Connexin43 Knockdown Accelerates Wound Healing but Inhibits Mesenchymal Transition after Corneal Endothelial Injury In Vivo | IOVS (original) (raw)
January 2008
Volume 49, Issue 1
Figure 1.
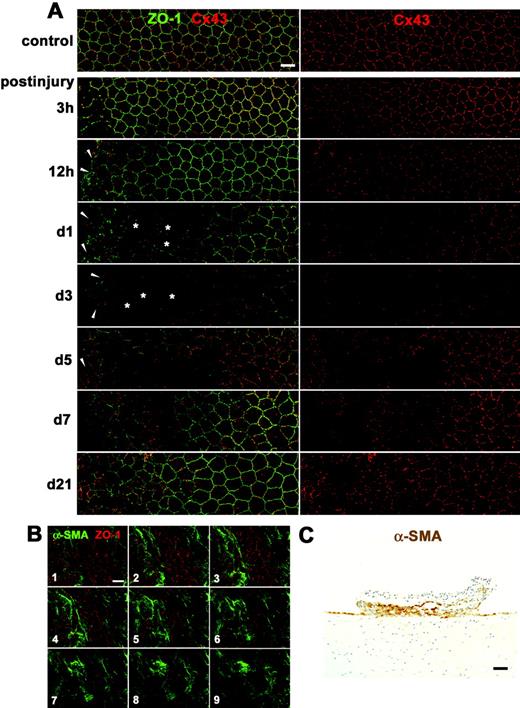
Changes in the rat corneal endothelium in vivo during wound healing after mechanical scrape injury with a 30-gauge needle. (A) Double immunolabeling for ZO-1 and Cx43 of a surface preparation (wounds on the left) during wound healing (3 hours to 21 days after injury) shows both enlarged corneal endothelial cells (  ) and multilayered small, elongated cells (arrowheads) and drastic changes in Cx43 expression in those cells. (B) Serial confocal images (1–9, from the surface to the deeper region) of double immunolabeling for ZO-1 and α-SMA taken at 0.7-μm intervals on day 5 after injury reveal that multilayered, small, elongated cells were α-SMA-positive myofibroblasts. (C) Immunohistochemistry for α-SMA using a cross section on day 5 after injury shows formation of a retrocorneal fibrous membrane consisting of α-SMA-positive myofibroblasts arranged in multiple cell layers posterior to Descemet’s membrane. Nuclei were stained with hematoxylin. Scale bars: (A, B) 20 μm; (C) 100 μm.
) and multilayered small, elongated cells (arrowheads) and drastic changes in Cx43 expression in those cells. (B) Serial confocal images (1–9, from the surface to the deeper region) of double immunolabeling for ZO-1 and α-SMA taken at 0.7-μm intervals on day 5 after injury reveal that multilayered, small, elongated cells were α-SMA-positive myofibroblasts. (C) Immunohistochemistry for α-SMA using a cross section on day 5 after injury shows formation of a retrocorneal fibrous membrane consisting of α-SMA-positive myofibroblasts arranged in multiple cell layers posterior to Descemet’s membrane. Nuclei were stained with hematoxylin. Scale bars: (A, B) 20 μm; (C) 100 μm.
Figure 1.
Changes in the rat corneal endothelium in vivo during wound healing after mechanical scrape injury with a 30-gauge needle. (A) Double immunolabeling for ZO-1 and Cx43 of a surface preparation (wounds on the left) during wound healing (3 hours to 21 days after injury) shows both enlarged corneal endothelial cells (  ) and multilayered small, elongated cells (arrowheads) and drastic changes in Cx43 expression in those cells. (B) Serial confocal images (1–9, from the surface to the deeper region) of double immunolabeling for ZO-1 and α-SMA taken at 0.7-μm intervals on day 5 after injury reveal that multilayered, small, elongated cells were α-SMA-positive myofibroblasts. (C) Immunohistochemistry for α-SMA using a cross section on day 5 after injury shows formation of a retrocorneal fibrous membrane consisting of α-SMA-positive myofibroblasts arranged in multiple cell layers posterior to Descemet’s membrane. Nuclei were stained with hematoxylin. Scale bars: (A, B) 20 μm; (C) 100 μm.
) and multilayered small, elongated cells (arrowheads) and drastic changes in Cx43 expression in those cells. (B) Serial confocal images (1–9, from the surface to the deeper region) of double immunolabeling for ZO-1 and α-SMA taken at 0.7-μm intervals on day 5 after injury reveal that multilayered, small, elongated cells were α-SMA-positive myofibroblasts. (C) Immunohistochemistry for α-SMA using a cross section on day 5 after injury shows formation of a retrocorneal fibrous membrane consisting of α-SMA-positive myofibroblasts arranged in multiple cell layers posterior to Descemet’s membrane. Nuclei were stained with hematoxylin. Scale bars: (A, B) 20 μm; (C) 100 μm.
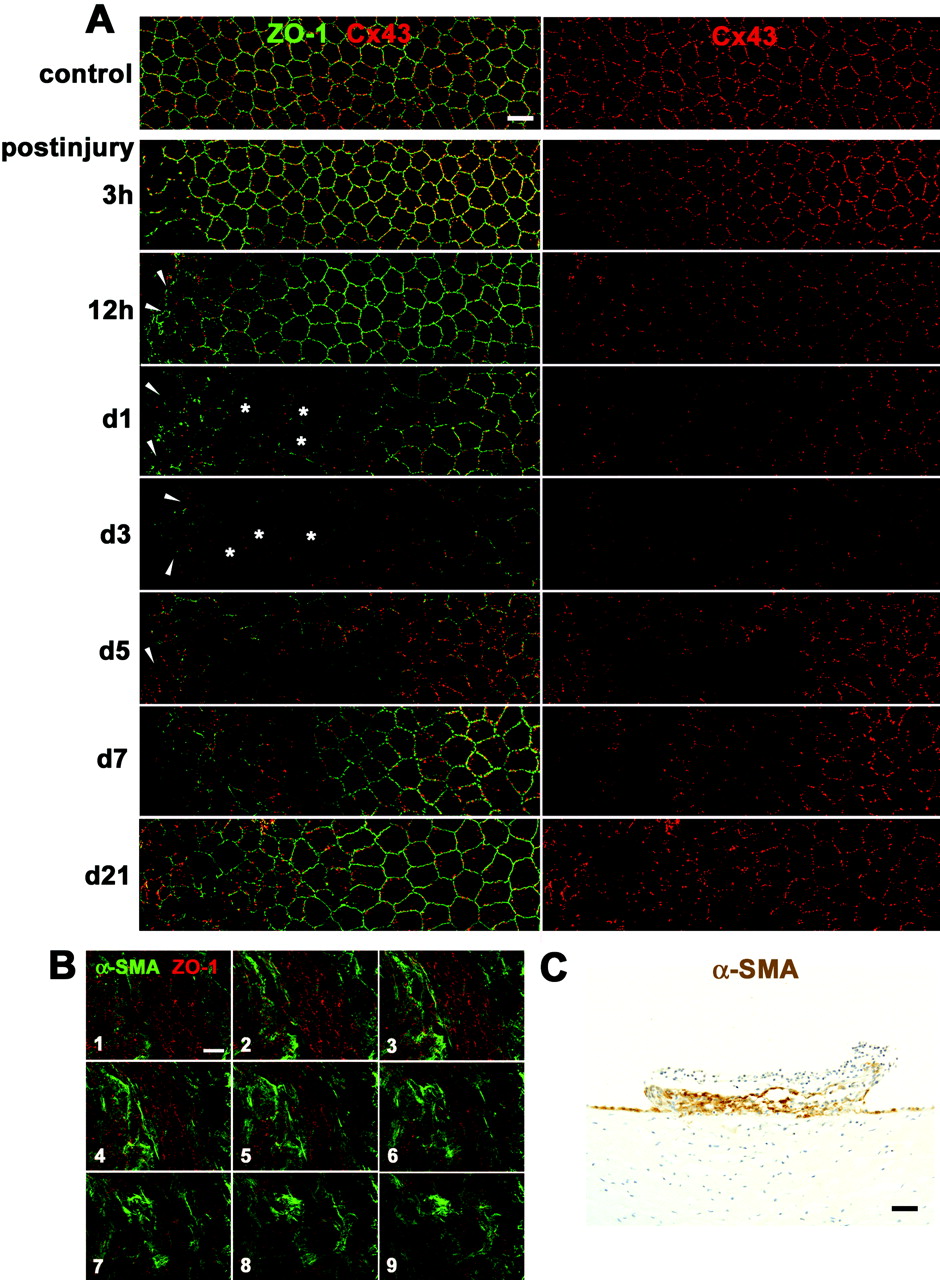
Figure 2.
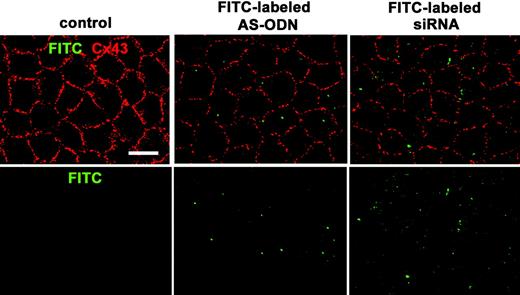
Cx43 AS-ODN or siRNA injected into the anterior chamber of the rat eye was delivered into the corneal endothelium in vivo and downregulated the Cx43 protein. A single injection (40 μM, 20 μL) of FITC-labeled Cx43 AS-ODN or FITC-labeled Cx43 siRNA into the anterior chamber was performed without injury. Cx43 immunolabeling was performed with the anti-Cx43 antibody and Alexa 594-labeled secondary antibody. FITC and Alexa 594 were observed 1 day after injection in a surface preparation by confocal microscope. Clear, small, punctate FITC labeling was seen in the endothelium. Substantial reductions in Cx43 immunolabeling were found in Cx43 AS-ODN- or siRNA-treated corneas compared with the control corneas. Scale bar, 20 μm.
Figure 2.
Cx43 AS-ODN or siRNA injected into the anterior chamber of the rat eye was delivered into the corneal endothelium in vivo and downregulated the Cx43 protein. A single injection (40 μM, 20 μL) of FITC-labeled Cx43 AS-ODN or FITC-labeled Cx43 siRNA into the anterior chamber was performed without injury. Cx43 immunolabeling was performed with the anti-Cx43 antibody and Alexa 594-labeled secondary antibody. FITC and Alexa 594 were observed 1 day after injection in a surface preparation by confocal microscope. Clear, small, punctate FITC labeling was seen in the endothelium. Substantial reductions in Cx43 immunolabeling were found in Cx43 AS-ODN- or siRNA-treated corneas compared with the control corneas. Scale bar, 20 μm.
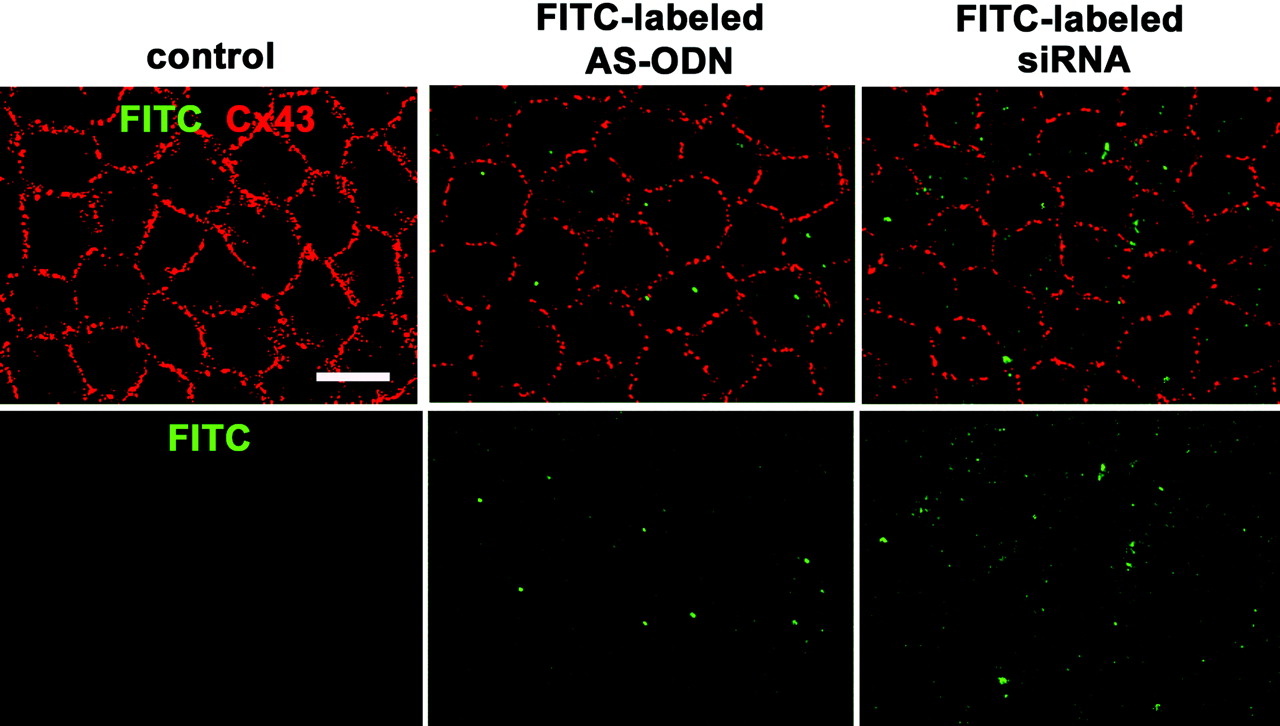
Figure 3.
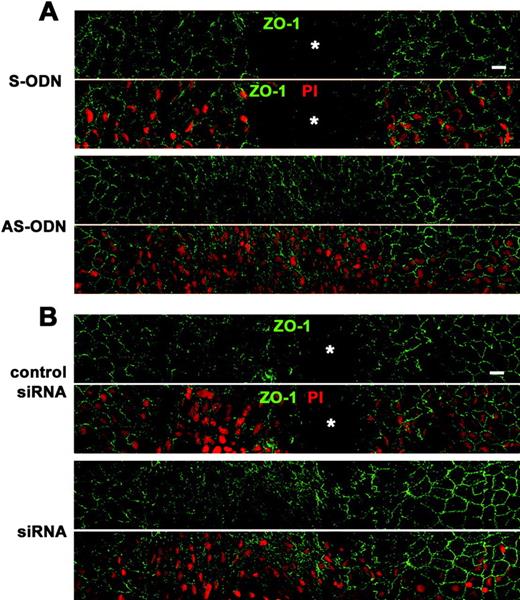
Cx43 AS-ODN or siRNA accelerated wound closure of the rat corneal endothelium after scrape injury. A single injection (40 μM, 20 μL) of Cx43 AS-ODN or siRNA into the anterior chamber was applied simultaneously with injury. (A, B) Immunolabeling for ZO-1 and nuclear staining with PI of a surface preparation on day 3 after injury (wounds at the center) reveal that the corneal surface with the single application of either AS-ODN (A) or siRNA (B) was fully covered with endothelial cells without intervening spaces, indicating complete wound closure. In contrast, spaces without cells (  ) were observed in the control eyes S-ODN or nonsense siRNA (control siRNA), demonstrating that closure was not complete. Scale bar, 20 μm.
) were observed in the control eyes S-ODN or nonsense siRNA (control siRNA), demonstrating that closure was not complete. Scale bar, 20 μm.
Figure 3.
Cx43 AS-ODN or siRNA accelerated wound closure of the rat corneal endothelium after scrape injury. A single injection (40 μM, 20 μL) of Cx43 AS-ODN or siRNA into the anterior chamber was applied simultaneously with injury. (A, B) Immunolabeling for ZO-1 and nuclear staining with PI of a surface preparation on day 3 after injury (wounds at the center) reveal that the corneal surface with the single application of either AS-ODN (A) or siRNA (B) was fully covered with endothelial cells without intervening spaces, indicating complete wound closure. In contrast, spaces without cells (  ) were observed in the control eyes S-ODN or nonsense siRNA (control siRNA), demonstrating that closure was not complete. Scale bar, 20 μm.
) were observed in the control eyes S-ODN or nonsense siRNA (control siRNA), demonstrating that closure was not complete. Scale bar, 20 μm.
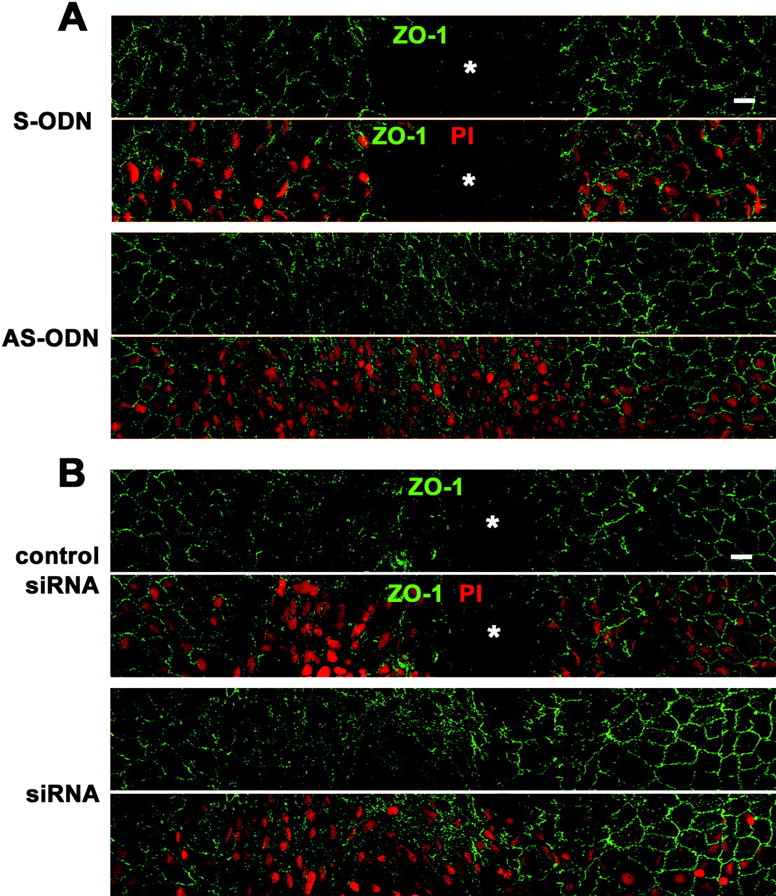
Figure 4.
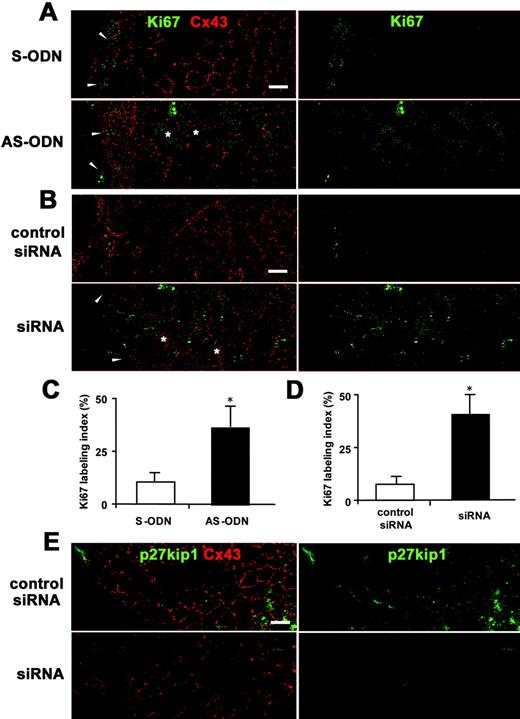
Cx43 AS-ODN or siRNA increased the proliferative activity of the rat corneal endothelium after scrape injury. (A, B) Double immunolabeling for Ki67 and Cx43 in a surface preparation on day 1 after injury (wounds on the left) revealed that not only small, elongated cells at the border of the wound (arrowheads), but also enlarged irregular-shaped endothelial cells (  ) on the periphery of the wound were Ki67-positive in the corneas treated with AS-ODN (A) or siRNA (B). In contrast, only small, elongated cells at the border of the wound were immunolabeled with Ki67 in the control eyes (S-ODN or control siRNA). (C, D) Ki67-labeling indexes in the corneas on day 1 after a single injection of AS-ODN (C) or siRNA (D) simultaneously with injury are shown. Mean ± SD; n = 5. *P < 0.001 versus control eyes (S-ODN or control siRNA); Student’s _t_-test. (E) Double immunolabeling for p27kip1 and Cx43 using surface preparations on day 1 after injury (wounds on the left) shows that Cx43 siRNA downregulated both p27kip1 and Cx43 expression. Scale bar, 20 μm.
) on the periphery of the wound were Ki67-positive in the corneas treated with AS-ODN (A) or siRNA (B). In contrast, only small, elongated cells at the border of the wound were immunolabeled with Ki67 in the control eyes (S-ODN or control siRNA). (C, D) Ki67-labeling indexes in the corneas on day 1 after a single injection of AS-ODN (C) or siRNA (D) simultaneously with injury are shown. Mean ± SD; n = 5. *P < 0.001 versus control eyes (S-ODN or control siRNA); Student’s _t_-test. (E) Double immunolabeling for p27kip1 and Cx43 using surface preparations on day 1 after injury (wounds on the left) shows that Cx43 siRNA downregulated both p27kip1 and Cx43 expression. Scale bar, 20 μm.
Figure 4.
Cx43 AS-ODN or siRNA increased the proliferative activity of the rat corneal endothelium after scrape injury. (A, B) Double immunolabeling for Ki67 and Cx43 in a surface preparation on day 1 after injury (wounds on the left) revealed that not only small, elongated cells at the border of the wound (arrowheads), but also enlarged irregular-shaped endothelial cells (  ) on the periphery of the wound were Ki67-positive in the corneas treated with AS-ODN (A) or siRNA (B). In contrast, only small, elongated cells at the border of the wound were immunolabeled with Ki67 in the control eyes (S-ODN or control siRNA). (C, D) Ki67-labeling indexes in the corneas on day 1 after a single injection of AS-ODN (C) or siRNA (D) simultaneously with injury are shown. Mean ± SD; n = 5. *P < 0.001 versus control eyes (S-ODN or control siRNA); Student’s _t_-test. (E) Double immunolabeling for p27kip1 and Cx43 using surface preparations on day 1 after injury (wounds on the left) shows that Cx43 siRNA downregulated both p27kip1 and Cx43 expression. Scale bar, 20 μm.
) on the periphery of the wound were Ki67-positive in the corneas treated with AS-ODN (A) or siRNA (B). In contrast, only small, elongated cells at the border of the wound were immunolabeled with Ki67 in the control eyes (S-ODN or control siRNA). (C, D) Ki67-labeling indexes in the corneas on day 1 after a single injection of AS-ODN (C) or siRNA (D) simultaneously with injury are shown. Mean ± SD; n = 5. *P < 0.001 versus control eyes (S-ODN or control siRNA); Student’s _t_-test. (E) Double immunolabeling for p27kip1 and Cx43 using surface preparations on day 1 after injury (wounds on the left) shows that Cx43 siRNA downregulated both p27kip1 and Cx43 expression. Scale bar, 20 μm.
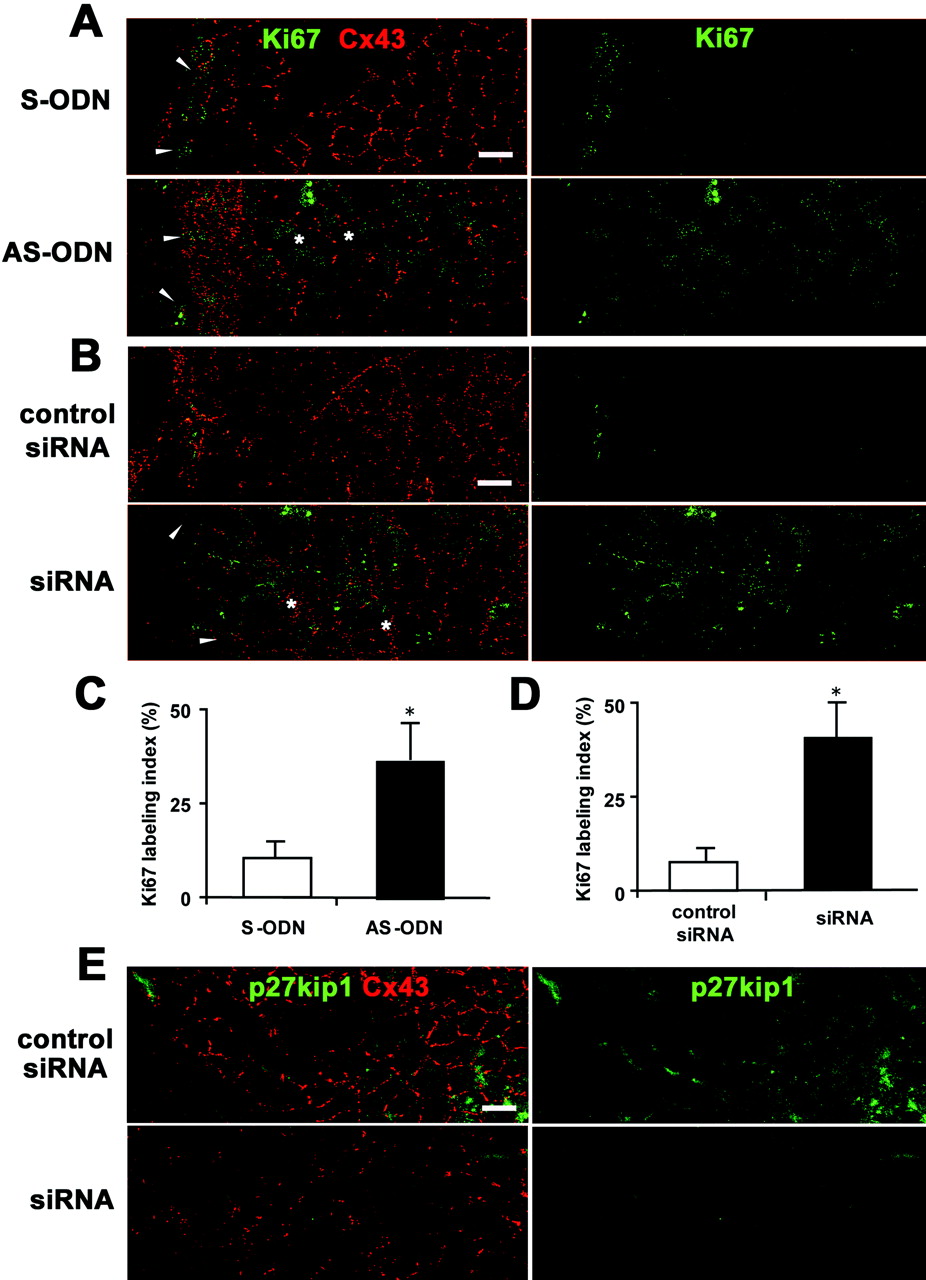
Figure 5.
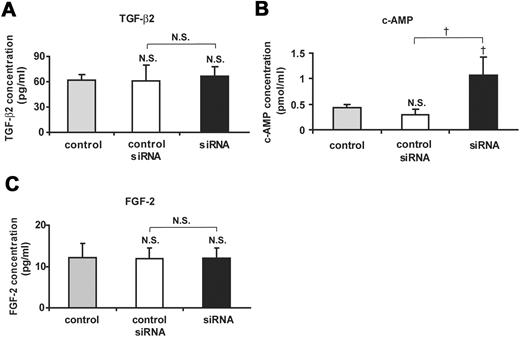
Measurement of the concentrations of TGF-β2 (A), cAMP (B), and FGF-2 (C) in the aqueous humor from Cx43 siRNA-treated and control siRNA-treated eyes at day 1 after injury and from nontreated control eyes. Mean ± SD; n = 3. †P < 0.05 compared with control or control siRNA; NS, not significant compared with control or control siRNA (Scheffé’s test).
Figure 5.
Measurement of the concentrations of TGF-β2 (A), cAMP (B), and FGF-2 (C) in the aqueous humor from Cx43 siRNA-treated and control siRNA-treated eyes at day 1 after injury and from nontreated control eyes. Mean ± SD; n = 3. †P < 0.05 compared with control or control siRNA; NS, not significant compared with control or control siRNA (Scheffé’s test).
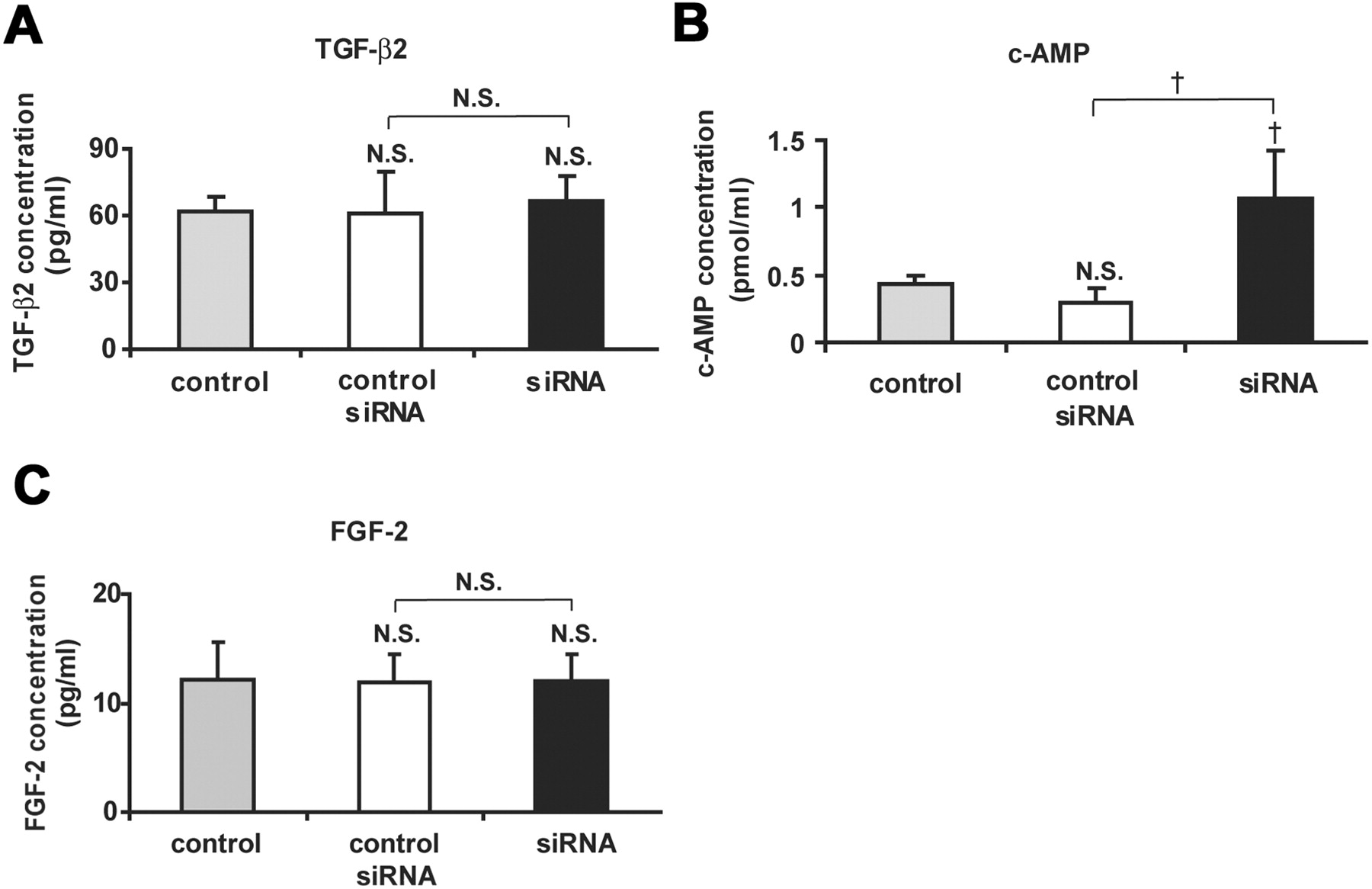
Figure 6.
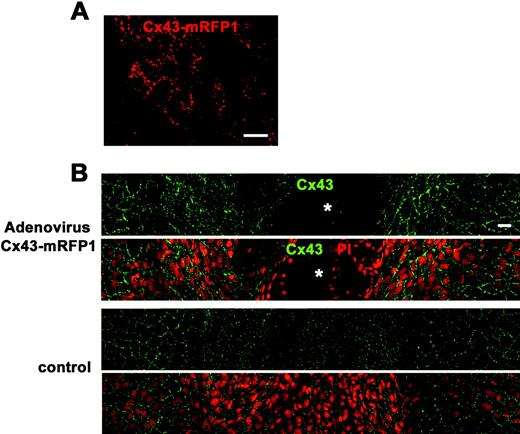
Cx43 overexpression by adenovirus containing CMV-Cx43-mRFP1 delayed wound closure of the rat corneal endothelium. (A) Cx43-mRFP1 fluorescence without fixation or any staining on day 3 after injection of 10 μL solution of recombinant adenovirus containing CMV-Cx43-mRFP (5 × 108 pfu/mL) into the anterior chamber without injury. Small, punctate Cx43-mRFP was seen between the endothelial cells. (B) Immunolabeling for Cx43 and nuclear staining with PI of a surface preparation on day 5 after injury (wounds at the center) revealed spaces without cells (  ) in the cornea treated with adenovirus containing CMV-Cx43-mRFP1, demonstrating that wound closure was not complete. In contrast, the control corneal surface was fully covered with endothelial cells without intervening spaces, indicating complete wound closure. Overexpression of Cx43 was seen in the corneal endothelium treated with adenovirus containing CMV-Cx43-mRFP1 compared with the control. Scale bar, 20 μm.
) in the cornea treated with adenovirus containing CMV-Cx43-mRFP1, demonstrating that wound closure was not complete. In contrast, the control corneal surface was fully covered with endothelial cells without intervening spaces, indicating complete wound closure. Overexpression of Cx43 was seen in the corneal endothelium treated with adenovirus containing CMV-Cx43-mRFP1 compared with the control. Scale bar, 20 μm.
Figure 6.
Cx43 overexpression by adenovirus containing CMV-Cx43-mRFP1 delayed wound closure of the rat corneal endothelium. (A) Cx43-mRFP1 fluorescence without fixation or any staining on day 3 after injection of 10 μL solution of recombinant adenovirus containing CMV-Cx43-mRFP (5 × 108 pfu/mL) into the anterior chamber without injury. Small, punctate Cx43-mRFP was seen between the endothelial cells. (B) Immunolabeling for Cx43 and nuclear staining with PI of a surface preparation on day 5 after injury (wounds at the center) revealed spaces without cells (  ) in the cornea treated with adenovirus containing CMV-Cx43-mRFP1, demonstrating that wound closure was not complete. In contrast, the control corneal surface was fully covered with endothelial cells without intervening spaces, indicating complete wound closure. Overexpression of Cx43 was seen in the corneal endothelium treated with adenovirus containing CMV-Cx43-mRFP1 compared with the control. Scale bar, 20 μm.
) in the cornea treated with adenovirus containing CMV-Cx43-mRFP1, demonstrating that wound closure was not complete. In contrast, the control corneal surface was fully covered with endothelial cells without intervening spaces, indicating complete wound closure. Overexpression of Cx43 was seen in the corneal endothelium treated with adenovirus containing CMV-Cx43-mRFP1 compared with the control. Scale bar, 20 μm.
Figure 7.
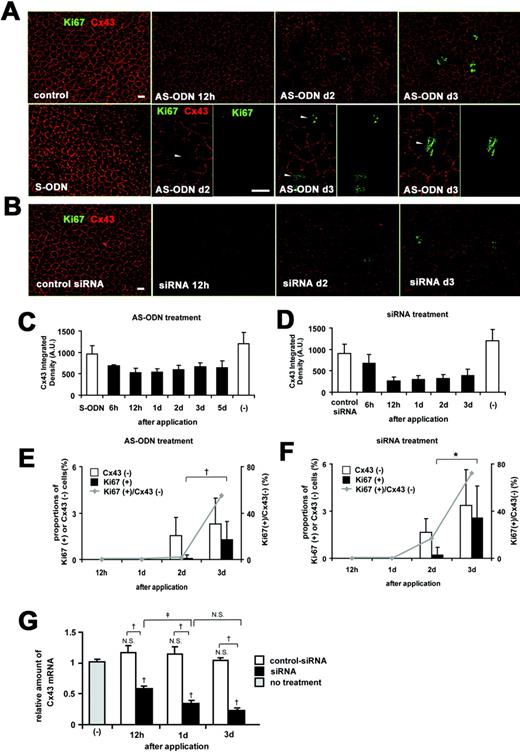
Cx43 AS-ODN or siRNA knocked down Cx43 expression and induced the proliferative activity of the rat corneal endothelium in vivo without injury. A single injection (40 μM, 20 μL) of Cx43 AS-ODN or siRNA into the anterior chamber was applied without injury. (A, B) Double immunolabeling for Ki67 and Cx43 of a surface preparation revealed that Cx43 AS-ODN (A) or siRNA (B) decreased Cx43 expression from 12 hours until day 3. A few Ki67-positive endothelial cells were present on day 2, and their number increased on day 3 after Cx43 AS-ODN or siRNA treatment. Cx43-negative cells (arrowheads) were found in the cornea on days 2 to 3 after Cx43 AS-ODN or siRNA treatment. Scale bar, 20 μm. (C, D) Morphometry for Cx43 fluorescence showed that Cx43 AS-ODN (C) or siRNA (D) decreased Cx43 expression starting at 6 hours, reaching a maximum decrease at 12 hours, and continuing until at least day 3. Mean ± SD; n = 2 to 16 for various time points. (E, F) Morphometry of Cx43-negative cells and Ki67-positive cells and the ratio of Ki67-positive cells to Cx43-negative cells revealed that no Ki67-positive endothelial cells were present at either 12 hours or day 1 after Cx43 AS-ODN or siRNA treatment, but that the number of Cx43-negative cells and Ki67-positive cells increased from days 2 to 3, that all Ki67-positive cells were Cx43-negative, and that the ratio increased from days 2 to 3. Mean ± SD; n = 4 to 16 for various time points. †P < 0.01; *P < 0.001; Welch’s t_-test. (G) Real-time RT-PCR analysis for Cx43 mRNA in the endothelium and Descemet’s membrane from Cx43 siRNA-treated, control siRNA-treated, and nontreated (control) corneas. Mean ± SD; n = 3. †_P < 0.01 compared with control or control siRNA; ‡P < 0.05; NS, not significant (Scheffé’s test).
Figure 7.
Cx43 AS-ODN or siRNA knocked down Cx43 expression and induced the proliferative activity of the rat corneal endothelium in vivo without injury. A single injection (40 μM, 20 μL) of Cx43 AS-ODN or siRNA into the anterior chamber was applied without injury. (A, B) Double immunolabeling for Ki67 and Cx43 of a surface preparation revealed that Cx43 AS-ODN (A) or siRNA (B) decreased Cx43 expression from 12 hours until day 3. A few Ki67-positive endothelial cells were present on day 2, and their number increased on day 3 after Cx43 AS-ODN or siRNA treatment. Cx43-negative cells (arrowheads) were found in the cornea on days 2 to 3 after Cx43 AS-ODN or siRNA treatment. Scale bar, 20 μm. (C, D) Morphometry for Cx43 fluorescence showed that Cx43 AS-ODN (C) or siRNA (D) decreased Cx43 expression starting at 6 hours, reaching a maximum decrease at 12 hours, and continuing until at least day 3. Mean ± SD; n = 2 to 16 for various time points. (E, F) Morphometry of Cx43-negative cells and Ki67-positive cells and the ratio of Ki67-positive cells to Cx43-negative cells revealed that no Ki67-positive endothelial cells were present at either 12 hours or day 1 after Cx43 AS-ODN or siRNA treatment, but that the number of Cx43-negative cells and Ki67-positive cells increased from days 2 to 3, that all Ki67-positive cells were Cx43-negative, and that the ratio increased from days 2 to 3. Mean ± SD; n = 4 to 16 for various time points. †P < 0.01; *P < 0.001; Welch’s t_-test. (G) Real-time RT-PCR analysis for Cx43 mRNA in the endothelium and Descemet’s membrane from Cx43 siRNA-treated, control siRNA-treated, and nontreated (control) corneas. Mean ± SD; n = 3. †_P < 0.01 compared with control or control siRNA; ‡P < 0.05; NS, not significant (Scheffé’s test).
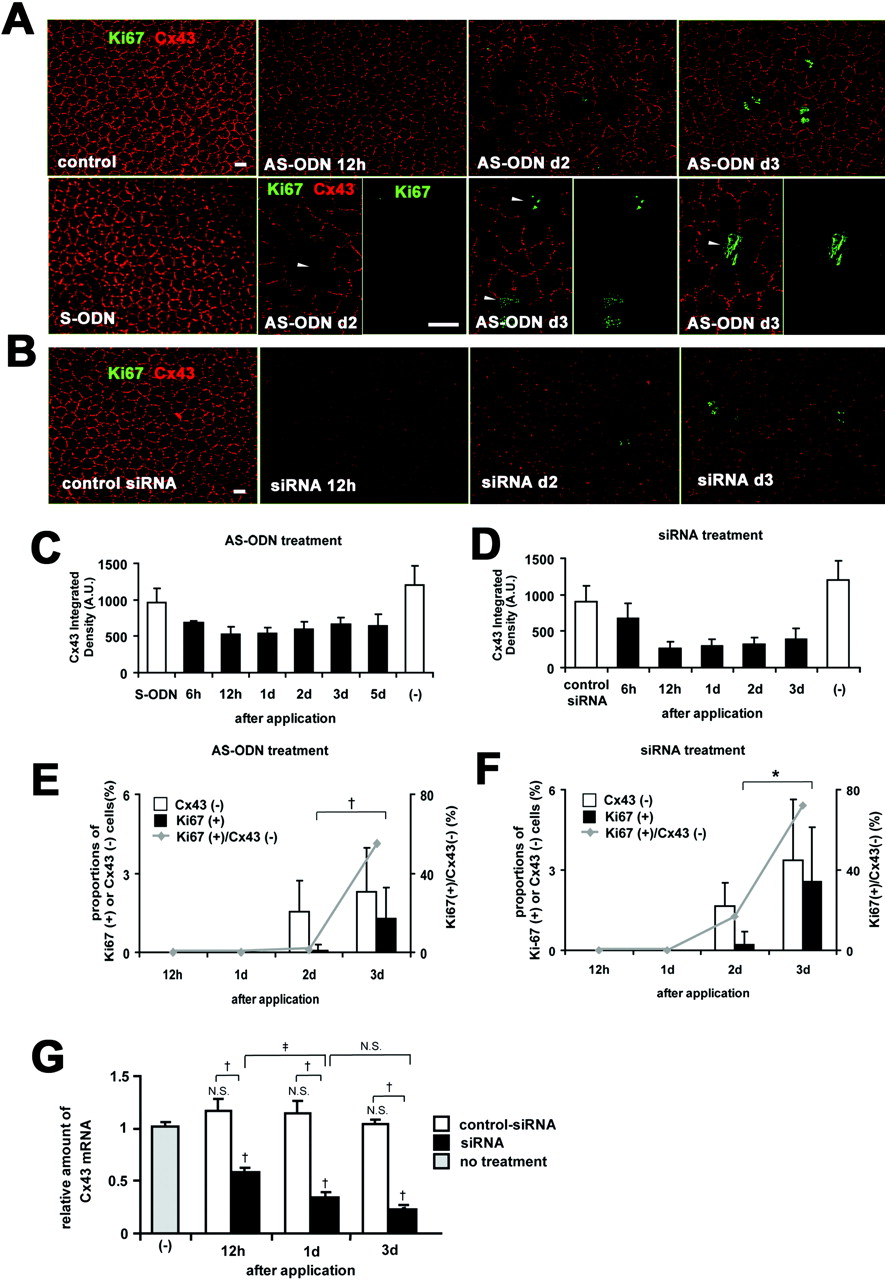
Figure 8.
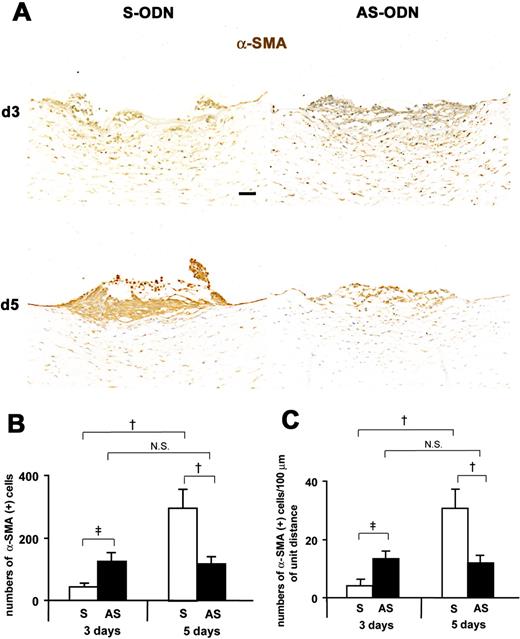
Cx43 AS-ODN treatment inhibits EMT and formation of retrocorneal fibrous membrane in the rat cornea after scrape injury. (A) Immunohistochemistry for α-SMA in cross sections on days 3 and 5 after injury revealed that on day 3, the wound was closed in the AS-ODN-treated cornea but remained open in the S-ODN-treated one. On day 5, the wounds were completely closed in both corneas. No increases in the number of α-SMA-positive myofibroblasts between days 3 and 5 were found in the AS-ODN-treated cornea, whereas the number of myofibroblasts in the S-ODN-treated cornea was markedly increased during the same period. Scale bar, 100 μm. (B, C) Morphometry for α-SMA-positive myofibroblasts. The data are expressed as the number of α-SMA-positive myofibroblasts per retrocorneal lesion (B) and the number of α-SMA-positive myofibroblasts per 100 μm of the linear horizontal distance across the retrocorneal lesion occupied by multilayered α-SMA-positive myofibroblasts (C). On day 5 after injury, the number of myofibroblasts in Cx43 AS-ODN-treated corneas was less than half that in S-ODN-treated corneas. Although more α-SMA-positive myofibroblasts were found in Cx43 AS-ODN-treated corneas than in the S-ODN-treated corneas on day 3 after injury, the number of myofibroblasts in Cx43 AS-ODN-treated corneas on day 3 was still less than half that in the S-ODN-treated corneas on day 5, and no increases in their number were observed from days 3 to 5. Mean ± SD; n = 5. †P < 0.01; ‡P < 0.05 (Scheffé’s test).
Figure 8.
Cx43 AS-ODN treatment inhibits EMT and formation of retrocorneal fibrous membrane in the rat cornea after scrape injury. (A) Immunohistochemistry for α-SMA in cross sections on days 3 and 5 after injury revealed that on day 3, the wound was closed in the AS-ODN-treated cornea but remained open in the S-ODN-treated one. On day 5, the wounds were completely closed in both corneas. No increases in the number of α-SMA-positive myofibroblasts between days 3 and 5 were found in the AS-ODN-treated cornea, whereas the number of myofibroblasts in the S-ODN-treated cornea was markedly increased during the same period. Scale bar, 100 μm. (B, C) Morphometry for α-SMA-positive myofibroblasts. The data are expressed as the number of α-SMA-positive myofibroblasts per retrocorneal lesion (B) and the number of α-SMA-positive myofibroblasts per 100 μm of the linear horizontal distance across the retrocorneal lesion occupied by multilayered α-SMA-positive myofibroblasts (C). On day 5 after injury, the number of myofibroblasts in Cx43 AS-ODN-treated corneas was less than half that in S-ODN-treated corneas. Although more α-SMA-positive myofibroblasts were found in Cx43 AS-ODN-treated corneas than in the S-ODN-treated corneas on day 3 after injury, the number of myofibroblasts in Cx43 AS-ODN-treated corneas on day 3 was still less than half that in the S-ODN-treated corneas on day 5, and no increases in their number were observed from days 3 to 5. Mean ± SD; n = 5. †P < 0.01; ‡P < 0.05 (Scheffé’s test).
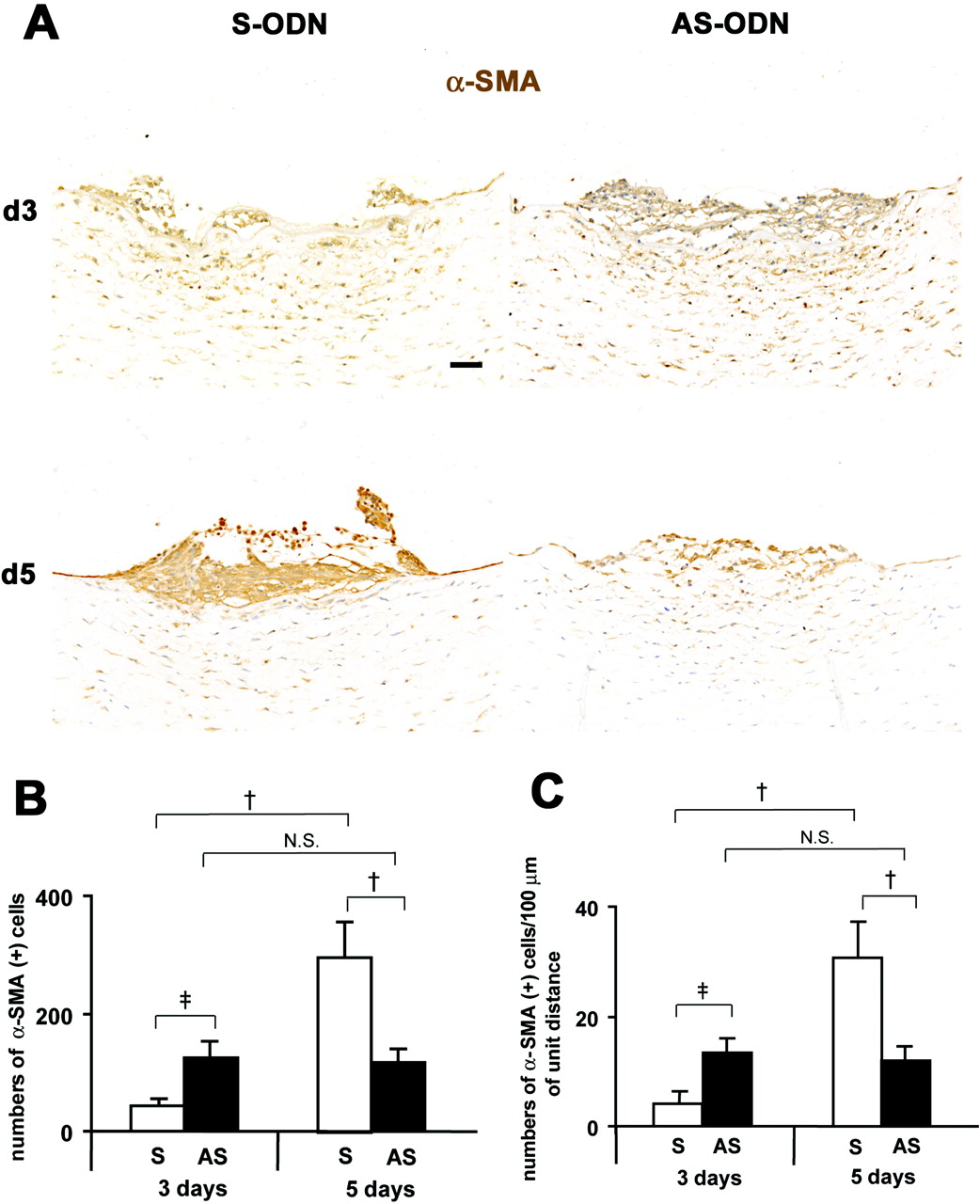
Table 1.
Acceleration of Postinjury Wound Closure in the Rat Corneal Endothelium by Cx43 AS-ODN or siRNA Treatment
Table 1.
Acceleration of Postinjury Wound Closure in the Rat Corneal Endothelium by Cx43 AS-ODN or siRNA Treatment
| Treatment | Complete Wound Closure on Postinjury Day 3 |
|---|---|
| Cx43 S-ODN | 0/6 |
| Cx43 AS-ODN | 5/6* |
| Control siRNA | 0/6 |
| Cx43 siRNA | 5/6, † |
Table 2.
Delay in Postinjury Wound Closure in the Rat Corneal Endothelium by Recombinant Adenovirus Containing CMV-Cx43-mRFP1
Table 2.
Delay in Postinjury Wound Closure in the Rat Corneal Endothelium by Recombinant Adenovirus Containing CMV-Cx43-mRFP1
| Treatment | Complete Wound Closure on Postinjury Day 5 |
|---|---|
| Control | 5/5 |
| Adenovirus containing CMV-Cx43-mRFP1 | 2/5* |
Copyright 2008 The Association for Research in Vision and Ophthalmology, Inc.
