Sex-specific differences in the prognostic value of METS-IR for long-term outcomes in patients with MASLD and advanced liver fibrosis: a nationwide study (original) (raw)
Abstract
Objective
Insulin resistance (IR) plays a critical role in shaping long-term outcomes in patients with metabolic dysfunction-associated steatotic liver disease (MASLD). Recent findings suggest that biological sex may influence the onset and progression of MASLD, yet it remains unclear whether sex modifies the link between IR and mortality in those with MASLD and advanced liver fibrosis.
Methods
We analyzed data from 14,081 MASLD patients (7327 men and 6754 women) drawn from the 2001–2018 cycles of the National Health and Nutrition Examination Survey (NHANES). Participants were categorized based on sex-specific deciles of the Metabolic Score for Insulin Resistance (METS-IR). Kaplan–Meier survival analysis and Cox proportional hazards models were used to assess the association between METS-IR and all-cause mortality. Restricted cubic spline (RCS) modeling explored potential non-linear relationships.
Results
Marked sex-related disparities were identified in clinical and metabolic characteristics. Elevated METS-IR significantly predicted increased all-cause mortality in females with MASLD (log-rank p < 0.001), whereas this trend was not evident in males (p = 0.54). Multivariable Cox models showed that higher METS-IR independently correlated with mortality in women with MASLD and advanced fibrosis, but not in their male counterparts.
Conclusion
The prognostic significance of METS-IR differs by sex in MASLD. Elevated METS-IR independently increases long-term mortality risk in females, supporting the need for sex-specific risk evaluation in managing metabolic liver disease.
Similar content being viewed by others
Introduction
In 2023, the term metabolic dysfunction-associated steatotic liver disease (MASLD) was introduced to replace non-alcoholic fatty liver disease (NAFLD), emphasizing the key role of metabolic disturbances in disease onset and progression [1]. MASLD now affects more than 30% of adults worldwide, with incidence rates rising steadily each year [2]. MASLD can progress to fibrosis and cirrhosis [[3](/article/10.1186/s40001-025-03783-x#ref-CR3 "Dominik N, Nixdorf L, Schwarz M, Hofer Bs, Hartl L, Balcar L, et al. Udff and auto Pswe accurately assess liver steatosis and fibrosis risk in obese patients with Masld. Ultraschallmed. 2025. https://doi.org/10.1055/A-2592-1431
.")\], especially in patients with combined type 2 diabetes \[[4](/article/10.1186/s40001-025-03783-x#ref-CR4 "Muzurović E, Topić G, Todorović N, Rizzo M, Zečević K. The prevalence and correlates of advanced fibrosis in patients with and without diabetes mellitus and metabolic dysfunction-associated steatotic liver disease: a cross-sectional study. J Diabetes Complications. 2025;39:109147.
https://doi.org/10.1016/J.Jdiacomp.2025.109147
.")\]. In addition to liver-related issues, MASLD is strongly linked to a range of extrahepatic conditions, such as chronic kidney disease, cardiovascular complications, and certain malignancies, thereby exerting a substantial impact on both public health and medical resources \[[5](#ref-CR5 "Miao L, Targher G, Byrne Cd, Cao Yy, Zheng Mh. Current status and future trends of the global burden of Masld. Trends Endocrinol Metab. 2024;35(8):697–707."),[6](#ref-CR6 "Lee Hh, Lee Ha, Kim Ej, et al. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut. 2024;73(3):533–40."),[7](#ref-CR7 "Gao J, Li Y, Zhang Y, et al. Severity and remission of metabolic dysfunction-associated fatty/steatotic liver disease with chronic kidney disease occurrence. J Am Heart Assoc. 2024;13(5):E032604."),[8](/article/10.1186/s40001-025-03783-x#ref-CR8 "Chan Ke, Ong E, Chung Ch, et al. Longitudinal outcomes associated with metabolic dysfunction-associated steatotic liver disease: a meta-analysis of 129 studies. Clin Gastroenterol Hepatol. 2024;22(3):488-498.E14.")\].Growing research highlights pronounced sex-related disparities in liver function and disease evolution. These variations stem from both inherent biological sex and sociocultural gender influences, which shape the initiation, progression, and clinical outcomes of hepatic disorders [9]. As a result, multiple investigations have uncovered sex-specific determinants that play roles in the development and advancement of MASLD [10,11,12,13,14,15,16]. Gaining insight into these sex-based distinctions is essential for refining risk assessment, halting disease advancement, and uncovering novel therapeutic strategies tailored by sex.
Insulin resistance (IR) is widely acknowledged as a pivotal driver in the initiation and progression of metabolic dysfunction-associated steatotic liver disease (MASLD) [17]. Persistent IR promotes hepatic fat accumulation by enhancing de novo lipogenesis while simultaneously reducing fatty acid oxidation efficiency [18]. This leads to an overload of triglycerides and free fatty acids in hepatocytes, which in turn worsens insulin resistance through pathways such as inflammation, oxidative damage, endoplasmic reticulum stress, and lipotoxic responses [19].
Recent studies have demonstrated sex-related variations in both the risk patterns and underlying mechanisms of type 1 diabetes, type 2 diabetes, and prediabetic states [20, 21]. Moreover, differences between males and females have been observed in how insulin resistance indices correlate with the likelihood of developing kidney failure [22]. Nevertheless, it is still uncertain whether biological sex influences the link between insulin resistance and long-term prognosis in individuals with MASLD and advanced stages of liver fibrosis.
To fill this knowledge gap, we utilized the Metabolic Score for Insulin Resistance (METS-IR), a recently developed surrogate index of insulin resistance that does not rely on insulin measurements [23]. Using a post hoc analysis of a large, nationally representative longitudinal dataset, our study aimed to explore whether the prognostic utility of METS-IR for predicting all-cause mortality differs between sexes in patients with MASLD and those with advanced liver fibrosis.
Methods
Data source and study population
This investigation was conducted as a secondary analysis based on data from the National Health and Nutrition Examination Survey (NHANES), which is administered by the U.S. National Center for Health Statistics (NCHS). NHANES adopts a sophisticated, multistage probability sampling approach to gather health and nutrition information from a nationally representative, non-institutionalized civilian population. The dataset is publicly available at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. The survey protocol was reviewed and approved by the NCHS Institutional Review Board, with all participants providing informed consent. Relevant ethics documentation is accessible online.
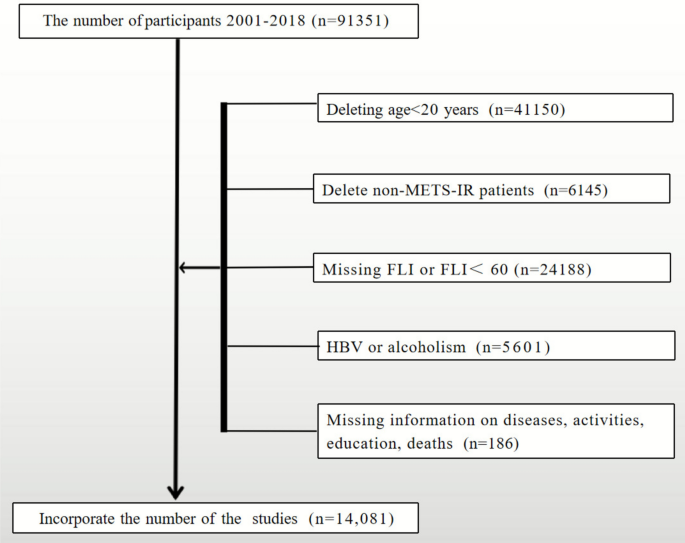
We analyzed data from the NHANES 2001–2018 survey cycles, which initially included 91,351 individuals. After implementing inclusion and exclusion criteria (illustrated in Fig. 1), a final sample of 14,081 MASLD cases was selected for analysis, consisting of 7327 male and 6754 female participants.
Fig. 1
Flow diagram outlining the selection process of study participants
Definition of METS-IR
The Metabolic Score for Insulin Resistance (METS-IR) is a validated index that estimates insulin resistance without the need for direct insulin measurement. It is computed using the following formula:
METS-IR = ln {[(2 × fasting blood glucose (mg/dL) + triglyceride level (mg/dL)) × body mass index (kg/m2)] ÷ ln (high-density lipoprotein cholesterol (HDL-C) in mg/dL)}.
Definition of MASLD and advanced liver fibrosis
Due to the lack of imaging data for hepatic steatosis across most NHANES cycles, we employed the Fatty Liver Index (FLI) to identify cases of steatotic liver disease (SLD), following previously validated methodologies [24, 25]. Participants with an FLI score of 60 or above were categorized as having hepatic steatosis.
MASLD diagnosis was based on the 2023 Delphi consensus, which requires both hepatic steatosis (FLI ≥ 60) and the presence of at least one cardiometabolic risk factor [26, 27], Additionally, participants with alternative liver disease etiologies—such as viral hepatitis, autoimmune or genetic liver conditions, drug-induced liver injury, or excessive alcohol intake (≥ 30 g/day in men, ≥ 20 g/day in women)—were excluded [25].
Assessment of advanced liver fibrosis relied on non-invasive biomarkers, namely the NAFLD Fibrosis Score (NFS), Fibrosis-4 Index (FIB-4), and AST-to-Platelet Ratio Index (APRI), as described in earlier studies[PMID: 35334881]. Participants were considered at high risk for advanced fibrosis if they exceeded any of the following thresholds: APRI > 1, FIB-4 > 2.67, or NFS > 0.676 [26, 28, 29].
Outcome definition
The primary outcome of interest was all-cause mortality among individuals diagnosed with MASLD and those categorized as having a high risk of advanced liver fibrosis. Mortality information was obtained from the NHANES Linked Mortality File, with updates extending through December 31, 2019. This dataset was matched to the National Death Index (NDI) via a probabilistic linkage process. Causes of death were classified according to the 10th Revision of the International Classification of Diseases (ICD-10). The duration of follow-up was determined from the date of the participant’s initial NHANES interview until either the date of death or December 31, 2019, depending on which occurred first.
Statistical analysis
All statistical analyses were performed using R version 4.2.0 and EmpowerStats software (www.empowerstats.com). A two-sided p value < 0.05 was considered statistically significant. In accordance with NHANES analytical guidelines, all analyses incorporated appropriate sampling weights, clustering, and stratification to account for the complex survey design. For combined analysis across the nine NHANES cycles (2001–2018), adjusted sampling weights were calculated by dividing the 2-year cycle weights by nine [23], and survey design parameters were applied using the survey package in R.
Continuous variables were described as weighted means with 95% confidence intervals (CIs) and compared using weighted linear regression. Categorical variables were summarized as weighted proportions with 95% CIs and analyzed using weighted chi-square tests.
To evaluate the association between METS-IR and all-cause mortality in patients with MASLD and advanced liver fibrosis, multivariable Cox proportional hazards regression models were employed. Four models were constructed:
Model 1 (crude): No covariates adjusted.
Model 2: Adjusted for age, race/ethnicity, PIR, and education level.
Model 3: Adjusted for all covariates listed in Table 1.
Table 1 Cox regression analysis of METS-IR with all-cause mortality in patients of MASLD and advanced liver fibrosis by gender
Additionally, we constructed a sensitivity model (Model 4) further adjusting for serum total bilirubin (TBIL), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT), to account for liver function and endogenous antioxidant capacity.
Kaplan–Meier survival curves were constructed to visualize differences in survival across METS-IR levels, and log-rank tests were used to compare survival distributions.
To identify potential threshold effects of METS-IR on all-cause mortality, restricted cubic spline (RCS) regression was performed within the multivariable Cox proportional hazards framework. Knots were placed at predefined percentiles of the METS-IR distribution. When a significant non-linear association was detected, a two-piecewise Cox regression model was further applied. The optimal cutoff value (inflection point) was determined using a maximum likelihood–based approach, in which the likelihood of models with different candidate inflection points was compared, and the point yielding the highest log-likelihood was selected. This procedure was conducted separately for each sex and disease subgroup, resulting in four subgroup-specific cutoff values used in Fig. 3. In addition, a subgroup analysis was conducted to assess the potential effect modification by relevant clinical confounders. For age stratification, 55 years was selected as a threshold to approximate menopausal transition in women, as the majority of females undergo menopause around this age. This cutoff has been commonly applied in epidemiological studies examining sex-specific metabolic and cardiovascular risks [[30](#ref-CR30 "Eb G. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–40. https://doi.org/10.1016/J.Ogc.2011.05.002
."),[31](#ref-CR31 "Ko Sh, Jung Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. 2021.
https://doi.org/10.3390/Nu13124556
."),[32](/article/10.1186/s40001-025-03783-x#ref-CR32 "Meyer Mr, Clegg Dj, Prossnitz Er, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf). 2011;203:259–69.
https://doi.org/10.1111/J.1748-1716.2010.02237.X
.")\]. Interaction analyses were conducted by including cross-product terms between sex and METS-IR in the multivariate Cox regression models to formally evaluate potential sex-specific differences in the associations.Results
Baseline characteristics
The final study population included 14,081 individuals diagnosed with MASLD, consisting of 7327 men and 6754 women. Of these, 969 males and 846 females met the criteria for advanced liver fibrosis. Throughout the follow-up period, all-cause mortality was recorded in 1294 men and 842 women.
Baseline characteristics, including demographic, clinical, and laboratory parameters, stratified by sex, are detailed in Supplementary Tables 1 and 2. Age distribution, metabolic profiles, and behavioral factors showed notable variation across METS-IR deciles for both sexes. Among female MASLD patients, higher METS-IR levels were significantly associated with increased all-cause mortality (p < 0.01), whereas this association was not statistically significant in males (p = 0.326). Furthermore, smoking prevalence in women rose consistently with increasing METS-IR deciles (p < 0.01), a pattern not observed in men (p = 0.79). In both sexes, elevated METS-IR levels were linked to higher serum uric acid and increased rates of hypertension, diabetes, asthma, and advanced liver fibrosis.
Prognostic role of METS-IR by sex
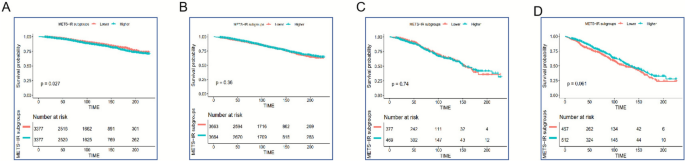
The Kaplan–Meier survival analysis indicated a strong association between higher METS-IR levels and elevated all-cause mortality among women with MASLD (Log-rank p < 0.001; Fig. 2A). Conversely, no statistically significant difference in survival was observed in men across METS-IR levels (Log-rank p = 0.54; Fig. 2B). In patients with advanced liver fibrosis, METS-IR appeared to have limited ability to discriminate mortality risk in either sex (Figs. 2C–D).
Fig. 2
Kaplan–Meier survival analysis of all-cause mortality by METS-IR levels: A Female MASLD patients; B Male MASLD patients; C Female patients with advanced liver fibrosis; D Male patients with advanced liver fibrosis
In fully adjusted multivariate Cox regression models (Model 3), increased METS-IR was independently linked to higher all-cause mortality risk in females with MASLD (HR: 1.77; 95% CI 1.28–2.47). However, this association was not statistically significant in male participants (HR: 1.20; 95% CI 0.92–1.56). A comparable sex-related trend was seen in those with advanced liver fibrosis: elevated METS-IR was significantly predictive of mortality in females (HR: 2.53; 95% CI 1.41–4.55), but not in males (HR: 0.64; 95% CI 0.39–1.06) (Table 1). In sensitivity analyses incorporating bilirubin, albumin, AST, ALT, and GGT (Model 4), the results were consistent with those of Model 3. Elevated METS-IR remained significantly associated with increased mortality risk in women with MASLD (HR: 1.80, 95% CI 1.30–2.49) and in those with advanced fibrosis (HR: 2.51, 95% CI 1.42–4.56), but no significant association was observed in men (Table 3). Importantly, formal tests for interaction confirmed significant sex differences in the prognostic effect of METS-IR on mortality. The p for interaction was 0.0059 in patients with MASLD and 0.0032 in those with advanced liver fibrosis, supporting the robustness and statistical significance of the observed sex-specific associations.
Restricted cubic spline and threshold effect analysis
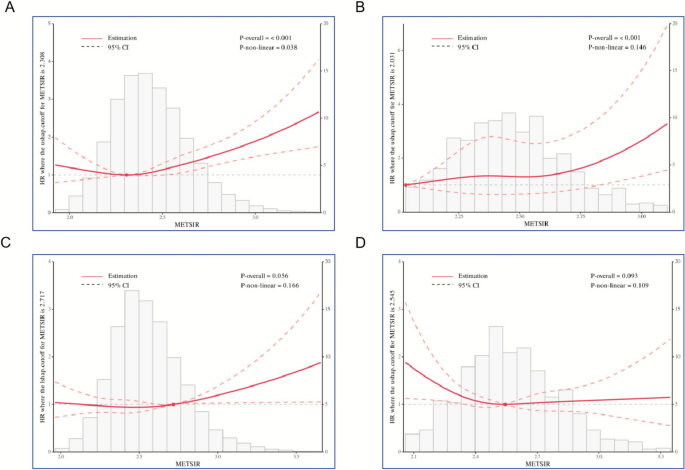
In women with MASLD, restricted cubic spline (RCS) modeling revealed a non-linear association between METS-IR and all-cause mortality (p for non-linearity = 0.038). Mortality risk escalated notably once METS-IR surpassed the threshold of 2.308 (Fig. 3A). For females within the advanced liver fibrosis subgroup, a linear positive trend between METS-IR and mortality was detected (p for non-linearity = 0.146), suggesting a gradual, dose-dependent increase in risk **(**Fig. 3B).
Fig. 3
Restricted cubic spline plots displaying the association between METS-IR and mortality: A MASLD in females; B Advanced liver fibrosis in females; C MASLD in males; D Advanced liver fibrosis in males. The red curves indicate the reference hazard ratio (HR), while shaded blue regions represent the 95% confidence intervals
In contrast, no statistically significant linear or non-linear relationships were found between METS-IR and mortality among male patients with MASLD or advanced liver fibrosis (all p-values > 0.05).
Subgroup analysis
Subgroup analyses demonstrated marked sex-based heterogeneity in the relationship between METS-IR and all-cause mortality among patients with MASLD and advanced liver fibrosis. In female MASLD patients, elevated METS-IR was significantly linked to higher mortality risk in those aged 55 years or older (HR = 1.29; 95% CI 1.01–1.84), as well as in White (HR = 1.77; 95% CI 1.16–2.71) and African American populations (HR = 2.37; 95% CI 1.04–5.37). A similar trend was observed among females with advanced fibrosis, where higher METS-IR levels were associated with increased mortality in those aged ≥ 55 (HR = 1.98; 95% CI: 1.07–3.65), Whites (HR = 1.77; 95% CI 1.16–2.71), and African Americans (HR = 2.37; 95% CI 1.04–5.37) (Table 2).
Table 2 Subgroup analysis of METS-IR with all-cause mortality in patients of MASLD and advanced liver fibrosis in women
Conversely, in male MASLD patients, higher METS-IR was unexpectedly associated with lower mortality in individuals aged ≥ 55 (HR = 0.70; 95% CI 0.53–0.93). Among males with advanced liver fibrosis, elevated METS-IR was also significantly linked to decreased all-cause mortality in both the ≥ 55 age group (HR = 0.44; 95% CI 0.26–0.72) and in White participants (HR = 0.44; 95% CI 0.23–0.84) (Table 3).
Table 3 Subgroup analysis of METS-IR with all-cause mortality in patients of MASLD and advanced liver fibrosis in men
Discussion
Using data from a nationally representative cohort of U.S. adults, this study is the first to explore the sex-specific association between the Metabolic Score for Insulin Resistance (METS-IR) and long-term all-cause mortality in individuals diagnosed with metabolic dysfunction-associated steatotic liver disease (MASLD) and advanced fibrosis. We observed that elevated METS-IR was significantly linked to increased mortality in women but not in men, implying that insulin resistance may exert sex-dependent prognostic effects in these conditions. The sex-specific divergence was statistically supported, as evidenced by significant p values for the interaction between sex and METS-IR (p for interaction = 0.0059 for MASLD; p = 0.0032 for advanced fibrosis). These results reinforce the notion that insulin resistance exerts a stronger adverse prognostic impact in females than in males, highlighting the importance of considering sex-specific differences in clinical risk assessment and management strategies.To the best of our knowledge, this is the most comprehensive analysis to date investigating sex-related disparities in this context using a large, longitudinal dataset.
The pathogenesis and progression of MASLD are influenced by a multifaceted combination of genetic factors, nutritional patterns, physical activity, and alterations in gut microbiota composition [33, 34]. Insulin resistance serves as a critical link connecting these variables to the accumulation of hepatic fat and subsequent disease progression [35, 36]. Previous studies have indicated that elevated insulin resistance markers, such as HOMA-IR, are associated with a higher likelihood of developing non-alcoholic steatohepatitis (NASH) and advancing to more severe fibrosis [37]. METS-IR, as an insulin-independent surrogate index, has likewise demonstrated notable diagnostic performance for NAFLD, with an area under the receiver operating characteristic curve (AUROC) of 0.824 [38].
In recent years, the prognostic implications of various insulin resistance–related indices have been the focus of growing research. Analyses based on NHANES data have shown that the triglyceride-glucose (TyG) index and its related parameters—such as TyG-WHtR, TyG-BMI, and TyG-WC—are useful predictors of both cardiovascular and overall mortality in individuals with MASLD [25, 39]. Findings from the Dalian Health Management Cohort indicated that TyG-BMI showed the strongest correlation with MASLD presence and progression to hepatic fibrosis [40]. Additional studies have corroborated these associations, linking TyG-based metrics with histologically confirmed liver fibrosis [41, 42]. Nonetheless, some contradictory evidence exists; for instance, data from a Korean population indicated that lower METS-IR scores were unexpectedly linked to increased risks of hepatocellular carcinoma and liver decompensation [43]. These inconsistencies highlight the importance of conducting analyses tailored to specific populations and ethnic backgrounds.
This study contributes novel evidence by demonstrating a significant and independent link between high METS-IR levels and increased long-term mortality risk among female patients with MASLD and advanced fibrosis. Several biological and behavioral mechanisms may underlie this sex-specific relationship:
Hormonal and postmenopausal factors
A substantial proportion of female participants were postmenopausal (with nearly 68% aged over 50), a demographic marked by estrogen deficiency. Consistent with this, we stratified our analyses at 55 years of age to approximate menopausal transition, recognizing that estrogen decline after menopause is closely linked to increased visceral adiposity, dyslipidemia, and heightened insulin resistance, thereby amplifying the prognostic role of METS-IR in women. Estrogen plays a protective role in maintaining insulin sensitivity, and its reduction post-menopause leads to visceral fat gain, lipid profile disturbances, and heightened insulin resistance [[31](/article/10.1186/s40001-025-03783-x#ref-CR31 "Ko Sh, Jung Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. 2021. https://doi.org/10.3390/Nu13124556
."), [32](/article/10.1186/s40001-025-03783-x#ref-CR32 "Meyer Mr, Clegg Dj, Prossnitz Er, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf). 2011;203:259–69.
https://doi.org/10.1111/J.1748-1716.2010.02237.X
."), [44](/article/10.1186/s40001-025-03783-x#ref-CR44 "Bell Ja, Santos Ferreira Dl, Fraser A, et al. Sex differences in systemic metabolites at four life stages: cohort study with repeated metabolomics. BMC Med. 2021;19(1):58.")\]. Visceral adiposity is more metabolically active and more strongly linked to IR than subcutaneous fat \[[45](/article/10.1186/s40001-025-03783-x#ref-CR45 "Williams Cm. Lipid metabolism in women. Proc Nutr Soc. 2004;63(1):153–60.")\].Differences in body composition and activity levels
Compared to men, women typically exhibit greater total fat mass and engage in less physical activity [46], both of which impair insulin signaling. Exercise enhances insulin action via mechanisms such as improved capillary perfusion and GLUT4 translocation [47], suggesting that lower activity levels in women may amplify the adverse effects of IR.
Sex-specific inflammatory responses
Emerging evidence indicates that higher METS-IR is associated with elevated levels of inflammatory cytokines—including EGF, Eotaxin, and MCP-1—in women but not in men [48]. These cytokines contribute to systemic inflammation and IR [49], which may partially explain the stronger association between METS-IR and mortality in females.
Notably, while the prevalence of MASLD, metabolic-associated steatohepatitis (MASH), and advanced fibrosis tends to be higher in males [50], our results indicate that the adverse prognostic influence of insulin resistance—measured via METS-IR—appears more pronounced in females. These findings emphasize the necessity of incorporating sex as a key biological determinant in the risk assessment and clinical management of MASLD.
Importantly, in sensitivity analyses that included bilirubin, albumin, AST, ALT, and GGT as additional covariates, the association between METS-IR and mortality in females remained robust. Bilirubin and albumin are well-recognized markers of liver function and endogenous antioxidant capacity. Recent studies have reported sex-specific associations of bilirubin with cancer risk and interactions with lifestyle factors, suggesting potential biological mechanisms underlying sex-related heterogeneity [[51](#ref-CR51 "Jw S, Tm N, Jee Sh. Association between creatinine and lung cancer risk in men smokers: a comparative analysis with antioxidant biomarkers from the kcps-Ii cohort. Antioxidants (Basel). 2025. https://doi.org/10.3390/Antiox14050584
."),[52](#ref-CR52 "Shin Jw, Kim N, Minh Nt, Chapagain Dd, Jee Sh. Serum bilirubin subgroups and cancer risk: insights with a focus on lung cancer. Cancer Epidemiol. 2025;94:102727.
https://doi.org/10.1016/J.Canep.2024.102727
."),[53](/article/10.1186/s40001-025-03783-x#ref-CR53 "Jw S, Nt M, Jee Sh. Sex-specific associations of total bilirubin, albi, and palbi with lung cancer risk: interactions with smoking and alcohol. Healthcare (Basel). 2025.
https://doi.org/10.3390/Healthcare13111321
.")\]. These findings further support that the prognostic relevance of METS-IR in women is independent of traditional liver function parameters, thereby strengthening the robustness and clinical interpretability of our results.This study has several strengths, including a large, nationally representative sample, a long follow-up period, and comprehensive adjustment for confounding variables. The use of METS-IR, a validated and easily accessible IR surrogate, adds practical value for clinical screening and risk assessment.
However, several limitations must be acknowledged. First, due to the observational design, causal inferences cannot be made. Second, although we adjusted for many potential confounders, residual confounding from unmeasured variables (e.g., medication use, unrecorded comorbidities) cannot be excluded. Third, METS-IR was assessed at a single time point, limiting our ability to evaluate longitudinal changes or treatment effects. Fourth, our findings are based on a U.S. adult population and may not be generalizable to other ethnic or age groups. Further validation in diverse cohorts is warranted.Finally, both the Fatty Liver Index (FLI) and the Metabolic Score for Insulin Resistance (METS-IR) are surrogate, non-invasive indices rather than definitive diagnostic tools. FLI does not directly confirm hepatic steatosis by imaging or histology, and METS-IR reflects insulin resistance indirectly rather than through direct insulin-based measurements. Therefore, misclassification cannot be completely exclude.
Conclusion
In conclusion, this study highlights a clear sex-specific relationship between METS-IR and all-cause mortality among individuals with MASLD and advanced liver fibrosis. Elevated METS-IR was independently associated with increased mortality risk in women but showed no significant prognostic relevance in men. These findings underscore the importance of integrating sex-specific considerations into MASLD risk stratification and clinical decision-making. Future research, particularly prospective and mechanistic investigations, is warranted to uncover the underlying biological pathways and to confirm the prognostic value of METS-IR across varied populations.
Data availability
The datasets analyzed during the current study are publicly available from the National Health and Nutrition Examination Survey (NHANES) at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
References
- Rinella Me, Lazarus Jv, Ratziu V, et al. A multisociety delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–56.
Article CAS PubMed Google Scholar - Perazzo H, Ag P, Griep Rh. Changing From Nafld Through Mafld To Masld: Similar Prevalence And Risk Factors In A Large Brazilian Cohort. J Hepatol. 2024;80(2):E72–4.
Article PubMed Google Scholar - Dominik N, Nixdorf L, Schwarz M, Hofer Bs, Hartl L, Balcar L, et al. Udff and auto Pswe accurately assess liver steatosis and fibrosis risk in obese patients with Masld. Ultraschallmed. 2025. https://doi.org/10.1055/A-2592-1431.
Article Google Scholar - Muzurović E, Topić G, Todorović N, Rizzo M, Zečević K. The prevalence and correlates of advanced fibrosis in patients with and without diabetes mellitus and metabolic dysfunction-associated steatotic liver disease: a cross-sectional study. J Diabetes Complications. 2025;39:109147. https://doi.org/10.1016/J.Jdiacomp.2025.109147.
Article PubMed Google Scholar - Miao L, Targher G, Byrne Cd, Cao Yy, Zheng Mh. Current status and future trends of the global burden of Masld. Trends Endocrinol Metab. 2024;35(8):697–707.
Article CAS PubMed Google Scholar - Lee Hh, Lee Ha, Kim Ej, et al. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut. 2024;73(3):533–40.
Article CAS PubMed Google Scholar - Gao J, Li Y, Zhang Y, et al. Severity and remission of metabolic dysfunction-associated fatty/steatotic liver disease with chronic kidney disease occurrence. J Am Heart Assoc. 2024;13(5):E032604.
Article PubMed PubMed Central Google Scholar - Chan Ke, Ong E, Chung Ch, et al. Longitudinal outcomes associated with metabolic dysfunction-associated steatotic liver disease: a meta-analysis of 129 studies. Clin Gastroenterol Hepatol. 2024;22(3):488-498.E14.
Article PubMed Google Scholar - Lefebvre P, Staels B. Hepatic sexual dimorphism - implications for non-alcoholic fatty liver disease. Nat Rev Endocrinol. 2021;17(11):662–70.
Article PubMed Google Scholar - Dileo E, Saba F, Parasiliti-Caprino M, Rosso C, Bugianesi E. Impact of sexual dimorphism on therapy response in patients with metabolic dysfunction-associated steatotic liver disease: from conventional and nutritional approaches to emerging therapies. Nutrients. 2025;17(3):477.
Article CAS PubMed PubMed Central Google Scholar - Bentanachs R, Blanco L, Montesinos M, et al. Adipose tissue protects against hepatic steatosis in male rats fed a high-fat diet plus liquid fructose: sex-related differences. Nutrients. 2023;15(18):3909.
Article CAS PubMed PubMed Central Google Scholar - Di Veroli B, Bentanachs R, Roglans N, et al. Sex differences affect the Nrf2 signaling pathway in the early phase of liver steatosis: a high-fat-diet-fed rat model supplemented with liquid fructose. Cells. 2024;13(15):1247.
Article PubMed PubMed Central Google Scholar - Meyer J, Teixeira Am, Richter S, et al. Sex differences in diet-induced Masld—are female mice naturally protected. Front Endocrinol (Lausanne). 2025;16:1567573.
Article PubMed Google Scholar - Mahmoud M, Kawtharany H, Awali M, Mahmoud N, Mohamed I, Syn Wk. The effects of testosterone replacement therapy in adult men with metabolic dysfunction-associated steatotic liver disease: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2025;16(1):E00787.
Article PubMed Google Scholar - Cherubini A, Della Torre S, Pelusi S, Valenti L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol Med. 2024;30(12):1126–36.
Article CAS PubMed Google Scholar - Zhang X, Nguyen Mh. Metabolic dysfunction-associated steatotic liver disease: a sexually dimorphic disease and breast and gynecological cancer. Metabolism. 2025;167:156190.
Article CAS PubMed Google Scholar - Maldonado-Rojas A, Zuarth-Vázquez Jm, Uribe M, Barbero-Becerra Vj. Insulin resistance and metabolic dysfunction-associated steatotic liver disease (Masld): pathways of action of hypoglycemic agents. Ann Hepatol. 2024;29(2):101182.
Article CAS PubMed Google Scholar - Targher G, Byrne Cd, Tilg H. Masld: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73(4):691–702.
Article CAS PubMed Google Scholar - Palma R, Pronio A, Romeo M, et al. The role of insulin resistance in fueling Nafld pathogenesis: from molecular mechanisms to clinical implications. J Clin Med. 2022;11(13):3649.
Article CAS PubMed PubMed Central Google Scholar - Muscogiuri G, Caporusso M, Caruso P, et al. Current evidence on gender-related risk factors for type 1 diabetes, type 2 diabetes and prediabetes: a reappraisal of the italian study group on gender difference in endocrine diseases. J Endocrinol Invest. 2025;48(3):573–85.
Article CAS PubMed Google Scholar - Kataki C. Sexual dimorphisms in endocrinopathies: their impact on the evolution of metabolic diseases. Mol Cell Endocrinol. 2025;601:112521.
Article CAS PubMed Google Scholar - Wang X, Zheng K, Hu X, Pei J. The impact of sex-related disparities on the association between triglyceride-glucose index and renal function decline in patients with type 2 diabetes: insights from the Accord Trial. Diabetes Res Clin Pract. 2025;224:112163.
Article CAS PubMed Google Scholar - Shen X, Chen Y, Chen Y, Liang H, Li G, Hao Z. Is the Mets-Ir index a potential new biomarker for kidney stone development. Front Endocrinol (Lausanne). 2022;13:914812.
Article PubMed Google Scholar - Park J, Kim G, Kim Bs, et al. The associations of hepatic steatosis and fibrosis using fatty liver index and bard score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2022;21(1):53.
Article PubMed PubMed Central Google Scholar - Min Y, Wei X, Wei Z, Song G, Zhao X, Lei Y. Prognostic effect of triglyceride glucose-related parameters on all-cause and cardiovascular mortality in the United States adults with metabolic dysfunction-associated steatotic liver disease. Cardiovasc Diabetol. 2024;23(1):188.
Article CAS PubMed PubMed Central Google Scholar - Han Y, Tang J, Wu N, et al. Association between the Life’s Essential 8 health behaviors score and all-cause mortality in patients with metabolic dysfunction-associated steatotic liver disease. Metabolism. 2025;163:156096.
Article CAS PubMed Google Scholar - Lazarus Jv, Newsome Pn, Francque Sm, Kanwal F, Terrault Na, Rinella Me. Reply: a multi-society delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2024;79(3):E93-E94.
Article PubMed Google Scholar - Cheah Mc, Mccullough Aj, Goh Gb. Current modalities of fibrosis assessment in non-alcoholic fatty liver disease. J Clin Transl Hepatol. 2017;5(3):261–71.
PubMed PubMed Central Google Scholar - Li L, Huang Q, Yang L, et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: results from the NHANES 1999–2004. Nutrients. 2022;14(6):1224.
Article CAS PubMed PubMed Central Google Scholar - Eb G. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–40. https://doi.org/10.1016/J.Ogc.2011.05.002.
Article Google Scholar - Ko Sh, Jung Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. 2021. https://doi.org/10.3390/Nu13124556.
Article PubMed PubMed Central Google Scholar - Meyer Mr, Clegg Dj, Prossnitz Er, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf). 2011;203:259–69. https://doi.org/10.1111/J.1748-1716.2010.02237.X.
Article CAS PubMed Google Scholar - Konyn P, Ahmed A, Kim D. Causes and risk profiles of mortality among individuals with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29(Suppl):S43–57.
Article PubMed Google Scholar - Song W, Yoo Sh, Jang J, et al. Association between sarcopenic obesity status and nonalcoholic fatty liver disease and fibrosis. Gut Liver. 2023;17(1):130–8.
Article CAS PubMed Google Scholar - Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of insulin resistance in MAFLD. Int J Mol Sci. 2021;22(8):4156.
Article CAS PubMed PubMed Central Google Scholar - Birkenfeld Al, Shulman Gi. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713–23.
Article PubMed Google Scholar - Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59(3):550–6.
Article CAS PubMed Google Scholar - Lee Jh, Park K, Lee Hs, Park Hk, Han Jh, Ahn Sb. The usefulness of metabolic score for insulin resistance for the prediction of incident non-alcoholic fatty liver disease in Korean adults. Clin Mol Hepatol. 2022;28(4):814–26.
Article PubMed PubMed Central Google Scholar - Chen Q, Hu P, Hou X, et al. Association between triglyceride-glucose related indices and mortality among individuals with non-alcoholic fatty liver disease or metabolic dysfunction-associated steatotic liver disease. Cardiovasc Diabetol. 2024;23(1):232.
Article CAS PubMed PubMed Central Google Scholar - Song Z, Miao X, Liu S, et al. Associations between cardiometabolic indices and the onset of metabolic dysfunction-associated steatotic liver disease as well as its progression to liver fibrosis: a cohort study. Cardiovasc Diabetol. 2025;24(1):154.
Article CAS PubMed PubMed Central Google Scholar - Zhang F, Han Y, Wu Y, et al. Association between triglyceride glucose-body mass index and the staging of non-alcoholic steatohepatitis and fibrosis in patients with non-alcoholic fatty liver disease. Ann Med. 2024;56(1):2409342.
Article PubMed PubMed Central Google Scholar - Tutunchi H, Naeini F, Mobasseri M, Ostadrahimi A. Triglyceride glucose (Tyg) index and the progression of liver fibrosis: a cross-sectional study. Clin Nutr ESPEN. 2021;44:483–7.
Article PubMed Google Scholar - Lee Jh, Kwon Yj, Park K, et al. Metabolic score for insulin resistance is inversely related to incident advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Nutrients. 2022;14(15):3039.
Article CAS PubMed PubMed Central Google Scholar - Bell Ja, Santos Ferreira Dl, Fraser A, et al. Sex differences in systemic metabolites at four life stages: cohort study with repeated metabolomics. BMC Med. 2021;19(1):58.
Article CAS PubMed PubMed Central Google Scholar - Williams Cm. Lipid metabolism in women. Proc Nutr Soc. 2004;63(1):153–60.
Article CAS PubMed Google Scholar - Kautzky-Willer A, Harreiter J, Abrahamian H, et al. Sex And gender-specific aspects in prediabetes and diabetes mellitus-clinical recommendations (update 2019). Wien Klin Wochenschr. 2019;131(Suppl 1):221–8.
Article PubMed Google Scholar - Imierska M, Kurianiuk A, Błachnio-Zabielska A. The influence of physical activity on the bioactive lipids metabolism in obesity-induced muscle insulin resistance. Biomolecules. 2020;10(12):1665.
Article CAS PubMed PubMed Central Google Scholar - Boccardi V, Mancinetti F, Baroni M, et al. Metabolic score for insulin resistance (Mets-Ir) and circulating cytokines in older persons: the role of gender and body mass index. Nutrients. 2022;14(15):3228.
Article CAS PubMed PubMed Central Google Scholar - Chait A, Den Hartigh Lj. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22.
Article CAS PubMed PubMed Central Google Scholar - Petrick Jl, Florio Aa, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147(2):317–30.
Article CAS PubMed Google Scholar - Jw S, Tm N, Jee Sh. Association between creatinine and lung cancer risk in men smokers: a comparative analysis with antioxidant biomarkers from the kcps-Ii cohort. Antioxidants (Basel). 2025. https://doi.org/10.3390/Antiox14050584.
Article Google Scholar - Shin Jw, Kim N, Minh Nt, Chapagain Dd, Jee Sh. Serum bilirubin subgroups and cancer risk: insights with a focus on lung cancer. Cancer Epidemiol. 2025;94:102727. https://doi.org/10.1016/J.Canep.2024.102727.
Article PubMed Google Scholar - Jw S, Nt M, Jee Sh. Sex-specific associations of total bilirubin, albi, and palbi with lung cancer risk: interactions with smoking and alcohol. Healthcare (Basel). 2025. https://doi.org/10.3390/Healthcare13111321.
Article Google Scholar
Acknowledgements
Not applicable.
Author information
Author notes
- Binbin Song, Yuan Zhou, Rui Su and Yan Wang have contributed equally to this work.
Authors and Affiliations
- Department of Gastroenterology, The Affiliated Chuzhou Hospital of Anhui Medical University (The First People’s Hospital of Chuzhou), Chuzhou, Anhui, 239000, PR China
Binbin Song - Outpatient Clinic, The Affiliated Chuzhou Hospital of Anhui Medical University (The First People’s Hospital of Chuzhou), Chuzhou, Anhui, 239000, PR China
Yuan Zhou - Disinfection and Supply Centre, The Affiliated Chuzhou Hospital of Anhui Medical University (The First People’s Hospital of Chuzhou), Chuzhou, Anhui, 239000, PR China
Rui Su - Department of General Practice, Wuhu City Second People`S Hospital (Affiliated Wuhu Hospital of East China Normal University), Wuhu, Anhui, 241000, China
Yan Wang - Department of Gastrointestinal Surgery, The Affiliated Chuzhou Hospital of Anhui Medical University (The First People’s Hospital of Chuzhou), Chuzhou, Anhui, 239000, PR China
Song Chen & Wenjin Chen
Authors
- Binbin Song
- Yuan Zhou
- Rui Su
- Yan Wang
- Song Chen
- Wenjin Chen
Contributions
Binbin Song (BS): Writing-original draft, Conceptualization, Data curation and Investigation. Yuan Zhou (YZ): Writing-original draft, Methodology and Software Rui Su (RS): Writing-original draft, Visualization. Yan Wang (YW): Writing-original draft,Formal Analysis. ·Song Chen (SC): Writing-review & editing and editing, Methodology. Wen jin Chen (WC): Writing-review & editing, Project administration.
Corresponding authors
Correspondence toSong Chen or Wenjin Chen.
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was granted by the Research Ethics Review Board of the National Center for Health Statistics (NCHS). All participants provided informed consent prior to participation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, B., Zhou, Y., Su, R. et al. Sex-specific differences in the prognostic value of METS-IR for long-term outcomes in patients with MASLD and advanced liver fibrosis: a nationwide study.Eur J Med Res 31, 176 (2026). https://doi.org/10.1186/s40001-025-03783-x
- Received: 26 June 2025
- Accepted: 25 December 2025
- Published: 31 December 2025
- Version of record: 02 February 2026
- DOI: https://doi.org/10.1186/s40001-025-03783-x