial staging[34].
Assessment of nodal response to treatment
According to the recent revised RECIST criteria 1.1[35], pathologic nodes, identified as target lesions, must meet the criterion of a short axis of diameter of at least 15 mm on CT. Non-target lymph nodes measure between 10 and 15 mm and lymph nodes measuring less than 10 mm are considered normal.
Lymph nodes identified as target lesions should always have the actual short axis measurement recorded, even if the nodes regress to below 10 mm. This means that when lymph nodes are included as target lesions, the sum of lesions may not be zero even if complete response criteria are met, since a normal lymph node is defined as having a short axis of <10 mm. In order to qualify for complete response, each node must achieve a short axis <10 mm. For partial response, stable disease and progressive disease, the actual short axis measurement of the nodes is included in the sum of target lesions.
Advances in nodal staging
MR lymphography
MR lymphography is an imaging technique that helps to distinguish malignant and benign nodes based on the pattern and degree of contrast enhancement independent of nodal size or morphology. MR lymphography is performed after the administration of a lymphotrophic MR contrast agent, of which ultrasmall iron oxide particles (USPIO) have been most widely applied.
Following the administration of USPIO, the particles escape into the interstitial spaces and are transported by lymphatics into the lymph nodes. Within the lymph nodes, the USPIO particles are phagocytosed by nodal macrophages, which results in signal loss in normal nodes on T2*-weighted imaging (Fig. 7a,b). Malignant nodes are of high signal intensity on T2*-weighted images.
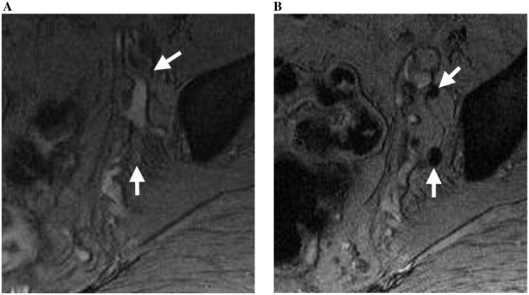
Figure 7.
Normal sidewall nodes (arrows) imaged using T2*-weighted MR imaging (MEDIC) (A) before and (B) 24 h after USPIO contrast administration in a 57-year-old man with rectal cancer. Prior to contrast administration, blood vessels and lymph nodes have relatively high signal intensity. Note signal darkening of the normal nodes after contrast, thus facilitating their detection.
In prostate cancer, the technique has shown very encouraging results for the detection of malignant nodes <10 mm in size, with a high diagnostic sensitivity and specificity compared with conventional imaging. The main potential advantage of the imaging technique is the ability to detect partially replaced non-enlarged malignant lymph nodes[36].
USPIO-enhanced MRI combined with diffusion-weighted MRI
A new imaging approach is using USPIO-enhanced MRI combined with diffusion-weighted MRI (USPIO/DW-MRI). A combined USPIO/DW-MRI approach has been found to have a high negative predictive value (86–93%), high accuracy (75–90%), and overall good sensitivity (60–80%) in finding nodal metastases in patients with either prostate cancer or bladder cancer or both[37]. These results were comparable to those obtained by evaluating USPIO images with and without DW-MRI. The major advantage of this new approach is that it is much quicker; 13 min for the combined USPIO/DW-MRI rather than 80 min.
Diffusion-weighted MR imaging
The image contrast on diffusion-weighted imaging is based on differences in the mobility of water protons between tissues, and reflects tissue cellularity and the integrity of cellular membranes. Tumour tissues are generally more cellular compared with the native tissues from which they originate, and thus they show high signal (restricted diffusion) on diffusion-weighted MR imaging. Diffusion-weighted MR imaging has been shown to improve the detection of lymph nodes. Fusion images created by the addition of DW-MRI to a conventional T1- or T2-weighted image can improve the detection of small nodes throughout the body. Early reports using diffusion-weighted imaging to identify malignant nodes in patients with head and neck and cervical cancers have been encouraging.
Conclusion
The accurate identification of malignant lymph nodes is a major challenge in diagnostic radiology. CT and MRI are limited in their ability to detect metastases in normal or minimally enlarged lymph nodes. By using the combination of size, shape, characteristics and site of lymph nodes identified on imaging, the radiologist can better indicate if a lymph node is likely to be metastatic. Functional imaging with PET-CT adds to the sensitivity and specificity of nodal evaluation in many tumours but has important limitations. Novel imaging techniques such as USPIO and diffusion-weighted imaging, alone or in combination, may further improve the diagnostic accuracy of nodal staging.
References
- 1.Steinkamp HJ, Cornehl M, Hosten N, Pegios W, Vogl T, Felix R. Cervical lymphadenopathy: ratio of long to short-axis diameter as a predictor of malignancy. Br J Radiol. 1995;68:266–70. doi: 10.1259/0007-1285-68-807-266. doi:10.1259/0007-1285-68-807-266. PMid:7735765. [DOI] [PubMed] [Google Scholar]
- 2.Na DG, Lim HK, Byun HS, Kim HD, Ko YH, Baek JH. Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. AJR Am J Roentgenol. 1997;168:1311–6. doi: 10.2214/ajr.168.5.9129432. [DOI] [PubMed] [Google Scholar]
- 3.Steinkamp HJ, Mueffelmann M, Böck JC, Thiel T, Kenzel P, Felix R. Differential diagnosis of lymph node lesions: a semiquantitataive approach with color Doppler ultrasound. Br J Radiol. 1998;71:828–33. doi: 10.1259/bjr.71.848.9828794. [DOI] [PubMed] [Google Scholar]
- 4.Choi MY, Lee JW, Jang KJ. Distinction between benign and malignant causes of cervical, axillary, and inguinal lymphadenopathy: value of Doppler spectral waveform analysis. AJR Am J Roentgenol. 1995;165:981–4. doi: 10.2214/ajr.165.4.7677005. [DOI] [PubMed] [Google Scholar]
- 5.Magarelli N, Guglielmi G, Savastano M, et al. Superficial inflammatory and primary neoplastic lymphadenopathy: diagnostic accuracy of power-Doppler sonography. Eur J Radiol. 2004;52:257–63. doi: 10.1016/j.ejrad.2003.10.020. doi:10.1016/j.ejrad.2003.10.020. PMid:15544903. [DOI] [PubMed] [Google Scholar]
- 6.Adibelli ZH, Unal G, Gül E, Uslu F, Koçak U, Abali Y. Differentiation of benign and malignant cervical lymph nodes: value of B-mode and color Doppler sonography. Eur J Radiol. 1998;28:230–4. doi: 10.1016/s0720-048x(97)00174-5. doi:10.1016/S0720-048X(97)00174-5. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder RJ, Maeurer J, Gath HJ, Willam C, Hidajat N. Vascularization of reactively enlarged lymph nodes analyzed by color duplex sonography. J Oral Maxillofac Surg. 1999;57:1090–5. doi: 10.1016/s0278-2391(99)90332-4. doi:10.1016/S0278-2391(99)90332-4. [DOI] [PubMed] [Google Scholar]
- 8.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–22. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 9.Magnusson, A. Size of normal retroperitoneal lymph nodes. Acta Radiol Diagn (Stockh) 1983;24:315–8. doi: 10.1177/028418518302400407. [DOI] [PubMed] [Google Scholar]
- 10.Einstein DM, Singer AA, Chilcote WA, Desai RK. Abdominal lymphadenopathy: spectrum of CT findings. Radiographics. 1991;11:457–72. doi: 10.1148/radiographics.11.3.1852937. [DOI] [PubMed] [Google Scholar]
- 11.Vinnicombe SJ, Norman AR, Nicolson V, Husband JE. Normal pelvic lymph nodes: evaluation with CT after bipedal lymphangiography. Radiology. 1995;194:349–55. doi: 10.1148/radiology.194.2.7824709. [DOI] [PubMed] [Google Scholar]
- 12.Gross BH, Glazer GM, Orringer MB, Spizarny DL, Flint A. Bronchogenic carcinoma metastatic to normal sized lymph nodes: frequency and significance. Radiology. 1988;166(1 Pt 1):71–4. doi: 10.1148/radiology.166.1.3336704. [DOI] [PubMed] [Google Scholar]
- 13.Kayser K, Bach S, Bülzebruck H, Vogt-Moykopf I, Probst G. Site, size and tumour involvement of resected extrapulmonary lymph nodes in lung cancer. J Surg Oncol. 1990;43:45–9. doi: 10.1002/jso.2930430112. doi:10.1002/jso.2930430112. PMid:2153261. [DOI] [PubMed] [Google Scholar]
- 14.Staples CA, Müller NL, Miller RR, Evans KG, Nelems B. Mediastinal nodes in bronchogenic carcinoma: comparison between CT and mediastinoscopy. Radiology. 1988;167:367–72. doi: 10.1148/radiology.167.2.3357944. [DOI] [PubMed] [Google Scholar]
- 15.McLoud TC, Bourgouin PM, Greenberg RW, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–23. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- 16.Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–7. doi: 10.1148/radiol.2272011747. doi:10.1148/radiol.2272011747. PMid:12732695. [DOI] [PubMed] [Google Scholar]
- 17.Matsukuma K, Tsukamoto N, Matsuyama T, Ono M, Nakano H. Preoperative CT study of lymph nodes in cervical cancer – its correleation with histological findings. Gynecol Oncol. 1989;33:168–71. doi: 10.1016/0090-8258(89)90544-1. doi:10.1016/0090-8258(89)90544-1. [DOI] [PubMed] [Google Scholar]
- 18.Yang WT, Lam WW, Yu MY, Cheung TH, Metreweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol. 2000;175:759–66. doi: 10.2214/ajr.175.3.1750759. [DOI] [PubMed] [Google Scholar]
- 19.Scatarige JC, Fishman EK, Kuhajda FP, Taylor GA, Siegelman SS. Low attenuation nodal metastases in testicular carcinoma. J Comput Assist Tomogr. 1983;7:682–7. doi: 10.1097/00004728-198308000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Barentsz JO, Jager GJ, van Vierzen PB, et al. Staging urinary bladder cancer after transurethral biopsy: value of fast dynamic contrast-enhanced MR imaging. Radiology. 1996;201:185–93. doi: 10.1148/radiology.201.1.8816542. [DOI] [PubMed] [Google Scholar]
- 21.Noworolski SM, Fischbein NJ, Kaplan MJ, et al. Challenges in dynamic contrast-enhanced MRI imaging of cervical lymph nodes to detect metastatic disease. J Magn Reson Imaging. 2003;17:455–62. doi: 10.1002/jmri.10280. doi:10.1002/jmri.10280. PMid:12655585. [DOI] [PubMed] [Google Scholar]
- 22.Husband JE, Koh DM. Bladder cancer. In: Husband JE, Reznek RH, editors. Imaging in oncology. London: Martin Dunitz Ltd; 2004. pp. 343–74. [Google Scholar]
- 23.Gollub MJ, Castellino RA. The cistern chylli: a potential mimic of retrocrural lymphadenopathy on CT scans. Radiology. 1996;199:477–80. doi: 10.1148/radiology.199.2.8668798. [DOI] [PubMed] [Google Scholar]
- 24.Schreurs LM, Pultrum BB, Koopmans KP, et al. Better assessment of nodal metastases by PET/CT fusion compared to side by side PET/CT in oesophageal cancer. Anticancer Res. 2008;28(3B):1867–73. [PubMed] [Google Scholar]
- 25.Tiguert R, Gheiler EL, Tefilli MV, et al. Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology. 1999;53:367–71. doi: 10.1016/s0090-4295(98)00518-4. doi:10.1016/S0090-4295(98)00518-4. [DOI] [PubMed] [Google Scholar]
- 26.Golimbu M, Morales P, Al-Askari S, Brown J. Extended pelvic lymphadenectomy for prostatic cancer. J Urol. 1979;121:617–20. doi: 10.1016/s0022-5347(17)56906-2. [DOI] [PubMed] [Google Scholar]
- 27.Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM, Kim KM. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR Am J Roentgenol. 2000;174:1551–7. doi: 10.2214/ajr.174.6.1741551. [DOI] [PubMed] [Google Scholar]
- 28.Spencer J, Golding S. CT evaluation of lymph node status at presentation of prostatic carcinoma. Br J Radiol. 1992;65:199–201. doi: 10.1259/0007-1285-65-771-199. doi:10.1259/0007-1285-65-771-199. PMid:1547445. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Choi BI, Lee HP, et al. Uterine cervical carcinoma: comparison of CT and MR findings. Radiology. 1990;175:45–51. doi: 10.1148/radiology.175.1.2315503. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology. 1994;190:807–11. doi: 10.1148/radiology.190.3.8115631. [DOI] [PubMed] [Google Scholar]
- 31.Williams AD, Cousins C, Soutter WP, et al. Detection of pelvic lymph node metastases in gynaecologic malignancy: a comparison of CT, MR imaging, and positron emission tomography. AJR Am J Roentgenol. 2001;177:343–8. doi: 10.2214/ajr.177.2.1770343. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda H, Nakagawa T, Shibuya H. Metastases to pelvic lymph nodes from carcinoma in the pelvic cavity: diagnosis using thin section CT. Clin Radiol. 1999;54:237–42. doi: 10.1016/s0009-9260(99)91158-3. doi:10.1016/S0009-9260(99)91158-3. [DOI] [PubMed] [Google Scholar]
- 33.Oyen RH, Van Poppel HP, Ameye FE, Van de Voorde WA, Baert AL, Baert LV. Lymph node staging of localised prostatic carcinoma with CT and CT guided fine needle aspiration biopsy: prospective study of 285 patients. Radiology. 1994;190:315–22. doi: 10.1148/radiology.190.2.8284375. [DOI] [PubMed] [Google Scholar]
- 34.Kostakoglu L, Leonard JP, Coleman M, Goldsmith SJ. The role of FDG-PET imaging in the management of lymphoma. Clin Adv Hematol Oncol. 2004;2:115–21. [PubMed] [Google Scholar]
- 35.Eisenhauera EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. PMid:19097774. [DOI] [PubMed] [Google Scholar]
- 36.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph node metastases in prostate cancer. N Engl J Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. doi:10.1056/NEJMoa022749. PMid:12815134. [DOI] [PubMed] [Google Scholar]
- 37.Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol. 2009;55:761–9. doi: 10.1016/j.eururo.2008.12.034. doi:10.1016/j.eururo.2008.12.034. PMid:19144456. [DOI] [PubMed] [Google Scholar]