Effect of a patient-centered drug review on polypharmacy in primary care patients: study protocol for a cluster-randomized controlled trial (original) (raw)
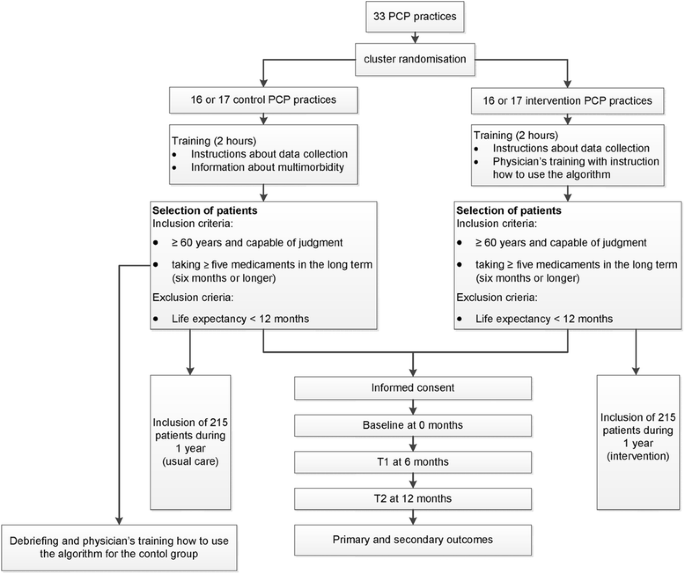
We will conduct a cluster-randomized controlled trial (randomization on PCP-practice level) with primary care physicians in the northern part of Switzerland (see Fig. 1). The design and methodology of our study is based on the experiences of a small pilot study.
Fig. 1
Study flow chart
Pilot study
The pilot study [[21](/articles/10.1186/s13063-015-0915-7#ref-CR21 "Neuner-Jehle S, Krones T, Senn O. Systematic elimination of prescribed medicines is acceptable and feasible among polymorbid family medicine patients. Praxis. 2014;103(6):317–22. doi: 10.1024/1661-8157/a001591
.")\] was conducted with 14 primary care physicians. An evaluation was conducted to test the logistics, baseline measurements and the feasibility of the study. The pilot study resulted in minor changes in the questionnaire and an important element of shared-decision-making was added: beside the four major clinical problems defined by the PCP the four most important complaints in a patient’s perspective were added. In order to detect potential undertreatment a special section was added entitled “medication started”. Recruitment and eligibility of primary care physicians
PCPs are eligible for participation if they provide care in the routine primary setting to unselected patients. About 1700 PCPs in the Canton of Zurich will be invited by a formal letter of the Institute of Primary Care of the University of Zurich.
PCPs who finally agree to participate will be listed in alphabetical order. If two PCPs of the same practice agree to participate, the first name in the alphabet receives a number which is valid also for the second one in order to avoid contamination between groups. Randomization will be stratified according to the practice size (e.g. single-handed versus group practice). The randomization scheme will be generated by using the website Randomization.com (http://www.randomization.com) and group assignment will be performed by a study nurse not involved in further data analysis. The PCPs with the corresponding numbers will be randomly allocated to the intervention and control group, respectively.
PCPs will be informed only after completion of the study about the group they have been randomized to (intervention or control group) in a debriefing session. At this time, an education session on how to use the algorithm will be offered to the control group.
If the number of the required 33 (see power calculation below) participating PCPs cannot be reached, the randomization will be done with the interested PCPs and the recruiting will be expanded to other Cantons of the northern area of Switzerland, followed by another randomization process.
Each PCP in the intervention and control groups will receive a financial allowance.
Patient recruitment
All PCPs are asked to approach, consecutively and regardless of the reason for the current consultation, patients aged 60 or older who are taking 5 or more long-term drugs.
Patient inclusion criteria
Be aged at least 60 years.
Be taking 5 or more long-term drugs (6 months or longer).
Patient exclusion criteria
Life expectancy less than 12 months.
Intervention
Blinding of PCPs is not possible in this study design and even participation in a study addressing a certain topic can influence a physician’s behavior. To achieve a degree of blinding, PCPs are not provided with specific information at invitation. They will receive a lecture (length: 2 hours) about multimorbidity in general and instructions for collecting data in a usual care group. PCPs from the control group will be told that the study purpose is to investigate best practices for physician-patient communication.
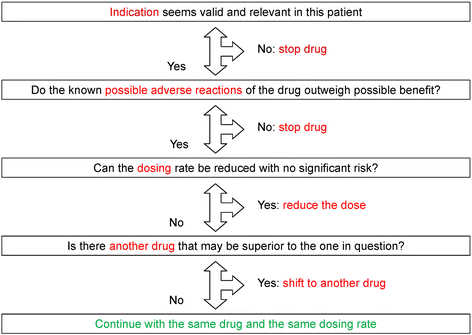
PCPs in the intervention group will undergo a physicians’ training with instruction on how to use the algorithm including a communication skills training. The adapted GPGP-algorithm used in our trial consists of four steps and is a patient-centered process. First, the drugs are listed together with the four main diagnoses as well as the four main disorders from the patient’s perspective. Then the following steps will be systematically followed (see Fig. 2):
Fig. 2
Improving drug therapy in primary care (adapted from [[20](/articles/10.1186/s13063-015-0915-7#ref-CR20 "Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–54. doi: 10.1001/archinternmed.2010.355
.")\] and \[[21](/articles/10.1186/s13063-015-0915-7#ref-CR21 "Neuner-Jehle S, Krones T, Senn O. Systematic elimination of prescribed medicines is acceptable and feasible among polymorbid family medicine patients. Praxis. 2014;103(6):317–22. doi:
10.1024/1661-8157/a001591
.")\])- Indication of the drug in this patient (identifying undertreatment or overtreatment)
- Do the known possible adverse reactions of the drug outweigh the possible benefits? (Potential adverse events)
- Can the dosing rate be reduced with no significant risk? (Dosing problems)
- Is there another drug that may be superior to the one in question?
Finally, the result of this analysis will be discussed together with the patient. For each drug the PCP will note whether the patient agrees with the recommendation.
After obtaining informed consent from the patient, a practice nurse or the PCP creates a list of the patient’s present medication. Then, the PCP defines the four major clinical problems and, together with the patient, the four most important complaints from the patients’ perspective. Physicians in the intervention group then decide, for every drug listed, whether the indication is correct, whether there are side effects, whether an alternative treatment would be suitable or whether a change in the dosage is indicated (key questions of the algorithm). After discussion with the patient, the PCP and patient decide together whether to stop a drug, to change the dosage of a drug or to switch to an alternative drug, with the option to restart if symptoms should increase or the disease deteriorate.
Primary outcomes measures
Change in the number of drugs (deprescribing rate) 12 months after applying the tool.
The primary outcome will be calculated on the basis of the medication list, provided by the practice nurse or the PCP at baseline (before the intervention) and 12 months afterwards.
Secondary outcome measures
Table 1 displays the secondary outcomes with the appropriate measuring method.
Table 1 Secondary outcomes and the appropriate measuring method
After the baseline assessment (including gender and living situation) systematic follow-up measurements will take place after 6 and 12 months.
Patient-reported outcomes
Quality of life (QoL) will be assessed using the EuroQol (EQ-5D-3 L). This is a generic preference-based health status measure that has been shown to be valid and reliable in a variety of populations and patients groups [22–25].
Furthermore, patients are asked to determine their QoL on a Likert scale from −2 to +2. They are asked about their main complaint and they have to indicate the intensity (no complaint to unsupportable) on a visual analogue pain scale.
Disease-specific parameters
PCPs of both groups measure the following disease-specific parameters at baseline, after 6 and after 12 months:
- Weight and blood pressure
- Glycated hemoglobin (HbA1c), for patients with diabetes only
- Thyroid-stimulating hormone (TSH), for patients with thyroid hormone substitution only
- Hemoglobin, for patients with iron substitution or vitamin B12 substitution only
Furthermore, the PCP evaluates the course of the main diseases (stable, improvement, aggravation).
Safety outcomes
Adverse events have to be reported by the PCP at the study center and the safety board. The following definition for adverse events is used:
- Complication of an existing disease (e.g. myocardial infarction in a coronary heart disease patient)
- Acute illness newly diagnosed
- Hospitalization or death
In case of adverse events the safety board can consult the medical history of the patient and, together with the PCP, it decides whether there is a plausible connection between intervention and adverse event.
The safety board is composed of a clinical ethicist, biostatistician and a geriatrician.
Data collection procedures
Patients will receive detailed written information on the aim of the study. After giving their written informed consent data will be collected by means of paper documents. For every patient a dossier with the different case report forms will be created. The forms are encoded; the code is stored at each primary care physicians’ practice. If a case-tracking is needed (in case of adverse events) it can be decoded. The transfer from paper to electronic data will be carried out through a research associate and controlled by a second research associate. Grouping of diagnoses and drugs will be organized by standardised databases.
Statistical analysis
The primary data analysis will follow the intent-to-treat (ITT) approach. This means that all available data from all individuals will be analyzed according to treatment group assignment, regardless of whether or not each individual actually received the assigned treatment.
The primary outcome, change in the number of drugs 12 months after applying the deprescribing tool, will be compared by using a t test for independent groups comparison. Hierarchical regression will be used to consider the cluster-design for potential confounder. Determinants associated with a change of the medication are investigated by exploratory, multivariate regression analysis.
For secondary outcomes, parametric (t test) or non-parametric tests (chi-square and Wilcoxon test) will be used where appropriate.
Descriptive statistics will be used to describe the study population. Drop-out and loss to follow-up will be described.
In case of study discontinuation the collected data that are hitherto available will be anonymized and evaluated.
The last observation carried forward (LOCF) method will be used for dealing with the missing data for patients who have dropped out. The drop-out rate and the causes of drop-outs will be compared between the two groups to investigate the possible influence of the intervention. To estimate the last observation carried forward-effect a per-protocol (PP) analysis will be applied.
Timeframe of the study
The recruitment of the PCP is planned over 2 months between March and May 2015.
Patients’ eligibility screening and patient inclusion is projected within a period of 4 months. The aim is to include 1 patient/week per PCP.
Ethics approval
The study protocol is approved by the Ethics Committee of the Canton of Zurich (reference KEK-ZH-number 2015–0595).
Patient informed consent
Previous to study participation, patients receive written and verbal information about the content and extent of the planned study from the PCPs. In case of acceptance, they sign the informed consent form.
Data security/disclosure of original documents
The patient names and all other confidential information fall under medical confidentiality rules and are treated according to appropriate Federal Data Security Laws. The patient names are not accessible to the study staff.
Sample size calculation
Assuming a 0.05 2-sided significance level, 30 clusters (on PCP-level) including 13 patients each would have an 80 % power to detect a difference of 1 drug, which we consider to be clinically relevant.
Based on previous studies [[14](/articles/10.1186/s13063-015-0915-7#ref-CR14 "Jager C, Freund T, Steinhauser J, Joos S, Wensing M, Szecsenyi J. A tailored implementation intervention to implement recommendations addressing polypharmacy in multimorbid patients: study protocol of a cluster randomized controlled trial. Trials. 2013;14:420. doi: 10.1186/1745-6215-14-420
.")\] we assumed an intracluster correlation coefficient (ICC) of 0.02 for the primary outcome and a standard deviation of 2.8 \[[21](/articles/10.1186/s13063-015-0915-7#ref-CR21 "Neuner-Jehle S, Krones T, Senn O. Systematic elimination of prescribed medicines is acceptable and feasible among polymorbid family medicine patients. Praxis. 2014;103(6):317–22. doi:
10.1024/1661-8157/a001591
.")\]. In consideration of a drop-out rate of 10 %, 33 PCPs (16 respectively 17 PCPs with 215 patients in each arm) are needed.