Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells (original) (raw)
FoxP3 is preferentially expressed in human CD4+CD25+ cells. To examine the expression of FoxP3 in human cells, peripheral blood mononuclear cells were purified from normal donors and specific subsets isolated on the basis of cell-surface phenotype. FoxP3 expression was ascertained by QPCR and immunoblotting. Both total CD4+ and CD8+ T cells expressed FoxP3; however, CD4+ cells express higher levels of FoxP3 (data not shown). B cells and nonlymphoid cells showed no FoxP3 expression. The expression in human CD8+ T cells differs from data seen in the mouse where only the CD4+ subset expressed detectable FoxP3 (17, 18, 20). As mouse FoxP3 has been shown to be preferentially expressed in the CD4+CD25+ cells, we further divided human CD4+ T cells based on CD25 expression and examined FoxP3 expression. Since only the highest 1–3% of CD25+ expressing cells in human CD4+ cells have been shown to be regulatory, these cells were used in our assays as the CD25+ population. As shown in Figure 1, a and b, only the CD25+ subset of human CD4+ T cells expressed FoxP3 mRNA and protein. Thus, as was seen in mice, only the putative TR population expresses detectable levels of FoxP3 within human CD4+ T cells. Interestingly, human FoxP3 protein migrates as a doublet under these conditions, one species of which comigrates with the protein expressed in 293T cells transfected with a human FoxP3 cDNA clone (see “control” lanes in Figures 2 and 3). It is not clear at this point whether these two species reflect post-translational modifications of FoxP3, the products of differentially spliced mRNAs, or some other possibility.
CD4+CD25+ regulatory cells from normal human peripheral blood express FoxP3. (a) Real-time QPCR analysis of FoxP3 gene expression relative to GAPDH expression in purified CD4+, CD4+CD25– (CD25–), and CD4+CD25+ (CD25+) T cells from a range of normal donors. (b) Western blot analysis of FoxP3 in purified CD4+, CD4+CD25–, and CD4+CD25+ cells from two separate donors. (c) Proliferation and suppression of purified CD4+CD25– and CD4+CD25+ T cells stimulated with soluble anti-CD3 and anti-CD28. These data are from one experiment but are representative of eight separate experiments with a suppression range of 60–95%.
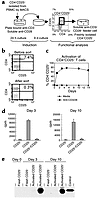
Activation of human CD4+CD25– T cells induces FoxP3 expression. Western blot analysis of FoxP3 expression in (a) freshly isolated, purified CD4+, CD4+CD25+, and CD4+CD25– T cells or CD4+CD25– T cells that have or have not (media) been activated with plate-bound anti-CD3/soluble anti-CD28 (anti-CD3/28) for 24 or 72 hours in the presence or absence of IL-2, (b) freshly isolated CD4+CD25– or CD4+CD25+ and activated CD4+CD25– PBMCs after sorting into CD4+CD25– and CD4+CD25+ T cells. (c) FACS plot showing percentage of cells in each population before sorting. Western blot analysis of FoxP3 expression in freshly isolated CD4+CD25– T cells or activated CD4+CD25– T cells either unsorted or sorted for CD25 and Annexin V staining. Cells were also gated and sorted on live cells by FCS versus SSC. Control cells were 293T cells transfected with a human FoxP3 cDNA clone. Parts a and b show results from one experiment but are representative of four separate experiments. Figure 2c shows results from one experiment.
CD4+CD25+ regulatory T cells can be induced by activation of human CD4+CD25– PBMCs. (a) Schematic for generation of regulatory cells from CD4+CD25– cells, including a typical FACS plot with percentages for activated CD4+CD25– cells before sorting. Cells were incubated on plate-bound Ab (Y) for 24 hours and then transferred to a new well without Ab for 9 days. (b) Dot plots showing CD4 versus CD25 staining on CD4+ PBL before and after sorting with CD25 MACS microbeads. (c) Percent of CD4+CD25+ cells over time when CD4+CD25– cells were stimulated with plate-bound anti-CD3/soluble anti-CD28 or media. (d) Proliferation and suppression of freshly isolated CD4+CD25– T cells by CD4+CD25+ cells, which were generated by activating CD4+CD25– PBMCs with plate-bound anti-CD3/soluble anti-CD28 for 3 or 10 days. (e) Western blot analysis of FoxP3 expression on day 3 or day 10 after activation of CD4+CD25– T cells. Control cells were 293T cells transfected with a human FoxP3 cDNA clone. These data are from one experiment and are representative of six separate experiments with a suppression range of 60–95%.
We next determined the function of the CD4+CD25+ T cells. For these experiments we used the CD4+CD25– and CD4+CD25+ peripheral blood T cells whose FoxP3 expression levels were shown in Figure 1 (a and b). These T cell subsets were assessed for their ability to respond to T cell receptor (TCR) stimulation, and for the ability of the CD25+ cells to suppress the in vitro activation of the CD25– cells. When cultured in the presence of feeder cells along with soluble anti-CD3 and anti-CD28, the CD4+CD25– cells responded with robust proliferation, whereas the CD4+CD25+ cells did not (Figure 1c). When the two populations were cocultured, the level of proliferation, as measured by 3H-thymidine incorporation, was dramatically reduced (Figure 1c). The level of suppression seen was correlated with the ratio of CD4+CD25–:CD4+CD25+ cells in the culture, with more CD25+ cells resulting in more suppression of CD25– cell proliferation. These results are not due to exhaustion of the resources within the culture because of the small number of cells in the culture and the fact that the addition of the same amount of CD25– cells instead of CD25+ cells does not cause suppression (see below). Similar results have been observed by other investigators for the CD4+CD25+ subset of human T cells (21–23). Thus, as has been seen in rodents, expression of FoxP3 correlated with TR activity.
TCR stimulation induces FoxP3 expression in CD4+CD25– human T cells. In addition to being a marker for TR cells, CD25 expression on CD4+ T cells is an indicator of cell activation. Specifically, stimulation of CD4+CD25– T cells through the TCR and CD28 leads to several outcomes including proliferation, cytokine production, and induction of cell-surface expression of CD25. It is, therefore, possible that some or all of the CD4+CD25+ T cells isolated from PBMCs are the result of recent activation. To assess the possibility that recently activated CD4 T cells express FoxP3, we determined FoxP3 expression in CD4+CD25– T cells stimulated through the TCR in vitro. CD4+CD25– T cells were purified from peripheral blood and stimulated with plate-bound anti-CD3 and soluble anti-CD28 in the presence or absence of IL-2, for 24 hours or 72 hours. At the indicated times, cells were harvested, lysed, and examined for FoxP3 expression by immunoblotting. As shown in Figure 2a, the starting population of CD4+CD25– T cells lacked detectable FoxP3 expression. However, after 24 hours of plate-bound anti-CD3/soluble anti-CD28 stimulation, FoxP3 expression was found in both cultures (± IL-2) and was considerably increased in the 72-hour cultures. To determine the nature of the FoxP3-expressing cells in these cultures, we purified CD25– and CD25+ cells from 72-hour cultures and examined FoxP3 expression. As shown in Figure 2b, only the CD25+ cells from these cultures expressed FoxP3. From these experiments we conclude that, unlike what has been described in the mouse, stimulation of CD4+CD25– T cells through the TCR results in the induction of FoxP3 expression and that this expression correlates with cell-surface expression of CD25.
We have recently shown that T cells that overexpress FoxP3 (from mice bearing a FoxP3 transgene) do not proliferate or produce cytokines upon TCR stimulation but undergo rapid apoptosis (ref. 24 and D.J. Kasprowicz and S.F. Ziegler, unpublished observations). Thus, one possible explanation for the results in Figure 2 is that FoxP3 is induced in those stimulated T cells undergoing activation-induced cell death. To ascertain whether FoxP3 expression correlated with activation-induced cell death, fresh human CD4+CD25– T cells were activated with plate-bound anti-CD3/soluble anti-CD28 for 72 hours and were either left unsorted or sorted into CD4+CD25–AnnexinV–, CD4+CD25+AnnexinV–, or CD4+CD25+AnnexinV+ (Figure 2c). FACS analysis shows that approximately 39% of the CD4+ cells are CD25+AnnexinV–, 59% CD25–AnnexinV–, 1.5% CD25+AnnexinV+, and 1% CD25–AnnexinV+. Annexin V staining was used as a measure of apoptosis. Lysates were prepared from the various populations, and FoxP3 expression was analyzed by immunoblotting. The number of CD4+CD25–AnnexinV+ cells was consistently insufficient for analysis by Western blotting. In all cases, FoxP3 expression segregated with CD25 expression and not with Annexin V staining. Therefore, in these T cell populations, FoxP3 expression did not mark cells undergoing activation-induced cell death. Therefore, in contrast to results seen in mice, FoxP3 can be expressed upon activation of CD4+CD25– T cells in humans (17, 18).
CD4+CD25+FoxP3+ T cells that arise from in vitro stimulation have TR activity. Since freshly isolated CD4+CD25+ T cells expressed FoxP3 and displayed regulatory activity, we asked whether CD25+ cells, arising from in vitro stimulation, also had suppressor cell activity. For these experiments, CD4+CD25+ and CD4+CD25– cells were isolated at days 3 and 10 of the culture as described above. At each time point, CD4+CD25– cells were freshly purified from the same donor and used in coculture suppression assays (Figure 3a). Figure 3b shows a representative dot plot of CD4+CD25– cells isolated by magnetic cell sorting (MACS). Several methods for activation of CD4+CD25– cells were employed; however, activation with plate-bound anti-CD3 and soluble anti-CD28 consistently resulted in a higher percentage of CD25+ cells after 10 days of culture (Figure 3c). Although both anti-CD3/CD28 bead-activated and plate-bound anti-CD3/soluble anti-CD28–activated CD4+CD25– cells induced FoxP3, the high signal strength of the plate-bound anti-CD3/soluble anti-CD28 was optimal for generation of T cells expressing FoxP3 (data not shown). Suppression assays were then performed with freshly isolated autologous CD4+CD25– T cells using soluble anti-CD3/CD28 along with feeder cells as has been done by others (18, 20, 21, 25). The CD4+CD25+ cells derived from CD4+CD25– cells activated with plate-bound anti-CD3/soluble anti-CD28 were able to suppress proliferation of freshly isolated CD4+CD25– cells (Figure 3d). Identical results were obtained when CD25– cells were FACS sorted instead of MACS sorted before the 10-day culture. In addition, CD25+ cells from the FACS sort were activated and found that less than 1% of these cells remained alive by day 10 after activation, thus negating the possibility that pre-existing CD25+ cells are expanding in these cultures. FoxP3 expression also correlated with suppressive activity (Figure 3e). These data demonstrate that CD4+CD25+ T cells with regulatory characteristics can arise from CD4+CD25– cells upon activation and that this regulatory activity is associated with the expression of FoxP3.
Furthermore, the suppressive activity of regulatory CD4+CD25+ generated from CD4+CD25– T cells is cell-contact dependent and cytokine independent. Separation of plate bound anti-CD3/soluble anti-CD28–activated CD4+CD25+ cells from freshly isolated CD4+CD25– cells by a transwell abrogates suppression of these cells (Figure 4a). As a control, activated-sorted CD4+CD25– cells were used in place of activated-sorted CD4+CD25+ cells. In contrast to results seen with activated-sorted CD25+ cells, in this assay, activated-sorted CD25– cells did not suppress proliferation. However, in one experiment, the CD4+CD25– cells isolated from activated cultures displayed suppressor activity and FoxP3 expression, suggesting that CD25 may not be a marker for all TR. Experiments are planned to explore this possibility. In addition, TR activity by generated CD25+ cells did not require IL-10 or TGF-β (Figure 4b). The addition of 10 μg/ml of anti–IL-10 or anti–TGF-β Ab has been shown to ablate suppressive activity of T regulatory type 1 (TR1) cells (26, 27), however, the same concentration of this Ab did not inhibit suppression by activated CD4+CD25+ cells generated from CD4+CD25– cells. These generated CD4+CD25+ regulatory cells are therefore similar to freshly isolated CD4+CD25+ TR from both humans and mice.
Activation-induced CD4+CD25+ regulatory T cells are cell-contact dependent and cytokine independent. Proliferation and suppression of freshly isolated CD4+CD25– T cells by CD4+CD25+ (a, left panel, and b) or CD4+CD25– (a, right panel) cells, which were generated by activation CD4+CD25– PBMCs with plate-bound anti-CD3/soluble anti-CD28 for 14 days. (a) CD4+CD25+ and CD4+CD25– cells from the same culture were sorted and cultured alone, together with freshly isolated CD25– cells, or separated by a transwell. Sorted cells are indicated in bold type and freshly isolated cells in normal type. (b) Cells were cultured in the presence of 10 μg/ml anti–IL-10, anti-TGF-β, or an isotype control Ab (RIgG2a). These data are from one experiment but are representative of two separate experiments.