Epithelial Tight Junction Structure in the Jejunum of Children with Acute and Treated Celiac Sprue (original) (raw)
Main
Celiac sprue is a disease associated with gluten sensitivity and hyperregenerative mucosal transformation, the so called “villus atrophy.” In active disease, diarrhea is usually a prominent symptom. The cause of the diarrhea has not been fully characterized, although a diminished villus area with a reduced transport capacity for the Na+-glucose cotransport may play a role. Epithelial barrier function may also be disturbed as indicated by in vivo permeability tests, where it has been shown that disaccharide permeability is increased over that of monosaccharides(1–3). This increased permeability is probably confined to the paracellular pathway, and the tight junction is that structure which largely determines permeability of the paracellular pathway. The increased paracellular permeability may not only contribute to diarrhea but also may be responsible for perpetuation ofthe active disease state by permitting passage of macromolecules (gluten) through the epithelium.
The aim of the present study was to investigate the epithelial barrier function in celiac disease in its most active state as observed in children with a flat mucosa and diarrhea and to directly correlate morphologic changes in epithelial tight junctions analyzed in disease activity by electron micrographs obtained by freeze fracture electron microscopy. Special attention was thereby paid to the question of whether strand discontinuities are an important feature in acute celiac sprue. Furthermore, morphometric analysis of tight junctions was performed on identical specimens that had been characterized electrophysiologically by impedance measurements in a miniaturized Ussing device adapted to small tissue specimens obtained by aspiration biopsy(4). This enabled us to gain insight into the functional relevance of the observed structural alterations in tight junction morphology.
The major findings in this study indicate that structural changes in epithelial cell tight junctions are compatible with a seriously impaired epithelial barrier function in the jejunum of children with acute (untreated) celiac sprue. A decreased number of tight junction horizontal strands and reduced meshwork depth directly parallels the decrease in epithelial resistance. Furthermore, strand discontinuities appeared in tight junctions from children with active disease. Gluten-free diet improved but did not restore this barrier disturbance to normal.
METHODS
Experimental groups. The study was approved by the human ethics committee of the children's university hospital (Kaiserin Auguste Victoria Kinderkrankenhaus, Freie Universität Berlin). Children ranged in age between 1 and 5 y.
Acute celiac sprue. Criteria for children in this group were 1) clinically symptomatic-all had diarrhea at the time of the biopsy, 2) complete hyperregenerative transformation of the jejunal mucosa (“flat mucosa”), 3) in subsequent 1-y follow-up the diagnosis had to be confirmed by clinical recovery under treatment with a gluten-free diet, 4) a complete normalization of the mucosal architecture in jejunal biopsies after 1 y of gluten-free nourishment, and 5) a morphologic response to 4 wk of reexposure to gluten (1 g/kg of body weight daily). Thus, the inclusion of tissue data into this group required a 1 y (plus 1 mo) follow-up period including rebiopsy. Tissue from the initial biopsy was studied in this group.
Gluten-free nourished celiac sprue. Criteria for children in this group were 1) symptoms of celiac disease (including diarrhea) during the time of the initial biopsy one year previously, 2) a flat mucosa in this initial biopsy, 3) clinical and complete morphologic recovery under a gluten-free diet (i.e. specimens showing partial villus atrophy were not included), and 4) a morphologic response to 4 wk of gluten reexposure thereafter. Therefore, all children in this group had three biopsies, and biopsy no. 2 obtained after 1 y of gluten-free diet was used for analysis in this group.
Control. The control group were children in whom biopsies were performed for diagnostic reasons to exclude monosymptomatic sprue,e.g. anemia, iron deficiency and/or a prior history of diarrhea, or growth retardation. Criteria for tissues in the control group were 1) no diarrhea at the time of the biopsy, 2) a normal mucosal architecture with a normal gluten-containing diet, and 3) no gliadin antibodies.
All diagnostic biopsies were performed in the university children's hospital. One-third of the tissue was used for conventional histologic analysis and two-thirds for impedance analysis in miniaturized Ussing chambers(4). After measuring tissue impedance, tissues were fixed for freeze fracture electron microscopy.
Freeze fracture electron microscopy. Morphometric analysis was performed at different locations along the surface-crypt axis. For this purpose the surface-crypt axis was arbitrarily divided into two levels, a villus (surface) region and a crypt region. Tight junction location along the surface-crypt axis was identified at low magnification on completely intact freeze fracture replicas, which were cross-fractured perpendicular to the surface, to allow for exact identification of crypt and villus/surface epithelium. The mucosa of acute celiac sprue patients did not possess villi(flat mucosa), and the surface region was compared with the villus position of the control jejunum.
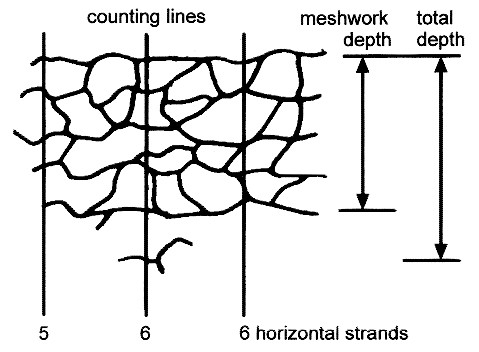
Freeze fracture analysis was performed as described previously(5–8). The tissues, obtained from eight control subjects and eight patients with acute celiac sprue and six with celiac sprue and on a gluten-free diet, were initially fixed at room temperature with phosphate-buffered 2% glutaraldehyde by simultaneously exposing the mucosal and serosal surface to the fixative. This was done while the tissue was still mounted in vitro in the Ussing chamber to guarantee an identical degree of stretch as in the electrophysiologic experiments. Tissue samples were frozen in Freon and liquid nitrogen(-100°C). Freeze fractures were shadowed with platinum and carbon in a Denton DV-502 apparatus and examined in a Philips 200 electron microscope. Morphometric analysis was performed using coded prints of freeze fracture electron micrographs (60 000-fold magnification) on all tight junction regions in which both a luminal and a contraluminal strand of the meshwork could be clearly identified. Vertical grid lines (counting lines, see Fig. 7) were drawn on the prints at 5-mm intervals(equivalent to 83 nm) perpendicular to the most luminal junctional strand. Along each vertical grid line the number of strand/grid line intersections in the main compact meshwork of the tight junction was counted and given as the number of horizontally oriented strands (strand number). The distance between the most luminal and most contraluminal strand along each vertical grid line was defined as the total depth of the tight junction (total depth). The distance between the most luminal and most contraluminal strand within the main compact meshwork was defined as the depth of the tight junctional meshwork (meshwork depth). The statistical evaluation of strand parameters is given in reference to the number of patients investigated.
Figure 7
Principle drawing of strands in a tight junction as obtained by freeze fracture electron microscopy. Vertical grid lines (counting lines) were drawn perpendicular to the most luminal strand at 83-nm intervals. Parameters for morphometrical analysis consist of the number of strands within the main compact meshwork of the tight junction (strand number) as well as the total depth of the tight junction (total depth) and the depth of the main compact meshwork (meshwork depth). Note that the aberrant strand below the main compact meshwork is transsected by the vertical grid line but is not counted. The total number of grid lines drawn and analyzed is important, because it reflects the longitudinal extent of junction analyzed.
For quantification of strand discontinuities, strand interruptions of more than 25 nm were counted within the main compact meshwork of the tight junction and given per 1000 nm of tight junctional strand length. This restriction to a 25-nm length was introduced to exclude small fixation artifacts from the analysis, although smaller strand discontinuities may certainly be of functional importance as well.
Statistics. The Statistical Package for Social Sciences (SPSS) was applied. All data are means ± SEM. Differences between groups were tested by one-way ANOVA (Student-Newman-Keuls; p < 0.05 was considered significant). In case of a significant difference, the significance level was determined by a two-tailed t test for unpaired data.
RESULTS
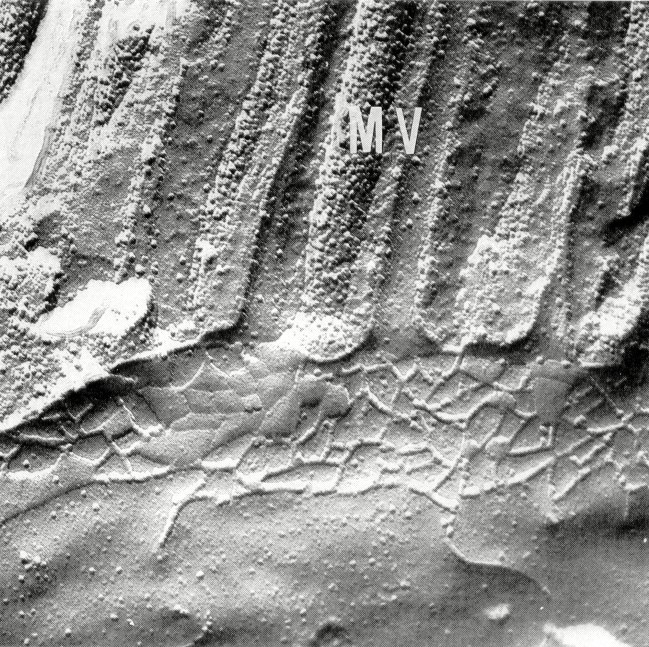
Tight junction structure in the jejunum of control subjects. Typical tight junctions from surface and crypt regions of control jejunum are shown in Figures 1 and 4, and the statistical evaluation is given in Tables 1–3. The number of horizontally oriented strands (strand number, Table 1), the depth of the main tight junctional meshwork (meshwork depth,Table 2), and the depth of the total tight junction (total depth, Table 3) continuously decreased from villus to crypt base. This decrease in tight junctional complexity has been previously noted in normal adult ileum by Marcial et al.(9).
Figure 1
Tight junction from the lower villus region of control jejunum. All electron micrographs are oriented so that microvilli(MV) are seen at the top. Magnification, ×70 000.
Figure 4
Tight junction from the lower crypt region of control jejunum. Magnification, ×70 000; MV, microvillus.
Table 1 Tight junctions in celiac sprue: number of horizontally oriented stands
Table 3 Tight junctions in celiac sprue: depth of the total tight junction
Table 2 Tight junctions in celiac sprue: depth of the main tight junctional meshwork
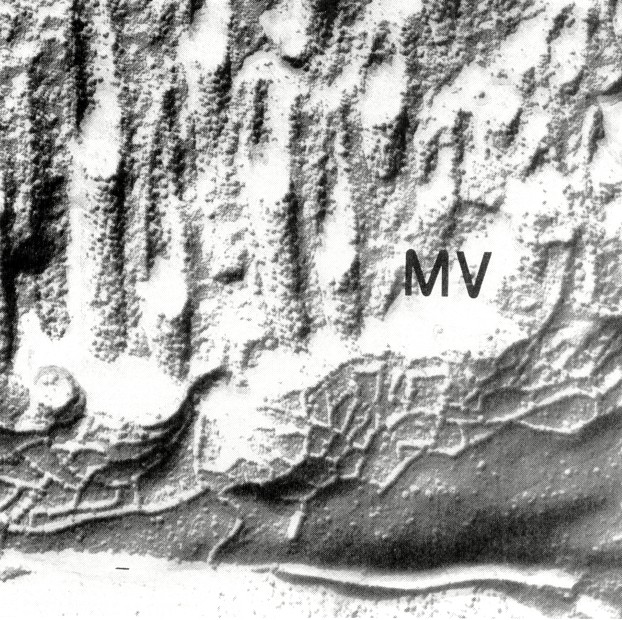
Tight junction structure in acute (untreated) celiac sprue. Typical tight junctions from surface and crypt base in acute celiac sprue are shown in Figures 2 and 5, and respective numerical data are presented in Tables 1–3. In acute celiac disease, strand number was found to be decreased at all regions along the surface-crypt axis when compared with control jejunum, although this decrease was more pronounced at the surface than in the crypt(Table 1). In keeping with this decrease in tight junctional strand count, there was a parallel reduction of the depth of the main tight junctional meshwork at all positions along the surface-crypt axis in acute celiac sprue (Table 2).
Figure 2
Tight junction from the surface epithelium in acute celiac sprue. Due to the lack of villi in acute celiac sprue (flat mucosa) only a “surface site” could be defined. Magnification, ×70 000; MV, microvillus; arrows indicate tight junction strand discontinuities not connected with the main compact meshwork.
Figure 5
Tight junction from the lower crypt region in acute celiac sprue. Magnification, ×70,000; MV, microvillus;arrows indicate aberrant tight junction strands.
Strand discontinuities in acute celiac sprue. In addition to the decreased strand number, there was also a complete loss of strands over short distances (of up to 50 nm) in tight junctions at the surface and in the crypts (Fig. 2 and Table 4). These strand discontinuities were much less frequent in tight junctions in the jejunum of healthy controls or gluten-free nourished sprue patients.
Aberrant strands. As a further structural feature in acute celiac sprue, aberrant strands appeared below the main tight junctional meshwork of crypt tight junctions. This explains why total depth exceeded meshwork depth in crypt tight junctions to a higher degree than in surface tight junctions (Tables 2 and 3). Furthermore, this is also the reason why the total tight junctional depth-despite the decreased depth of the main tight junctional meshwork-was not decreased in crypt tight junctions in acute celiac sprue when compared with control(Table 3), because the decrease in meshwork depth and the appearance of aberrant strands compensated for each other. Aberrant strands below the main tight junctional meshwork might be indicative of tight junction assembly and may be correlated to the increase in mitotic rate in celiac disease, because similar tight junction alterations have been described during cell proliferation(10).
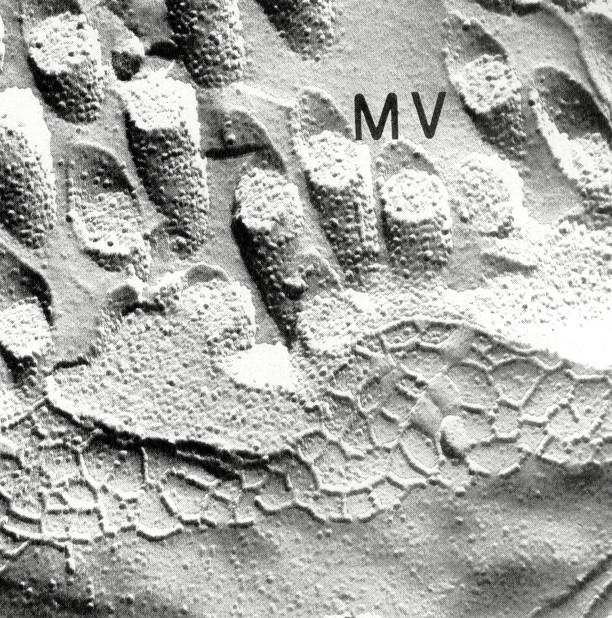
Tight junction structure in children with celiac sprue treated with gluten-free diets. Typical tight junctions from surface and crypt regions of children with celiac sprue treated with gluten-free diets are shown in Fig. 3 and 6 and the morphometrical analysis is given in Tables 1–3. Strand number was not completely restored to normal when compared with control jejunum(Table 1). Although strand number was not different from control at the surface, it was still decreased in the crypt level (p< 0.05). In the villus main meshwork depth was restored to normal(Table 2). As in acute sprue, aberrant strands below the main junctional meshwork especially in the crypt were frequent. When considered all together, tight junctions of gluten-free nourished children with celiac sprue exhibited alterations which may be described as“minimal changes.” These were mainly restricted to the crypt positions.
Figure 3
Tight junction from the lower villus region of a child with celiac sprue treated with a gluten-free diet. Magnification, ×70 000; MV, microvillus.
Figure 6
Tight junction from the lower crypt region of a child with celiac sprue treated with a gluten-free diet. Magnification, ×70 000; MV, microvillus.
DISCUSSION
Ionic conductance as well as permeability to mono- and disaccharides in the small intestine is mainly determined by the paracellular pathway and in particular by the “leakiness” of the tight junction(10). Therefore, it is reasonable to assume that tight junction alterations are responsible for the increased leakiness of the mucosa in celiac sprue observed in in vivo permeability tests(1–3) and corroborated by impedance analysis in the Ussing chamber(4).
A structural analysis of jejunal epithelial tight junctions using freeze fracture electron micrographs in celiac disease has been performed in adults by Madara and Trier(11). They also found changes compatible with a loss of barrier function. The present measurements were performed on mucosae from children who presented to the university children's hospital with diarrhea. Our observations confirm those of Madara and Trier(11) and make the following additional points:1) the tight junction was studied in a children's age group (range: 1-5 y), 2) these children had a highly active disease (all had complete “flat mucosae”), 3) measurements were extended to a group of children who had fully responded to gluten-free diet for 1 y (no“partial villus atrophy” was accepted in this group), and 4) and for the first time, a direct correlation could be made between morphometric and electrophysiologic data from the same tissue specimens.
Freeze fracture electron microscopy. In healthy controls, all measured parameters of tight junction structure declined toward a lower complexity along the surface-to-crypt axis, supporting the general view of a more leaky crypt epithelium and a more tight villus epithelium. This finding has been established in adult small intestine(9) and is now also confirmed in the jejunum of children. The strand count in control was almost identical in our children's group with that of adults studied by Madara and Trier(11), supporting the view that there is no significant age-dependent change in jejunal epithelial cell tight junction morphology.
Compared with control values, in acute celiac disease tight junction strand count and main meshwork depth were diminished at the surface and in the crypt. This decrease was more pronounced at the surface, thereby reversing the normal junctional morphologic complexity gradient in acute celiac sprue. Thus, the hyperregeneratively transformed mucosa of celiac sprue is characterized by an inverted gradient of the morphologic equivalent of tightness with a tighter crypt epithelium and a more leaky surface epithelium. The degree of the tight junction alterations in our study was more pronounced than that reported by Madara and Trier(11). In adults, they found a modest 20% decrease in strand count from 5.0 to 4.0 at the surface and no change at all in the crypt. The 20% decrease in adults compared with the 40% decrease(surface) in our study probably reflects the more active disease state in our children's group with complete flat mucosa. This type of tight junction alteration in celiac sprue represents a specific feature for the celiac sprue mucosa and is probably not secondarily due to diarrhea per se or the hyperregenerative mucosal transformation, because it was not observed in the diarrheal states caused by the experimental short bowel syndrome(5) or blind loop syndrome(7), the latter of which is also an example for a hyperregeneratively transformed mucosa.
Strand discontinuities were more frequent in the jejunal tight junction of children with acute celiac sprue. Strand discontinuities are difficult to interpret, because the extent of fixation can modify strand continuity(12). Also, even in normal epithelia (guinea pig colon) thinning of the tight junction meshwork and strand discontinuities have been reported(13). However, in this study all specimens were similarly fixed, which points against an artifact. Although strand discontinuities due to their low frequency may be less important for ionic permeability, they may enable macromolecules, e.g. gliadin, which are otherwise not absorbed to penetrate the epithelial barrier to a significant extent. In this manner, strand discontinuities could be a structural feature consistent with the “two-stage model” proposed by Ferguson et al.(14). Not every person possessing the disposition for celiac disease is necessarily ill. Once the epithelial barrier is disturbed, e.g. during a course of viral enteritis, gliadin can penetrate the epithelium, inducing an immune reaction, and celiac disease becomes acute. Subsequently, in acute celiac sprue the mucosal barrier remains seriously impaired in a circulus vitiosus allowing gliadin to be further taken up. This two-stage model can explain why children can later on be without any symptoms, even when eating a gluten-containing diet (although patients with celiac sprue have to stay on a gluten-free diet irrespective of clinical symptoms, to reduce the risk of malignancy/lymphoma). Thus, taken together the epithelial barrier defect in acute celiac disease may be an important cofactor in the immunologic pathogenesis of the disease, permitting increased passage of antigens(including gluten) into the submucosa.
Gluten-free nourished sprue patients. Under gluten-free diet tight junction parameters were returned to control values at the surface, but strand count was still slightly decreased in the crypts. Because the crypt base is the most conductive region along the surface-crypt axis in control jejunum, a decrease in tight junctional complexity should be functionally important at this location. However, alleviation of the symptoms of sprue under gluten-free diet stresses the importance of the restoration of strand discontinuities and of villar tight junction properties in spite of the still altered crypt properties.
Nevertheless, there may still be a modest barrier disturbance after treatment with a gluten-free diet. Although our present study did not investigate this directly, we would tend to interpret this not as a slight primary barrier defect but rather as “minimal damage” due to either traces of gluten in “gluten-free” food or due to incomplete patient compliance with diets.
Morphologic correlation with electrophysiologic measurements. Transepithelial resistance and tight junction structure by freeze fracture analysis were determined for the first time in parallel on identical specimens in celiac sprue. It turned out(4) that epithelial resistance (Ω·cm2) measured 20 ± 2 (control), 9± 1 (active celiac sprue), and 15 ± 1 (gluten-free). Thus, the alteration in tight junction structure toward a lower tight junction complexity in celiac sprue was paralleled by a decrease in epithelial resistance of 56% in acute celiac sprue and of 23% in gluten-free nourished sprue patients(4). Thus in treated sprue recovery was not complete, both morphologically and functionally.
Claude and Goodenough(15) in a meta analysis correlated the number of horizontally oriented strands in freeze fracture analysis to the electrical resistance and later Claude(16) postulated that this correlation was not linear but a power function. In contrast, in a recent study no significant difference in tight junctional strand count could be detected between Madin-Darby canine kidney cell monolayers of low and high conductance, indicating that also other factors can determine tight junction permeability(17). In our study, using a native leaky epithelium, the increase in ionic conductance was accompanied by a significant change in tight junction complexity (junctional depth and strand number), further supporting the concept that transepithelial resistance and tight junction strand number are related.
Diarrhea in celiac sprue. The epithelial barrier is a structural prerequisite for maintaining the milieu intérieur. The tight junction normally prevents absorbed solutes from leaking back into the lumen, depending on the electrochemical driving forces across the junction as well as the junctional permeability. If this barrier is defective, significant amounts of solutes and water may enter the lumen in an uncontrolled way, driven by hydrostatic pressure or concentration gradients. This may contribute alone or together with other mechanisms to the diarrheal state-the so-called “leak flux diarrhea.” Thus, a disturbance of the epithelial barrier function can per se cause diarrhea as for example has been postulated in Clostridium difficile infection(18, 19). More direct evidence for this concept was obtained by Fasano et al.(20), who showed that an attenuated Vibrio cholerae strain, which is depleted of the Cl- secretion-inducing cholera toxin gene, still induced significant watery diarrhea in human volunteers. This diarrhea is due to a second toxin(zonula occludens toxin), leading to a reversible decrease in epithelial resistance of ileum by altering tight junction structure(20). As a consequence, ions and water are lost into the intestinal lumen. Several factors can alter tight junction permeability as e.g. cAMP(21), cytokines(22, 23), complement components(24), hormones(25, 26), and the cytoskeleton(6), factors which could also be mediators in celiac disease. Therefore, an impaired epithelial barrier not only may cause unwanted uptake of noxious luminal contents, but it may also contribute to diarrhea by the leak flux mechanism.
Pathogenic mechanisms. How tight junction permeability could be altered in celiac sprue is yet not known. A possible mechanism in this respect may be that certain cytokines liberated from activated immune cells alter tight junction morphology and permeability, because we recently found a decline in the epithelial resistance induced by tumor necrosis factor α in the colonic epithelial cell line HT-29/B6(27).
Table 4 Tight junctions in celiac sprue: strand discontinuities
References
- Cobden I, Dickinson RJ, Rothwell J, Axon ATR 1978 Intestinal permeability assessed by excretion ratios of two molecules: results in coeliac disease. BMJ 2: 1060
Article CAS Google Scholar - Menzies IS, Laker MF, Pounder R, Bull J, Heyer S, Wheeler PG, Creamer B 1979 Abnormal intestinal permeability to sugars in villous atrophy. Lancet 2: 1107–1109
Article CAS Google Scholar - Ukabam SO, Cooper BT 1984 Small intestinal permeability to mannitol, lactulose, and polyethylene glycol 400 in celiac disease. Dig Dis Sci 29: 809–816
Article CAS Google Scholar - Schulzke JD, Schulzke I, Fromm M, Riecken EO 1995 Epithelial barrier and ion transport in celiac sprue: Electrical measurements on intestinal aspiration biopsies. Gut 37: 7777–7782
Article Google Scholar - Schulzke JD, Fromm M, Bentzel CJ, Zeitz M, Menge H, Riecken EO 1992 Epithelial ion transport in the experimental short bowel syndrome of the rat: Increased glucose-dependent Na-absorption is the main adaptive response. Gastroenterology 102: 497–504
Article CAS Google Scholar - Bentzel CJ, Hainau B, Ho S, Hui SW, Edelman A, Anagnostopoulos T, Benedetti EL 1980 Cytoplasmic regulation of tight-junction permeability: effect of plant cytokinins. Am J Physiol 239:C75–C89
Article CAS Google Scholar - Schulzke JD, Fromm M, Zeitz M, Menge H, Riecken EO, Bentzel C 1990 Regulation of the tight junction during impaired ion transport in the experimental blind loop syndrome of the rat. Res Exp Med 190: 59–68
Article CAS Google Scholar - Bentzel CJ, Fromm M, Palant CE, Hegel U 1987 Protamine alters structure and conductance of Necturus gallbladder tight junctions without major effect on the apical membrane. J Membr Biol 95: 9–20
Article CAS Google Scholar - Marcial MA, Carlson SL, Madara JL 1984 Partitioning of paracellular conductance along the ileal crypt-villus-axis: a hypothesis based on structural analysis detailed consideration of tight junction structure-function relationships. J Membr Biol 80: 59–70
Article CAS Google Scholar - Tice LW, Tice LW, Carter RL, Cahill MB 1979 Changes in tight junctions of rat intestinal crypt cells associated with changes in their mitotic activity. Tissue Cell 11: 293–316
Article CAS Google Scholar - Madara JL, Trier JS 1980 Structural abnormalities of jejunal epithelial cell membranes in celiac sprue. Lab Invest 43: 254–261
CAS PubMed Google Scholar - Staehelin LA 1973 Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci 13: 763–786
CAS PubMed Google Scholar - Luciano L, Reale E, Rechkemmer G, v Engelhardt W 1984 Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and distal colon of guinea pig. J Membr Biol 82: 145–156
Article CAS Google Scholar - Ferguson A, Arranz E, O'Mahony 1993 Clinical and pathological spectrum of coeliac disease-active, silent, latent, potential. Gut 34: 150–151
Article CAS Google Scholar - Claude P, Goodenough DA 1973 Fracture faces of zonulae occludentes from tight and leaky epithelia. J Cell Biol 58: 390–400
Article CAS Google Scholar - Claude P 1978 Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol 39: 219–232
Article CAS Google Scholar - Stevenson BR, Anderson JM, Goodenough DA, Mooseker MS 1988 Tight junction structure and ZO-1 content are identical in two strains of MDCK cells which differ in transepithelial resistance. J Cell Biol 107: 2401–2408
Article CAS Google Scholar - Hecht G, Pothoulakis C, LaMont JT, Madara JL 1988 Clostridium difficile Toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest 82: 1516–1524
Article CAS Google Scholar - Moore R, Pothoulakis C, LaMont JT, Carlson S, Madara JL 1990 Clostridium difficile toxin A increases intestinal permeability and induces Cl secretion. Am J Physiol 259:G165–G172
Article CAS Google Scholar - Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD 1991 Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA 88: 5242–5246
Article CAS Google Scholar - Duffey ME, Hainau B, Ho S, Bentzel CJ 1981 Regulation of epithelial tight junction permeability by cAMP. Nature 289: 451–453
Article Google Scholar - Madara JL, Stafford J 1989 Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 83: 724–727
Article CAS Google Scholar - Mullin J, Snock KV 1990 Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res 50: 2172–2176
CAS PubMed Google Scholar - Conyers G, Milks LC, Conklyn M, Showell H, Cramer EB 1990 A factor in serum lowers resistance and open tight junctions of MDCK cells. Am J Physiol 259:C577–C585
Article CAS Google Scholar - Lowe PJ, Miyal K, Steinbach JH, Hardison WGM 1988 Hormonal regulation of the hepatocyte tight junction permeability. Am J Physiol 255:G454–G461
CAS PubMed Google Scholar - McRoberts JA, Aranda R, Riley N, Kang H 1990 Insulin regulates the paracellular permeability of cultured intestinal epithelial cell monolayers. J Clin Invest 85: 1127–1134
Article CAS Google Scholar - Schmitz H, Epple HJ, Fromm M, Riecken EO, Schulzke JD 1995 Tumor necrosis factor-α (TNF-α) impairs barrier function in epithelial monolayers of HT-29/B6 cells. Gastroenterology 108:A322.
Google Scholar
Acknowledgements
The authors thank A. Fromm, U. Lempart, and S. Lüderitz for their excellent technical assistance.
Author information
Authors and Affiliations
- Departments of Gastroenterology, Kaiserin Auguste Victoria Kinderkrankenhaus, Freie Universität Berlin, Berlin, Germany
Jörg-Dieter Schulzke & Ernst-Otto Riecken - Clinical Physiology, Kaiserin Auguste Victoria Kinderkrankenhaus, Freie Universität Berlin, Berlin, Germany
Michael Fromm - Department of Medicine, Universitätsklinikum Benjamin Franklin, Freie Universität Berlin, Berlin
Carl J Bentzel - Department of Medicine, Kaiserin Auguste Victoria Kinderkrankenhaus, Freie Universität Berlin, Berlin, Germany
Carl J Bentzel - East Carolina University, Greenville, 27834, North Carolina
Ines Schulzke - Department of Pediatrics, Kaiserin Auguste Victoria Kinderkrankenhaus, Freie Universität Berlin, Berlin, Germany
Ines Schulzke
Authors
- Jörg-Dieter Schulzke
You can also search for this author inPubMed Google Scholar - Carl J Bentzel
You can also search for this author inPubMed Google Scholar - Ines Schulzke
You can also search for this author inPubMed Google Scholar - Ernst-Otto Riecken
You can also search for this author inPubMed Google Scholar - Michael Fromm
You can also search for this author inPubMed Google Scholar
Additional information
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (Schu 559/2-2, /4-1, and /6-1) and from the National Institutes of Health (Am 31552).
Prof. Dr. Jörg-Dieter Schulzke, Medizinische Klinik I, Gastroenterologie und Infektiologie, Universitätsklinikum Benjamin Franklin, Freie Universität Berlin, Hindenburgdamm 30, D-12200 Berlin, Germany.
Rights and permissions
About this article
Cite this article
Schulzke, JD., Bentzel, C., Schulzke, I. et al. Epithelial Tight Junction Structure in the Jejunum of Children with Acute and Treated Celiac Sprue.Pediatr Res 43, 435–441 (1998). https://doi.org/10.1203/00006450-199804000-00001
- Received: 04 July 1996
- Accepted: 08 December 1997
- Issue Date: 01 April 1998
- DOI: https://doi.org/10.1203/00006450-199804000-00001