Electrogenerated Chemiluminescence: An Oxidative‐Reduction Type ECL Reaction Sequence Using Tripropyl Amine (original) (raw)
© 1990 ECS - The Electrochemical Society
Journal of The Electrochemical Society,Volume 137,Number 10Citation Jonathan K. Leland and Michael J. Powell 1990 J. Electrochem. Soc. 137 3127DOI 10.1149/1.2086171
Article metrics
973 Total downloads
0 Video abstract views
1945-7111/137/10/3127
Abstract
A new electrogenerated chemiluminescence (ECL) reaction which utilizes tripropyl amine and 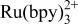 is presented. The mechanism of light generation appears general enough to include a range of amines and luminophores. An oxidative‐reduction mechanism is proposed. Upon electrochemical oxidation of both the luminophore and amine, a strong emission is observed. Voltammetric analysis reveals the potential for greatest light emission at the tripropyl amine oxidation. The emission is from the excited state of
is presented. The mechanism of light generation appears general enough to include a range of amines and luminophores. An oxidative‐reduction mechanism is proposed. Upon electrochemical oxidation of both the luminophore and amine, a strong emission is observed. Voltammetric analysis reveals the potential for greatest light emission at the tripropyl amine oxidation. The emission is from the excited state of 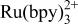 . An electron transfer reaction from the deprotonated tripropyl amine radical and
. An electron transfer reaction from the deprotonated tripropyl amine radical and 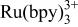 is the central reaction for excited state production. An estimate of the ECL efficiency cannot be made, due to the complex nature of the reaction.
is the central reaction for excited state production. An estimate of the ECL efficiency cannot be made, due to the complex nature of the reaction.
Export citation and abstractBibTeXRIS
Access this article
The computer you are using is not registered by an institution with a subscription to this article. Please choose one of the options below.
Login
Rent from
Make a recommendation
To gain access to this content, please complete the Recommendation Form and we will follow up with your librarian or Institution on your behalf.
For corporate researchers we can also follow up directly with your R&D manager, or the information management contact at your company. Institutional subscribers have access to the current volume, plus a 10-year back file (where available).