Integrin-mediated type II TGF-β receptor tyrosine dephosphorylation controls SMAD-dependent profibrotic signaling (original) (raw)
Increased UUO-mediated collagen I production in integrin α1–null mice. Since it is not known whether the collagen-binding receptor integrin α1β1 mediates kidney tubulointerstitial fibrosis, we performed UUO in BALB/c WT and integrin α1–null (Itga1–/–; referred to herein as α1KO) male mice. This experiment revealed that α1KO mice developed more severe injury than WT mice 7 days after UUO, which was characterized by worse tubular dilatation and matrix deposition as well as increased collagen I levels in the kidney medulla and the whole kidney (Figure 1, A–D).
Loss of integrin α1β1 leads to exacerbated fibrosis following UUO. (A) H&E and Trichrome staining of kidneys from WT and α1KO mice 7 days after UUO, showing more dilated tubules (asterisks), fibrosis (arrows), and collagen deposition (blue staining) in the injured α1KO mice. (B) Paraffin kidney sections from control and injured mice were stained with FITC-conjugated DBA (green) and collagen I (CI; red) antibodies to visualize CDs and degree of fibrosis, respectively. Increased deposition of collagen was evident in the injured α1KO mice. (C) Kidney lysates (20 μg/lane) from injured WT and α1KO mice (n = 3 and 5 shown, respectively) were analyzed by Western blot for levels of collagen I. (D) Collagen I and β-actin bands were quantified by densitometry analysis, and collagen I signal was expressed as the collagen I/β-actin ratio. Values are mean ± SEM of the indicated n. Scale bars: 100 μm (A); 40 μm (B).
Altered epithelial cell morphology in α1KO CD cells. Tubular kidney CD cells are the primary cellular target of UUO-mediated injury. Therefore, we generated primary cultures of WT and α1KO CD cells (Supplemental Figure 1A; supplemental material available online with this article; doi:10.1172/JCI71668DS1) and examined their contribution to the phenotype of α1KO mice. Although the epithelial and CD origin of the α1KO CD cells was confirmed by aquaporin-2 expression, they underwent EMT — characterized by a fibroblast-like phenotype, increased levels of αSMA, and loss of the epithelial markers ZO-1 and E-cadherin — when grown on plastic (Figure 2, A–D). Loss of E-cadherin expression in the α1KO CD cells was accompanied by increased levels of ZEB1 (Figure 2D), a suppressor of E-cadherin expression whose levels are positively regulated by TGF-β (27, 28).
α1KO CD cells undergo EMT. (A) Cell lysates (10 μg/lane) from serum-starved WT and α1KO CD cells were analyzed by Western blot for levels of aquaporin-2 (AQP2). Mesangial cells (MC) were used as a negative control. (B) Morphology of WT, α1KO, and α1KO-Rec CD cells grown on plastic. α1KO cells showed a fibroblast-like phenotype relative to the epithelial morphology observed in WT or α1KO-Rec cells. (C) CD cells were stained with anti–ZO-1 and anti-αSMA antibodies to visualize levels and localization of epithelial and myofibroblast markers. (D) Western blot analysis showing loss of epithelial markers (E-cadherin), increased myofibroblast markers (αSMA), and increased levels of E-cadherin suppressors (ZEB1) in α1KO compared with WT or α1KO-Rec CD cells (20 μg/lane cell lysates used for analysis). Scale bars: 20 μm (B); 10 μm (C).
To rule out the possibility that acquisition of mesenchymal characteristics in α1KO CD cells was because the cells were cultured on plastic, freshly isolated WT and α1KO CD cells were grown on transwells. Similar to cells grown on plastic, α1KO CD cells cultured for 3 days on transwells developed a fibroblast-like phenotype, characterized by increased levels of αSMA and loss of the epithelial marker ZO-1 (Supplemental Figure 2, A and B). Importantly, α1KO cells reconstituted with the human integrin α1 subunit cDNA (α1KO-Rec cells; Supplemental Figure 1B) showed a restored WT CD epithelial cell phenotype (Figure 2, B–D), which indicates that the EMT of the α1KO CD cells was caused by loss of integrin α1β1 expression, not by cell culture conditions.
Loss of integrin α1β1 leads to increased basal levels of activated SMAD2 and SMAD3. The EMT phenotype of α1KO CD cells resembled that of epithelial cells exposed to TGF-β ligand (29). To determine whether TGF-β pathways were activated in the absence of integrin α1β1, we analyzed the basal levels of activated SMAD2 and SMAD3 in CD cells grown on plastic or transwells and found significantly more phosphorylated SMAD2 (pSMAD2) and pSMAD3 as well as synthesis of collagens I and IV in α1KO versus WT and α1KO-Rec CD cells (Figure 3, A–D, and Supplemental Figure 2, C and D). Altered expression of TβRI and TβRII in the α1KO cells was excluded as the cause of elevated pSMAD2/3 levels (Figure 3A). In addition, TGF-β–mediated noncanonical signaling was also unaffected in α1KO CD cells (Supplemental Figure 3).
Increased TGF-β downstream signaling in α1KO CD cells and mice. (A and C) Cell lysates (20 μg/lane) from the indicated serum-starved cells were analyzed by Western blot for levels of pSMAD2, pSMAD3, TβRI, and TβRII (A) as well as collagens I and IV (C). (B and D) pSMAD2, pSMAD3, SMAD2, and SMAD3 (B) as well as collagen I, collagen IV, and β-actin (D) bands were quantified by densitometry analysis, and signals are expressed as a pSMAD/SMAD or collagen/β-actin ratio. Values are mean ± SEM of 3 independent experiments. *P ≤ 0.05 vs. WT; **P ≤ 0.05 vs. α1KO. (E) Paraffin kidney sections were stained with FITC-conjugated DBA (green) and anti-pSMAD3 (red) antibodies to visualize CDs and activated SMAD3, respectively. (F) Kidney lysates (20 μg/lane) from injured WT and α1KO mice (n = 3 and 5 shown, respectively) were analyzed by Western blot for levels of activated and total SMAD2 and SMAD3. (G) pSMAD2, pSMAD3, SMAD2, and SMAD3 bands were quantified by densitometry analysis; signal is expressed as pSMAD/SMAD ratio. Values are mean ± SEM of the indicated n. Scale bars: 40 μm (E, top); 20 μm (E, bottom).
Consistent with our in vitro findings, we also detected increased nuclear levels of activated SMAD3 in CD cells of UUO-injured α1KO mice and significantly higher levels of activated SMAD2 and SMAD3 in whole kidney lysates isolated from α1KO mice 7 days after UUO (Figure 3, E–G).
Blocking TβR signaling rescues the phenotype of α1KO CD cells. TGF-β signaling is transduced by the serine/threonine kinase receptors TβRI and TβRII. Upon activation by TβRII, TβRI triggers SMAD2 and SMAD3 activation (3, 4). Inhibition of TβR signaling in α1KO CD cells with the TβRI-specific inhibitor SB431542 reverted their EMT phenotype to an epithelial-like morphology, increased membrane localization of ZO-1, and decreased levels of pSMAD2, pSMAD3, αSMA, and collagens I and IV (Figure 4, A–C), which indicates that integrin α1β1 negatively regulates TβR-mediated fibrotic signaling in CD cells.
Inhibition of TβR signaling reverts EMT in α1KO CD cells. (A) Morphology of α1KO CD cells cultured on plastic in 0.2% serum with or without the TβRI inhibitor SB431542 (SB) for 4 days. (B) α1KO CD cells were cultured as in A, then stained with anti–ZO-1 antibodies to visualize its expression and localization. (C) Serum-starved WT and α1KO CD cells were treated with SB431542 at the indicated concentrations. After 24 hours, cell lysates (20 μg/lane) were analyzed by Western blot for levels of collagen I, collagen IV, αSMA, pSMAD2, SMAD2, pSMAD3, and SMAD3. Scale bars: 20 μm (A); 10 μm (B).
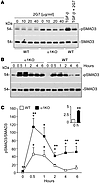
A possible mechanism for these effects is that loss of integrin α1β1 leads to increased levels of activated TGF-β. Similar levels of total and active TGF-β were detected in kidney lysates from WT and α1KO mice and in conditioned medium from WT and α1KO CD cells (Supplemental Figure 4, A–D), which suggests that increased levels of active TGF-β ligands were not responsible for the EMT phenotype. Furthermore, the normal α1KO CD cell surface expression of integrins involved in regulating levels and/or activation of TGF-β (e.g., integrins α2, αv, β3, and αvβ6; Supplemental Figure 4E) further excluded an increased release of extracellular matrix–bound TGF-β ligand. Moreover, inhibition of TGF-β activity with the pan–TGF-β blocking antibody 2G7 only slightly reduced the elevated levels of activated SMAD3 in α1KO CD cells (Figure 5A). Together, these findings suggest that the increased TGF-β–mediated signaling in α1KO CD cells is TGF-β ligand independent and thus cell autonomous.
Prolonged SMAD3 activation in α1KO CD cells stimulated with TGF-β. (A) Serum-starved WT and α1KO CD cells were treated with the indicated concentrations of the blocking anti–TGF-β antibody 2G7. After 24 hours, cell lysates (20 μg/lane) were analyzed by Western blot for levels of pSMAD3 and SMAD3. As controls, 24 hour serum-starved WT CD cells with or without 2G7 (40 μg/ml) were treated with TGF-β (5 ng/ml) 1 hour prior to harvesting. (B) Serum-starved WT and α1KO CD cells were treated with TGF-β (5 ng/ml) for the times indicated. Cell lysates (20 μg/lane) were analyzed by Western blot for levels of pSMAD3 and SMAD3. (C) pSMAD3 and SMAD3 bands were quantified by densitometry analysis, and pSMAD3 signal is expressed as the pSMAD3/SMAD3 ratio. Values are mean ± SEM of 3 independent experiments. *P ≤ 0.05 vs. untreated; **P ≤ 0.05 vs. WT.
We next compared the ability of WT and α1KO CD cells to respond to TGF-β1; α1KO CD cells showed increased and more sustained SMAD3 activation in response to TGF-β1 compared with that in WT CD cells (Figure 5, B and C). Although both SMAD2 and SMAD3 were phosphorylated in α1KO CD cells, in the following experiments we focused on SMAD3, as this transcription factor positively correlates to EMT, matrix accumulation, and renal fibrosis (30).
TβRII is highly phosphorylated on tyrosines in α1KO CD cells. Integrin α1β1 inhibits the activation and signaling of the EGF receptor by preventing tyrosine phosphorylation of its cytoplasmic tail (25, 31). Therefore, we decided to investigate whether integrin α1β1 can also block TGF-β signaling at the receptor level. As the TβRII cytoplasmic tail contains 5 phosphorylatable tyrosines (6, 7), we analyzed basal levels of TβRII tyrosine phosphorylation in serum-starved WT and α1KO CD cells. Cell lysates were immunoprecipitated with the anti-phosphotyrosine antibody 4G10 or with IgG isotype controls and subsequently immunoblotted with anti-phosphotyrosine (anti-pY99) or anti-mouse TβRII antibodies. Phosphorylated TβRII was only detectable in samples derived from α1KO CD cells (Figure 6A), which suggests that loss of integrin α1β1 expression increases tyrosine phosphorylation of TβRII.
TCPTP regulates TβRII tyrosine phosphorylation and signaling. (A) Cell lysates (0.5 mg) from serum-starved WT and α1KO CD cells were immunoprecipitated with the anti-phosphotyrosine antibody 4G10 (10 μg) or with mouse IgG isotype control antibody (10 μg) and analyzed by Western blot. A band corresponding to TβRII (~68 kDa) was visible only in α1KO cells incubated with 4G10. Tyrosine-phosphorylated products (50–100 kDa) were detected with anti-pY99 antibodies in both WT and α1KO CD cells. (B) Morphology of WT CD cells stably transfected with shRNAi control (ShC) or TCPTP shRNAi (Sh-TCPTP). (C and D) Cell lysates (20 μg/lane) from serum-starved WT CD cells transfected with ShC (1 clone shown) or Sh-TCPTP (3 clones shown) and treated with or without SB431542 (SB) were analyzed by Western blot for levels of TCPTP, pSMAD3, SMAD3, and collagen I. (E) WT CD cells were cultured on plastic in 0.2% serum with or without the TCPTP inhibitor compound 8 (TCPTP-I) for 4 days and then stained with anti–ZO-1 and anti-αSMA antibodies. (F) Serum-starved WT CD cells were treated with TCPTP inhibitor at the concentrations indicated. After 24 hours, cell lysates (20 μg/lane) were analyzed for levels of collagen IV, pSMAD3, and SMAD3. (G) Cell lysates (0.5 mg) from serum-starved WT CD cells transfected with control or TCPTP shRNAi (1 clone each shown) were immunoprecipitated and analyzed by Western blot as in A. A band corresponding to TβRII was more evident in lysates of CD cells transfected with TCPTP shRNAi. Scale bars: 20 μm (B and E).
Next we sought to determine whether tyrosine-phosphorylated TβRII increased the phosphorylation/activation of TβRI. We analyzed basal levels of TβRI serine and threonine phosphorylation in serum-starved WT and α1KO CD cells by immunoprecipitating cell lysates with anti-phosphoserine and anti-phosphothreonine antibodies followed by immunoblotting with either anti-phosphoserine or anti-mouse TβRI antibodies. Increased levels of pTβRI were detectable in samples derived from α1KO CD cells (Supplemental Figure 5A), which suggests that increased tyrosine phosphorylation of TβRII indeed leads to increased serine and threonine phosphorylation of TβRI.
The T cell protein tyrosine phosphatase prevents TβRII-mediated EMT. We next determined the mechanism by which integrin α1β1 inhibits tyrosine phosphorylation of TβRII. We and others have shown that TCPTP binds to the integrin α1 cytoplasmic tail, is activated in an integrin α1β1–dependent manner, and dephosphorylates tyrosines both on receptor tyrosine kinases (such as EGF, VEGF, and PDGF receptors) and on scaffolding proteins (such as caveolin-1) (24, 25, 31–33). Interestingly, analysis of the human and mouse TβRII cytoplasmic tails with PhosphoMotif Finder confirmed the presence of 5 potential tyrosine phosphorylation sites (Y259, Y284, Y336, Y424, and Y470) and identified 3 of them (Y284, Y336, and Y470) as substrates for TCPTP dephosphorylation (Supplemental Figure 6, A–C). In support of a role for TCPTP in TGF-β signaling, shRNAi-mediated downregulation of TCPTP or treatment with the selective TCPTP inhibitor compound 8 (34) induced EMT of WT CD cells, with increased SMAD3 activation, loss of ZO-1 at the plasma membrane, and increased expression of αSMA and collagens I and IV, which were efficiently prevented by treatment with the TβRI inhibitor SB431542 (Figure 6, B–F).
shRNAi-mediated depletion of TCPTP in WT CD cells also increased serine and threonine phosphorylation of TβRI and tyrosine phosphorylation of TβRII (Figure 6G and Supplemental Figure 5B), which indicates that TCPTP inhibits TβR signaling and EMT of WT CD cells.
We next determined whether TCPTP activation diminishes TβR-mediated signaling in α1KO CD cells. Activation of TCPTP with the polyamine spermidine (33) in α1KO CD cells reverted their fibroblast-like morphology to an epithelial phenotype and decreased basal levels of activated SMAD3 and expression of collagen I (Figure 7, A and B). In addition, immunoprecipitation of lysates of spermidine-treated α1KO CD cells with the anti-phosphotyrosine antibody 4G10 failed to immunoprecipitate TβRII (Figure 7C), which suggests that spermidine-mediated TCPTP activation decreased tyrosine phosphorylation of TβRII.
Spermidine ameliorates TβR-activated signaling in α1KO CD cells and mice. (A) Morphology of α1KO CD cells cultured on plastic in 0.2% serum with or without spermidine (SP) for 4 days (B) Serum-starved α1KO CD cells were treated with spermidine for 24 hours at the concentrations indicated. Cell lysates (20 μg/lane) were analyzed by Western blot for levels of pSMAD3, SMAD3, and collagen I. (C) Cell lysates (0.5 mg) from serum-starved WT and α1KO CD cells, treated or not for 24 hours with 2.5 μM spermidine, were incubated with 4G10 (10 μg) or mouse IgG isotype control antibody (10 μg). Immunoprecipitation products were then analyzed as in Figure 6A. Lanes were run on the same gel but were noncontiguous (black line). (D) H&E and Trichrome staining of kidneys from α1KO mice untreated or treated with spermidine (30 μM via gavage) at 7 days post-UUO, showing less injury and collagen deposition (blue staining) in the spermidine-treated group. (E and G) Kidney lysates (10 μg/lane) from injured α1KO (n = 4) and spermidine-treated α1KO (n = 3) mice were analyzed by Western blot for levels of collagen I, collagen IV, pSMAD3, and SMAD3. (F and H) Collagen I, collagen IV, β-actin, pSMAD3, and SMAD3 bands were quantified by densitometry analysis; signal is expressed as the collagen I/β-actin or pSMAD/SMAD ratio. Values are mean ± SEM of the indicated n. Scale bars: 20 μm (A); 100 μm (D).
To confirm the in vitro finding and the relevance of TCPTP activation in kidney fibrosis, we treated α1KO mice with spermidine at the time of UUO. At 7 days after UUO, we observed a significantly attenuated injury response, characterized by decreased tubular dilatation and tubulointerstitial fibrosis, decreased expression of collagens I and IV, and diminished SMAD3 activation (Figure 7, D–H).
Polyamines such as spermidine and integrin α1β1 regulate macrophage functions and tissue infiltration (35–37). As macrophages contribute to UUO-mediated fibrosis, we quantified the number of F4/80-positive cells in the papilla of injured α1KO mice untreated or treated with spermidine. Since we found comparable numbers of macrophages in these 2 groups (Supplemental Figure 7), we concluded that the beneficial effects of spermidine are likely due to reduced TβR-mediated signaling in resident cells, rather than altered immunological responses.
Tyrosine phosphatase directly binds and dephosphorylates TβRII. Since TCPTP binds the integrin α1 subunit (24, 31) and we observed evidence in support of its role in TβRII tyrosine dephosphorylation, we hypothesized that TCPTP, integrin α1β1, and TβRII form a ternary protein complex. Immunoprecipitating the integrin α1 subunit from lysates of α1KO-Rec CD cells and immunoblotting the precipitate with specific antibodies against integrin α1, TβRII, and TCPTP confirmed that they indeed formed a complex (Supplemental Figure 8A). In addition, immunofluorescence staining performed on frozen sections of human kidneys revealed that integrin α1, TβRII, and TCPTP colocalized in CDs (Supplemental Figure 8, B–D).
To further confirm that TCPTP can directly bind and dephosphorylate TβRII, we performed ELISA using recombinant full-length TCPTP and human glutathione S-transferase–TβRII cytoplasmic domain (GST-TβRIICD) as well as tyrosine-phosphorylated GST-TβRIICD (GST-pYTβRIICD) (Supplemental Figure 9, A–C), and in vitro dephosphorylation assays with GST-pYTβRIICD and a constitutively active form of TCPTP (TCPTP-37). Whereas GST alone showed no binding to TCPTP, GST-TβRIICD and GST-pYTβRIICD demonstrated robust binding to immobilized TCPTP (Figure 8A). Furthermore, TCPTP-37 efficiently dephosphorylated GST-pYTβRIICD in a dose-dependent manner (Figure 8, B and C). Importantly, addition of the phosphatase inhibitor sodium vanadate to the reaction mixture prevented TCPTP-37–mediated GST-pYTβRIICD dephosphorylation (Figure 8B), which suggests that TβRII is a TCPTP substrate.
TCPTP directly binds and dephosphorylates the cytoplasmic tail of TβRII. (A) Immobilized TCPTP (5 μg/ml) was incubated with GST, GST-TβRIICD, or GST-pYTβRIICD at the indicated concentrations. Bound proteins were detected with anti-GST antibodies. Shown is 1 experiment performed in triplicate, representative of 2 independent experiments performed with similar results. Values are mean ± SD. *P ≤ 0.05 vs. GST-pYTβRIICD; **P ≤ 0.05 vs. GST. (B) GST-pYTβRIICD (~50 ng) was incubated with TCPTP-37 at the indicated concentrations, with or without the tyrosine phosphatase inhibitor sodium vanadate. After 10 minutes at 30°C, samples were analyzed by Western blot for levels of phosphorylated (anti-pY99) and total (anti-TβRII) GST-pYTβRIICD. (C) pY99 and TβRII bands were quantified by densitometry. Values represent pY99/TβRII ratio relative to samples incubated without TCPTP (assigned as 1). (D and E) GST-pYTβRIICD or GST-pYTβIIY284/336/470ACD (~50 ng) was incubated with TCPTP-37 at the indicated concentrations. After 10 minutes at 30°C, samples were analyzed by Western blot for levels of phosphorylated (anti-pY99) and total (anti-TβRII) GST-conjugated recombinant proteins. (F) pY99 and TβRII bands were quantified by densitometry. Values (mean ± SEM of 3 experiments) represent pY99/TβRII ratio relative to samples incubated without TCPTP (assigned as 1). *P ≤ 0.05 vs. 0 ng/ml.
As the cytoplasmic domain of TβRII contains 3 potential TCPTP dephosphorylatable tyrosines (Y284, Y336, and Y470; Supplemental Figure 6 and Supplemental Figure 9A), we investigated whether these 3 sites can be dephosphorylated by TCPTP in vitro. We treated GST-pYTβRIICD recombinant proteins carrying the triple Y284/336/470A mutations (GST-pYTβRIIY284/336/470ACD) with TCPTP-37. GST-pYTβRIIY284/336/470ACD retained a basal tyrosine phosphorylation signal, due to the remaining phosphorylatable sites TβRIIY259 and TβRIIY424 in the cytoplasmic domain (Figure 8D, Supplemental Figure 6, and Supplemental Figure 9A). However, TCPTP-37, at doses that efficiently dephosphorylated GST-pYTβRIICD, failed to dephosphorylated GST-pYTβRIIY284/336/470ACD (Figure 8, D–F), which suggests that Y284, Y336, and/or Y470 in the TβRII cytoplasmic domain are the target tyrosine residues of TCPTP in vitro.
TβRIIY336 and TβRIIY470 control cell morphology and SMAD activation. We next defined the relative contribution of TβRIIY284, TβRIIY336, and/or TβRIIY470 in TβR-mediated activation of SMAD-dependent signaling and EMT. To this end, we crossed α1KO mice with floxed TβRII mice (Itga1–/– Tgfbr2fl/fl; referred to herein as α1KO TβRIIfl/fl), isolated CD cells, and deleted the floxed TβRII alleles using adenoviral Cre (adeno-Cre) infection. The resulting cells were then transfected with human TβRII carrying single or multiple Y-to-A mutations in the TCPTP dephosphorylation sites Y284, Y336, or Y470. While α1KO TβRIIfl/fl CD cells displayed a fibroblast-like phenotype, adeno-Cre treatment resulted in an epithelial morphology (Figure 9A), similar to α1KO CD cells treated with SB431542 or spermidine (Figure 4A and Figure 7A). As expected, adeno-Cre–treated α1KO TβRIIfl/fl CD cells transfected with WT TβRII switched to a fibroblast-like phenotype (Figure 9A). Transfection of adeno-Cre–treated α1KO TβRIIfl/fl CD cells with single-mutant TβRIIY284A, TβRIIY336A, or TβRIIY470A also reverted to a fibroblast-like phenotype, while cells expressing TβRIIY284/336/470A retained the epithelial phenotype (Figure 9A). Interestingly, transfection of adeno-Cre–treated α1KO TβRIIfl/fl CD cells with TβRII carrying 2 Y-to-A substitutions revealed that only expression of TβRIIY336/470A prohibited the conversion of the epithelial cell morphology to a fibroblast-like phenotype (Figure 9A).
Y336 and Y470 in the cytoplasmic tail of TβRII regulate EMT. (A) α1KO TβRIIfl/fl CD cells were left untreated (–Cre) or treated with adeno-Cre (Cre) to downregulate TβRII. Cells were then transfected with empty vector (Cre/Vo), WT TβRII (Cre/TβRII), or TβRII constructs mutated in 1 or more of the 3 tyrosines (as indicated), and their morphology was evaluated. (B) HEK293 cells were transiently transfected with empty pEGFP-N2 vector (EGFP), WT TβRII (TβRII/EGFP), or TβRII constructs mutated in 1 (TβRIIY284A/EGFP and TβRIIY470A/EGFP) or multiple (TβRIIY336/470A/EGFP and TβRIIY284/336/470A/EGFP) tyrosines. After 72 hours, the membrane localization (asterisks) of the various TβRII constructs was evaluated by analyzing the cells under an epifluorescence microscope. (C and D) Cell lysates (20 μg/lane) from the serum-starved CD cell populations indicated were analyzed by Western blot for levels of TβRII, pSMAD3, SMAD3, collagen IV, and E-cadherin. Lanes were run on the same gel but were noncontiguous (black lines). (E) The indicated CD cell populations were serum starved for 24 hours, then treated or not with TGF-β1 for 30 minutes. Cell lysates (20 μg/lane) were analyzed by Western blot for levels of TβRII, pSMAD3, and SMAD3. (F and G) Cell lysates (0.5 mg) from the serum-starved CD cell populations indicated were immunoprecipitated with anti-human TβRII antibodies (2 μg) (F) or with 4G10 (10 μg) or mouse IgG isotype control antibody (10 μg) (G), then analyzed by Western blot. A band corresponding to TβRII was more tyrosine phosphorylated (F) and more evident (G) in lysates of CD cells expressing WT than Y284/336/470A TβRII. Scale bars: 20 μm (A); 5 μm (B).
Next, we analyzed subcellular localization of WT TβRII or TβRII carrying single or multiple Y-to-A substitutions fused to EGFP by transiently transfecting expression constructs into HEK293 cells. These constructs localized to the plasma membrane (Figure 9B), excluding aberrant localization of mutant TβRII as a cause for impaired TβRII/TβRI function.
Cell signaling analysis performed on transiently transfected CD cells expressing comparable levels of WT and Y-to-A mutated TβRII revealed that TβRIIY336 and TβRIIY470 controlled SMAD3 activation. CD cells carrying the single TβRIIY336A or TβRIIY470A mutation displayed significantly reduced levels of pSMAD3 compared with cells expressing WT TβRII or TβRIIY284A (Figure 9C and Supplemental Figure 10). Consistent with their epithelial morphology (Figure 9A), only cells expressing the TβRIIY336/470A and TβRIIY284/336/470A mutations showed E-cadherin levels similar to those of adeno-Cre–treated α1KO TβRIIfl/fl CD cells (Figure 9D and Supplemental Figure 10), which suggests that both Y336 and Y470 are required for regulation of E-cadherin expression. Finally, cells carrying all 3 Y-to-A mutations showed collagen IV levels similar to those of adeno-Cre–treated α1KO TβRIIfl/fl CD cells (Figure 9D and Supplemental Figure 10), which indicates that TβRII tyrosine phosphorylation–mediated regulation of collagen synthesis is both SMAD dependent and independent.
We next determined the ability of the TβRII mutants to activate SMAD3 in response to TGF-β1. For TGF-β1–mediated signaling, we focused on CD cells expressing WT, TβRIIY284A (which should promote SMAD3 phosphorylation via Y336 and Y470), and TβRIIY284/336/470A (which should not promote SMAD3 activation due to loss of Y336 and Y470). Mock-treated and adeno-Cre–infected α1KO TβRIIfl/fl CD cells were used as positive and negative controls, respectively. Western blotting revealed comparable expression levels of WT and Y-to-A mutated TβRII. As expected, TGF-β1 promoted SMAD3 activation only in mock-treated α1KO TβRIIfl/fl CD cells, or adeno-Cre–treated α1KO TβRIIfl/fl CD cells transfected with either WT or TβRIIY284A (Figure 9E). In contrast, TGF-β1 failed to stimulate SMAD3 phosphorylation in TβRIIY284/336/470A expressing cells (Figure 9E), which suggests that Y336 and Y470 regulate TGF-β1–mediated activation of canonical signaling.
Finally, we sought to determine whether Y284, Y336, and/or Y470 are phosphorylated in α1KO CD cells. We immunoprecipitated TβRII in cell lysates from adeno-Cre–treated α1KO TβRIIfl/fl CD cells transfected with either WT TβRII or TβRIIY284/336/470A and immunoblotted the gel-separated precipitates with anti-human TβRII or anti-pY99 antibodies, respectively. Adeno-Cre–treated α1KO TβRIIfl/fl CD cells served as negative control. Although CD cells expressing WT TβRII and TβRIIY284/336/470A contained comparable levels of TβRII, only cells expressing WT TβRII showed substantial TβRII tyrosine phosphorylation (Figure 9F). Similarly, reciprocal immunoprecipitation with the anti-phosphotyrosine antibody 4G10 followed by anti-TβRII immunoblotting revealed that only CD cells transfected with WT TβRII showed tyrosine phosphorylation (Figure 9G). Together, these data confirmed that Y284, Y336, and/or Y470 can be phosphorylated in vivo.