Immunohistochemical Localization of Protein 3-Nitrotyrosine and S-nitrosocysteine in a Murine Model of Inhaled Nitric Oxide Therapy (original) (raw)
- Article
- Published: 01 June 2000
- Raymond Foust III1,
- Andrew Gow1,
- Mark Arkovitz2,
- Andrew L Salzman3,
- Csaba Szabo3,
- Bernard Vayert4,
- Michel Geffard4 &
- …
- Harry Ischiropoulos1
Pediatric Research volume 47, pages 798–805 (2000)Cite this article
Abstract
Inhaled nitric oxide (INO) therapy is currently used clinically to selectively dilate the pulmonary vasculature and to help treat persistent pulmonary hypertension and bronchopulmonary dysplasia in the neonate. However, in the presence of oxygen or superoxide, nitric oxide forms potentially harmful reactive nitrogen species. Using an experimental mice model, we examined the effects of concurrent hyperoxia and INO on protein tyrosine nitration and cysteine _S_-nitrosylation in pulmonary tissue. Data showed enhanced 3-nitrotyrosine staining within the airway epithelium and alveolar interstitium of mice lungs treated with hyperoxia, which did not increase significantly with INO administration. Within the alveolar interstitium, 3-nitrotyrosine staining was localized to macrophages. _S_-Nitrosocysteine staining in airway epithelium was significantly enhanced with INO administration regardless of oxygen content. These data suggest that the formation of protein _S_-nitrosocysteine is the major protein modification during administration of INO.
Similar content being viewed by others
Main
Respiratory disease, including PPHN and BPD, contributes to the morbidity and mortality associated with neonatal intensive care therapy. New treatment modalities, such as INO, are being developed to prevent both the development and long-term morbidity of these disease processes. INO acts as a direct pulmonary vasodilator when given to patients with pulmonary hypertension (1–4).
In both PPHN and BPD, INO is usually administered in the presence of both therapeutic hyperoxia and concurrent pulmonary inflammation. Both hyperoxia and pulmonary inflammation are associated with the overproduction of superoxide radical and hydrogen peroxide (5). The reaction between .NO and superoxide results in the diffusion-limited formation of peroxynitrite, an oxidant capable of damaging both the alveolar epithelium as well as pulmonary surfactant (6–8). In the presence of carbon dioxide, the major product of the reaction of peroxynitrite with biologic targets, specifically proteins, is the formation of 3-nitrotyrosine (9, 10). Under inflammatory conditions, protein tyrosine nitration can also occur through the oxidation of nitrite by myeloperoxidase and hydrogen peroxide (11). Tyrosine nitration of proteins has been shown to alter the function of several proteins in vitro (12) and a few proteins in vivo (13, 14).
Another important reaction of .NO with proteins is the _S_-nitrosylation of cysteine residues (15). The formation of _S_-nitrosocysteine residues on proteins is critical in regulating cell homeostasis and death via the _S_-nitrosylation of critical cysteine residues in such proteins as glyceraldehyde-3-phosphate dehydrogenase (16), the ryanodine receptor (17), and caspase-3 (18, 19).
To help further delineate the potential side effects of INO therapy, we used a mouse model of hyperoxia, which has previously been used to examine pulmonary compliance and nitric oxide synthase expression (20, 21). The goal of this study was to examine the effect of hyperoxia with and without INO on the production and localization of 3-nitrotyrosine and _S_-nitrosocysteine. We hypothesized that 1) hyperoxic conditions would increase the production of 3-nitrotyrosine and _S_-nitrosocysteine;2) INO would increase 3-nitrotyrosine production and decrease _S_-nitrosocysteine production under hyperoxic conditions; and 3) nitration and _S_-nitrosylation of proteins would be localized to the airway epithelium where exposure to the inhaled gases was highest.
METHODS
Female FVBN mice (Harlan, IN, U.S.A.), age 6–8 wk (20–23 g), were used for all experiments. Animals were housed and fed in accordance with guidelines set forth by the Children's Hospital Research Foundation Institutional Animal Care and Use Committee. All animals were allowed to acclimatize for a minimum of 5 d before experimental use.
Exposure to hyperoxia.
Mice were placed into 1.5-ft3 Plexiglas chambers and exposed to hyperoxia (>95% O2). Each chamber received oxygen at 4.0 L/min flow, and oxygen concentration was measured continuously with an oxygen afferent limb of the system (Catalyst Research, Owings Mills, MD, U.S.A.). During the exposure period, carbon dioxide concentration in the chamber was less than 1%. Normoxic control animals were maintained in open cages. All animals were allowed food and water ad libitum.
Administration of INO.
Nitric oxide was administered at a continuous rate at a concentration of 20 ppm. The concentration of .NO and .NO2 was monitored continuously in the efferent limb with a chemiluminescence monitor. All monitors were calibrated every 24 h.
Lung fixation.
After 72 h of exposure, animals were killed with a lethal i.p. injection of pentobarbital at 50 mg/kg. A laparotomy was then performed, and the abdominal aorta was transected to exsanguinate the animals. A sternotomy was performed, and the left atrium was then excised. The pulmonary artery was cannulated, and the vasculature was perfused with 15 mL of sterile PBS. The trachea was then cannulated with a 24-gauge blunt tip needle, and the lungs were inflated with approximately 1.5 mL of 4% paraformaldehyde solution, made in 0.1 M sodium cacodylate buffer (pH = 7.4). Next, the trachea was ligated, and the lungs were immersed completely in 15 mL of 4% paraformaldehyde solution. Thirty minutes later, the lungs were switched to 15 mL of fresh paraformaldehyde solution, left for 30 min, and switched to a fresh aliquot of paraformaldehyde solution. The lungs were then incubated for approximately 16 h at room temperature and transferred again to 15 mL of fresh paraformaldehyde solution.
Immunostaining with affinity-purified polyclonal 3-nitrotyrosineand _S_-nitrosocysteine antibody.
After fixation, the lungs were cryoprotected by placing them in 30% sucrose solution for 24 h, and frozen with Freon 22 in liquid nitrogen. Eight-micrometer-thick lung slices were then placed on slides and incubated four times for 3 min each in 1 mg/mL sodium borohydride. Sodium borohydride will not reduce 3-nitrotyrosine to aminotyrosine but will significantly reduce tissue autofluorescence. The tissues were then incubated for 30 min in blocking solution, made up of 0.3% Triton X-100, 5% fatty acid-free BSA, and 10% normal goat serum in 0.05 M PBS (pH = 7.2).
Tissues were incubated overnight at 4°C with the anti-3-nitrotyrosine or anti-_S_-nitrosocysteine antibody diluted at 1:100 concentration in blocking solution. The polyclonal antibody for 3-nitrotyrosine was produced in the laboratory of J. S. Beckman (University of Alabama in Birmingham, U.S.A.), and the affinity and specificity of the antibody has been previously reported (22). Rabbit polyclonal antibodies directed against conjugated NO-cysteine were raised and characterized according to Geffard et al. (23). Cysteine (Cys) was linked to BSA via glutaraldehyde (G). After a reduction step, Cys-G-BSA was nitrosylated using NaNO2. Purified NO-Cys-G-BSA was injected s.c. into rabbits. The avidity and specificity of the antibodies were evaluated using an adapted ELISA method (24).
Anti-rabbit goat secondary antibody labeled with FITC was diluted at 1:200 concentration in blocking solution and incubated with the tissues for 1 h. A 5-min washing with 0.05 M PBS (pH = 7.2) was performed between each step until blocking solution was introduced to the tissues, upon which 0.05 M PBS (pH = 7.2) with 0.3% Triton X-100 was used for the washing solution. The slides were then examined under an Olympus fluorescence inverted microscope. Representative computer-assisted images were obtained under identical conditions at the same setting using the Metamorph imaging system (Universal Imaging Corp., West Chester, PA), with exposure times of 1500 ms for the anti-3-nitrotyrosine-stained tissues and 2200 ms for the anti-_S_-nitrosocysteine-stained tissues. Magnification of the images was taken at ×200.
To compare the magnitude of antibody binding for 3-nitrotyrosine and _S_-nitrosocysteine, we used computer-assisted image quantification through the Metamorph system. Four to six random images from different slides were analyzed under each condition by counting fluorescent intensity of the area selected. One-sided t test was used to compare differences for the various experimental groups and two-sided t test was used to compare differences between the negative control groups and normoxic lung tissue. Equivalent areas of analysis were used for each tissue of interest as assessed by one-way ANOVA analysis. A significance level of p < 0.05 was used for all comparisons.
Negative and positive controls for both 3-nitrotyrosine and _S_-nitrosocysteine were prepared at the same time. For 3-nitrotyrosine, negative controls were obtained by the addition of 0.5 M dithionite in 0.1 N NaOH to lung tissue from the hyperoxia plus nitric oxide group. Positive controls were obtained by the addition of peroxynitrite to the same tissue. For _S_-nitrosocysteine, negative controls were made by the addition of 0.5% HgCl2 in 0.05 M PBS (pH = 7.2) to lung tissue from the hyperoxia plus nitric oxide group. Positive controls were obtained by the addition of 0.1 M sodium nitrite mixed at a 1:1 concentration with 0.1 N HCl to the same tissue.
RESULTS
Histologic evaluation of lung tissue.
Three slides for each treatment group were stained independently, with representative images presented in this section. Histologic evaluation of the tissue revealed mild changes in the lung tissue exposed to hyperoxic conditions. Alveolar interstitium showed some mild thickening of the alveolar septa and mild increase in the number of alveolar macrophages, without an increase in other types of inflammatory cells. There was no evidence of hyaline membranes in the tissue samples. The administration of INO with hyperoxia did not appear to alter the histologic appearance of the lung tissue when compared with the hyperoxia group. When exposed to INO alone, there were no remarkable histologic alterations noted. These data are consistent with previous studies using this model. Minimal changes in pulmonary compliance and lung histology and no evidence of pulmonary edema were reported after 72 h of hyperoxic exposure (21).
Immunohistochemical localization of 3-nitrotyrosine.
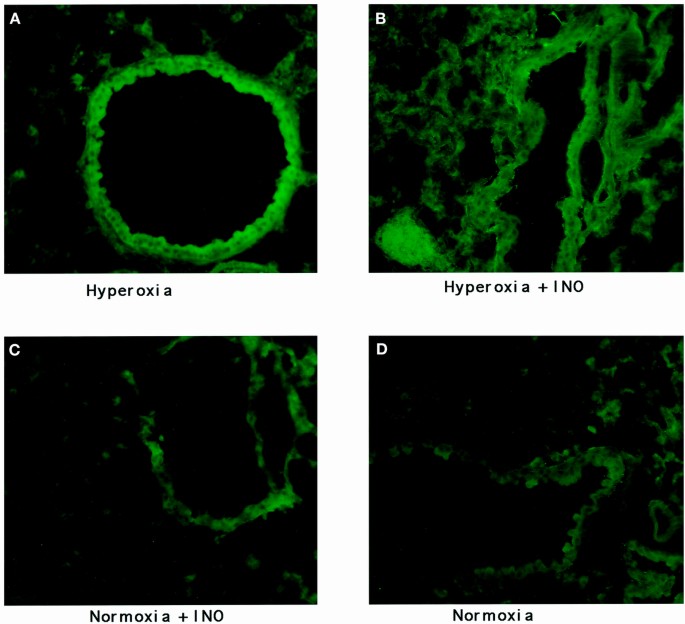
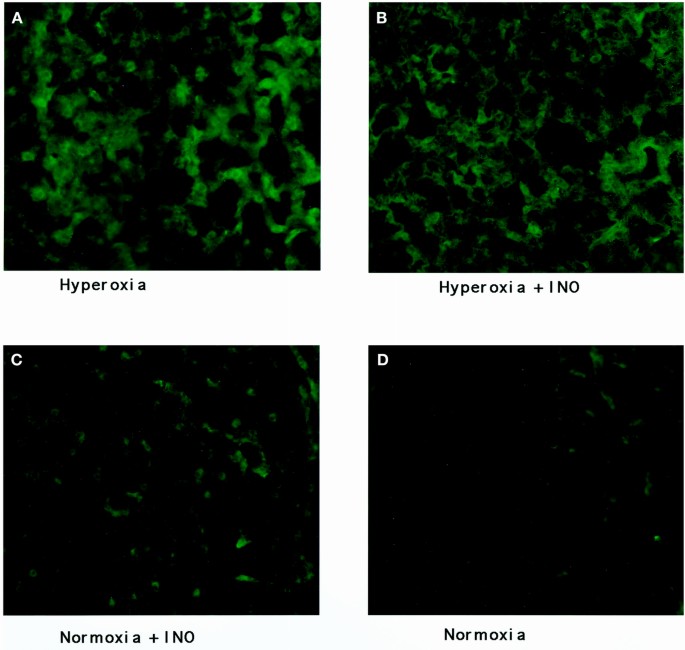
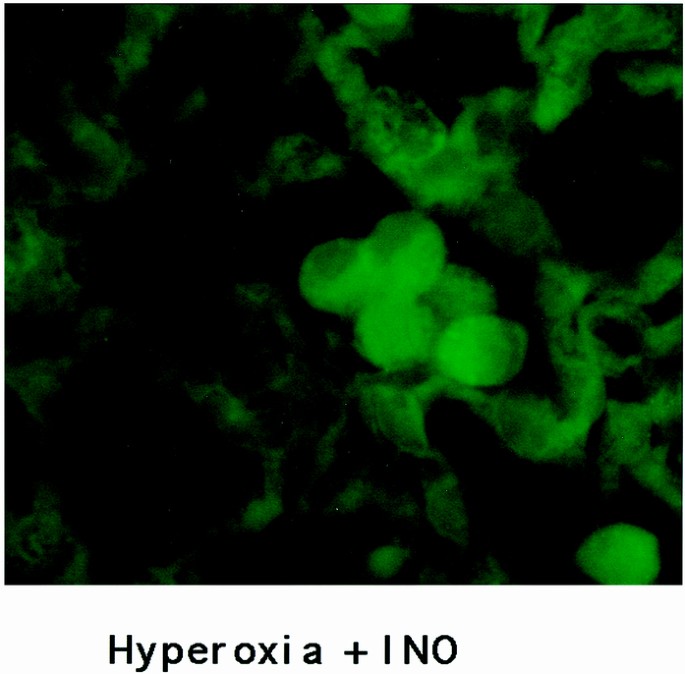
Representative images of the staining of the four groups of mouse lung tissue for 3-nitrotyrosine are seen in Figures 1 and 2, with the results of image quantification analysis shown in Table 1. An increased staining within the airway epithelial cells of both bronchioles and larger airways, as shown in Figure 1, can be seen in hyperoxic conditions when compared with normoxic conditions. INO with concurrent hyperoxia did not appear to increase the staining of the airway epithelium when compared with hyperoxia alone. Alveolar staining, as seen in Figure 2, showed increased staining for 3-nitrotyrosine in the hyperoxic lung tissue. Again, the addition of INO to hyperoxia did not cause any substantial increase in staining. Staining of lung tissue after chemical reduction of 3-nitrotyrosine to aminotyrosine with dithionite showed the same staining for 3-nitrotyrosine as the normoxic lungs (8.6 × 105 ± 1.3 × 105 gray-scale units for negative controls;p = 0.45). Examination of the lung tissue under higher magnification revealed increased staining in alveolar macrophages (Fig. 3). Increased staining for 3-nitrotyrosine in the vasculature was observed in both hyperoxia groups when compared with the normoxic lung tissues. The addition of INO increased staining of the vasculature when compared with normoxic lung tissue, but the difference was not significant (Table 1).
Figure 1. 3
Nitrotyrosine staining of airway epithelium. Images of airway epithelium taken from mice (A) exposed to hyperoxia; (B) exposed to hyperoxia plus 20 ppm of INO; (C) exposed to 20 ppm of INO; and (D) exposed to air. The fluorescence intensity is enhanced in airway epithelium of hyperoxic-exposed lungs.
Figure 2. 3
Nitrotyrosine staining of alveolar interstitium. Images of alveolar interstitium taken from mice (A) exposed to hyperoxia; (B) exposed to hyperoxia plus 20 ppm of INO; (C) exposed to 20 ppm of INO; and (D) exposed to air. Fluorescence intensity is enhanced in alveolar interstitium from hyperoxic-exposed lungs with no apparent increase in fluorescence intensity with INO exposure (×200).
Table 1 Computer-assisted image quantification of 3-nitrotyrosine-stained tissues All values are expressed in total gray-scale units as mean ± SD. *p < 0.05 when compared with normoxic conditions.
Figure 3. 3
Nitrotyrosine staining of alveolar macrophage. Epifluorescence image of alveolar macrophage taken from the murine lung exposed to hyperoxia plus 20 ppm of INO (×900).
Immunohistochemical localization of _S_-nitrosocysteine.
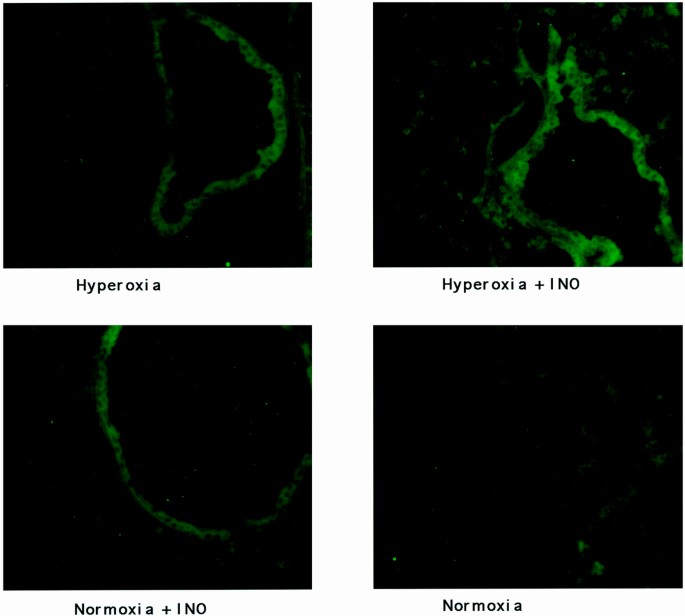
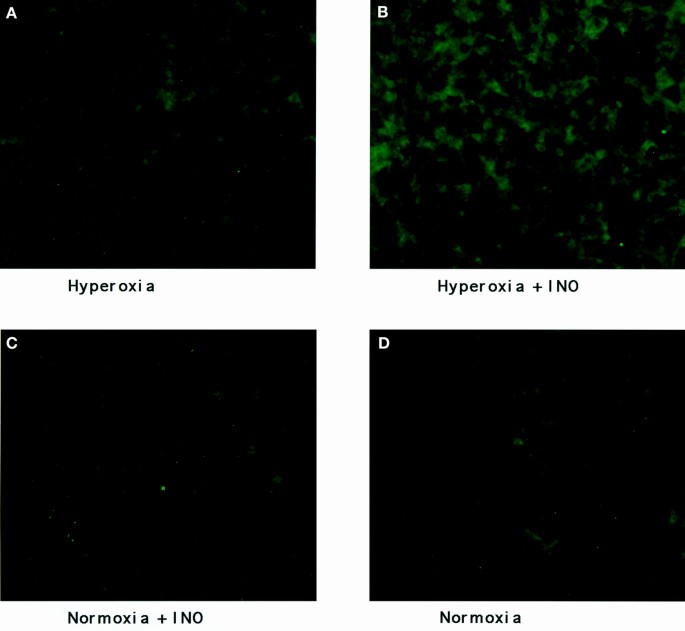
Representative images of _S_-nitrosocysteine staining are seen in Figures 4 and 5, with the results of image quantification analysis shown in Table 2. In Figure 4, an increased staining for _S_-nitrosocysteine was observed in airway epithelium of animals treated with hyperoxia and both INO groups as compared with normoxic conditions. INO administration with hyperoxia enhanced the staining of airway epithelium (Table 2). Examination of the alveolar interstitium shown in Figure 5 showed increased staining only in the hyperoxia plus INO group when compared with hyperoxia alone and INO alone. Vascular epithelium showed no increase in staining for hyperoxia alone or INO alone when compared with normoxia, but an increase in staining for the hyperoxia plus INO group was observed when compared with normoxia. Treatment of slides from the hyperoxia plus INO group with HgCl2 to remove .NO from _S_-nitrosocysteine resulted in a significant reduction in staining (9.6 × 105 ± 2.3 × 105 gray-scale units for alveoli interstitium after treatment with HgCl2;p = 0.008 compared with the hyperoxia plus INO group).
Figure 4
_S_-Nitrosocysteine staining of airway epithelium. Images of airway epithelium taken from mice (A) exposed to hyperoxia; (B) exposed to hyperoxia plus 20 ppm of INO; (C) exposed to 20 ppm of INO; and (D) exposed to air. Fluorescence intensity is increased in airway epithelium taken from lungs exposed to both hyperoxia and inhaled nitric oxide (×200).
Figure 5
_S_-Nitrosocysteine staining of alveolar interstitium. Images of alveolar interstitium taken from mice (A) exposed to hyperoxia; (B) exposed to hyperoxia plus 20 ppm of INO; (C) exposed to 20 ppm of INO; and (D) exposed to air. Fluorescence intensity is increased in alveolar interstitium taken from lungs exposed to hyperoxia plus INO (×200).
Table 2 Computer-assisted image quantification of S-nitrosocysteine-stained tissues All values are expressed in total gray-scale units as mean ± SD. *p < 0.05 when compared with normoxic conditions. †p < 0.05 when compared with hyperoxic conditions.
Comparison of the localization of 3-nitrotyrosine with _S_-nitrosocysteine.
The localization of protein nitration in a murine model showed that oxygen administration at >95% substantially enhanced 3-nitrotyrosine staining in bronchiolar epithelium and alveolar interstitium, especially within the perivascular macrophages. INO administration at 20 ppm did not increase 3-nitrotyrosine staining in the presence of hyperoxia or normoxia. In contrast to 3-nitrotyrosine staining, _S_-nitrosocysteine staining of airway epithelium was increased with the administration of INO. Alveolar interstitium staining was increased only in the presence of hyperoxia and INO. Therefore, the major difference between the test groups is that INO in the presence or absence of hyperoxia increased _S_-nitrosocysteine staining of airway epithelium and alveolar interstitium without increasing 3-nitrotyrosine staining.
DISCUSSION
Hyperoxia increases 3-nitrotyrosine production as part of the mechanism for oxidative-mediated lung injury (25). As we initially hypothesized, an increased production of 3-nitrotyrosine in lung tissue from mice exposed to hyperoxic conditions when compared with normoxia was apparent (Figs. 1 and 2). This increase could be secondary to increased peroxynitrite formation, which may increase under hyperoxic conditions because of elevations in superoxide production. We have recognized that the formation of peroxynitrite is largely dependent on the level and location of superoxide release (13), and that the catalytic reaction of peroxynitrite with carbon dioxide significantly enhances the nitration yield of tyrosine residues in proteins even in the presence of such antioxidants as glutathione and ascorbate (9, 10). Nitration of proteins may be alternatively formed from activated neutrophils through the oxidation of nitrite by myeloperoxidase and hydrogen peroxide (11). Although other studies using this model have not shown an increase in the number of neutrophils in the lung of animals treated with hyperoxia at the time of examination (21), resident neutrophils normally present in the lung and activated by hyperoxia may contribute to 3-nitrotyrosine formation. Therefore, formation of peroxynitrite or activation of resident neutrophils may contribute to the increased staining of 3-nitrotyrosine in the lung after hyperoxia exposure.
Tyrosine nitration has been shown to adversely affect function of a number of proteins, including surfactant protein-A (8) and manganese superoxide dismutase (14). Also, 3-nitrotyrosine production was found to be elevated in infants who later went on to develop BPD. The plasma concentration of 3-nitrotyrosine was directly related to the amount of supplemental oxygen that these infants received, suggesting a relationship between the amount of oxidative stress and 3-nitrotyrosine production (26).
In our study, exposure to INO appeared to increase _S_-nitrosocysteine formation in the presence or absence of hyperoxia, whereas it did not alter the formation of 3-nitrotyrosine under the same conditions. The lack of increased formation of 3-nitrotyrosine with the addition of INO to hyperoxia may be explained by the following:1) formation of nitrating agents is dependent on superoxide and hydrogen peroxide, and the addition of INO is not expected to increase the levels of these compounds beyond those produced by hyperoxia;2) it is also possible that in the presence of excess .NO, secondary reactions with peroxynitrite may lead to the formation of nitrosating, but not nitrating, species (27); and 3) reduced cysteine residues may be critical in regulating the reactivity of .NO, possibly preventing the formation of nitrating species.
In vitro, INO has been shown to have both beneficial and pathologic effects on lung physiology. Lower doses of INO (<20 ppm) have been shown to reduce hyperoxia-induced alterations in surfactant activity and aggregation (28); to improve pulmonary edema, cell injury, and lipid peroxidation associated with hyperoxia (29–31); and to reduce the toxicity of hyperoxia in adult rats (29). However, other studies using higher doses of INO (50–200 ppm) have shown the opposite effect, with impairment of surfactant function (32, 33), increased inflammation and lung injury (30), and reduced induction of Na, K-ATPase in newborn piglets (34). However, a recent study by Nelin et al. (35) showed an improved survival rate for newborn rats in hyperoxia when given 100 ppm of INO.
Clinical trials evaluating the effect of INO in PPHN have demonstrated an improved arterial Po2 and reduced extracorporeal membrane oxygenation utilization, although mortality was unchanged (1–4). These improvements were enhanced in situations where lung volumes were optimized, particularly the use of high-frequency oscillatory modes of ventilation in infants whose PPHN was secondary to respiratory distress syndrome or meconium aspiration syndrome (3). Preliminary studies using INO for infants aged 1–7 mo with BPD found that 11 of 16 infants had a significant increase in arterial Po2 after 1 h of treatment, and 11 of 16 infants showed a >15% reduction in fraction of inspired oxygen after 72 h of therapy (36).
Finally, our data showed increased staining for 3-nitrotyrosine in alveolar macrophages compared with _S_-nitrosocysteine, especially in hyperoxia. Macrophages have been suggested as one site for the production of peroxynitrite, both in vitro (12) and in patients with idiopathic pulmonary fibrosis (37). The macrophage, whose influx is increased in situations of oxidative stress, may contribute to the formation of peroxynitrite. The macrophage, though, may also act as a potential pathway of clearance of nitrated proteins, which are also removed via proteolysis or specific nonproteolytic pathways not yet defined.
In summary, our experimental data on the localization and production of tyrosine nitrated adducts and cysteine _S_-nitrosylated adducts in a murine model of INO therapy suggest that 3-nitrotyrosine production in airway epithelium and alveolar interstitium is enhanced with hyperoxic conditions, and that the administration of INO leads to increased production of _S_-nitrosocysteine with no apparent change in 3-nitrotyrosine formation. These data imply that the formation of protein _S_-nitrosocysteine is a major protein modification during the administration of INO. They also suggest that the interaction of .NO with reduced thiol groups of proteins is critical in maintaining the balance between protein tyrosine nitration and protein cysteine nitrosylation and possibly preventing cellular and tissue injury. This may help to explain some of the beneficial effects of INO and the lack of toxicity when administered in the presence of hyperoxia.
Abbreviations
INO:
inhaled nitric oxide
.NO:
nitric oxide
PPHN:
persistent pulmonary hypertension
BPD:
bronchopulmonary dysplasia
References
- Roberts JD Jr, Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM 1997 Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med 336: 605–610
Article CAS Google Scholar - The Neonatal Inhaled Nitric Oxide Study Group. 1997 Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336: 597–604
- Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, DeLemos RA, Sardesai S, McCurnin DC, Moreland SG, Cutter GR, Abman SH 1997 Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 131: 55–62
Article CAS Google Scholar - Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, Rhines J, Chang CT 1998 Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics 101: 325–334
Article CAS Google Scholar - Turrens JF, Freeman BA, Levitt JG, Crapo JD 1982 The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys 217: 401–410
Article CAS Google Scholar - Beckman JS, Koppenol WH 1996 Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol 271: C1424–C1437
Article CAS Google Scholar - Royall JA, Kooy NW, Beckman JS 1995 Nitric oxide-related oxidants in acute lung injury. New Horiz 3: 113–122
CAS PubMed Google Scholar - Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S 1993 Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol 265: L555–L564
CAS PubMed Google Scholar - Denicola A, Trujillo M, Freeman BA, Radi R 1996 Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidation reactions. Arch Biochem Biophys 333: 49–58
Article CAS Google Scholar - Gow A, Duran D, Thom SR, Ischiropoulos H 1996 Carbon dioxide catalyzed protein tyrosine nitration by peroxynitrite. Arch Biochem Biophys 333: 42–48
Article CAS Google Scholar - Van der Vliet A, Eiserich JP, Halliwell B, Cross CE 1997 Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. J Biol Chem 272: 7617–7625
Article CAS Google Scholar - Ischiropoulos H 1998 Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys 356: 1–11
Article CAS Google Scholar - Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H 1998 Inactivation of tyrosine hydroxylase following exposure to peroxynitrite and MPTP. Proc Natl Acad Sci USA 95: 7659–7663
Article CAS Google Scholar - MacMillan Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA 1996 Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA 93: 11853–11858
Article CAS Google Scholar - Simon DI, Mullins ME, Jia L, Gaston B, Singel DJ, Stamler JS 1996 Polynitrosylated proteins: characterization, bioactivity, and functional consequences. Proc Natl Acad Sci USA 93: 4736–4741
Article CAS Google Scholar - Molina Y, Vedia L, McDonald B, Reep B, Brune B, Di Silvio M, Billiar TR, Lapetina EG 1992 Nitric oxide-induced _S_-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem 267: 24929–24932
Google Scholar - Xu L, Eu JP, Meissner G, Stamler JS 1998 Activation of the cardiac calcium release channel (ryanodine receptor) by poly-_S_-nitrosylation. Science 279: 234–237
Article CAS Google Scholar - Kim YM, Talanian RV, Billiar TR 1997 Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272: 31138–31148
Article CAS Google Scholar - Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stammler JS 1999 Fas-induced caspase denitrosylation. Science 284: 651–654
Article CAS Google Scholar - Arkovitz MS, Szabo C, Garcia VF, Wong HR, Wispe JR 1997 Differential effects of hyperoxia on the inducible and constitutive isoforms of nitric oxide synthase in the lung. Shock 7: 345–350
Article CAS Google Scholar - Arkovitz MS, Garcia VF, Szabo C, McConnell K, Bove K, Wispe JR 1997 Decreased pulmonary compliance is an early indicator of pulmonary oxygen injury. J Surg Res 67: 193–198
Article CAS Google Scholar - Beckman JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR 1994 Extensive nitration of protein tyrosine in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler 375: 81–88
Article Google Scholar - Geffard M, Dulluc J, Heinrich-Rock AM 1985 Antisera against the indolealkylamines: tryptophan, 5-hydroxytryptophan, 5-hydroxytryptamine, 5-methoxytryptophan, and 5-methoxytryptamine tested by an enzyme-linked immunosorbent assay method. J Neurochem 44: 1221–1228
Article CAS Google Scholar - Mnaimneh S, Geffard M, Veyret B, Vincendeau P 1997 Albumin nitrosylated by activated macrophages possesses antiparasitic effects neutralized by anti-NO-acetylated-cysteine antibodies. J Immunol 158: 308–314
CAS PubMed Google Scholar - Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S 1994 Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest 94: 2404–2413
Article Google Scholar - Banks BA, Ischiropoulos H, McClelland M, Ballard PL, Ballard RA 1998 Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics 101: 870–874
Article CAS Google Scholar - Wink DA, Cook JA, Kim SY, Vodovotz Y, Pacelli R, Krishna MC, Russo A, Mitchell JB, Jourd'heuil D, Miles AM, Grisham MB 1997 Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide-derived reactive intermediates. J Biol Chem 272: 11147–11151
Article CAS Google Scholar - Issa A, Lappalainen U, Kleinman M, Bry K, Hallman M 1999 Inhaled nitric oxide decreases hyperoxia-induced surfactant abnormality in preterm rabbits. Pediatr Res 45: 247–254
Article CAS Google Scholar - Gutierrez HH, Nieves B, Chumley P, Rivera A, Freeman BA 1996 Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administered nitric oxide. Free Radic Biol Med 21: 43–52
Article CAS Google Scholar - Garat C, Jayr C, Eddahibi S, Laffon M, Meignan M, Adnot S 1997 Effects of inhaled nitric oxide or inhibition of endogenous nitric oxide formation on hyperoxic lung injury. Am J Respir Crit Care Med 155: 1957–1964
Article CAS Google Scholar - Chetham PM, Sefton WD, Bridges JP, Stevens T, McMurtry IF 1997 Inhaled nitric oxide pretreatment but not posttreatment attenuates ischemia-reperfusion-induced pulmonary microvascular leak. Anesthesiology 86: 895–902
Article CAS Google Scholar - Matalon S, DeMarco V, Haddad IY, Myles C, Skimming JW, Schurch S, Cheng S, Cassin S 1996 Inhaled nitric oxide injures the pulmonary surfactant system of lambs in vivo. Am J Physiol 270: L273–L280
CAS PubMed Google Scholar - Robbins CG, Horowitz S, Merritt TA, Kheiter A, Tierney J, Narula P, Davis JM 1997 Recombinant human superoxide dismutase reduces lung injury caused by inhaled nitric oxide and hyperoxia. Am J Physiol 272: L903–L907
CAS PubMed Google Scholar - Youssef JA, Thibeault DW, Rezaiekhaligh MH, Mabry SM, Norberg MI, Truog WE 1999 Influence of inhaled nitric oxide and hyperoxia on Na, K-ATPase expression and lung edema in newborn piglets. Biol Neonate 75: 199–209
Article CAS Google Scholar - Nelin LD, Welty SE, Morrisey JF, Gotuaco C, Dawson CA 1998 Nitric oxide increases the survival of rats with high oxygen exposure. Pediatr Res 43: 727–732
Article CAS Google Scholar - Banks BA, Seri I, Ischiropoulos H, Merrill J, Rychik J, Ballard RA 1999 Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics 103: 610–618
Article CAS Google Scholar - Saleh D, Barnes PF, Giaid A 1997 Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 155: 1763–1769
Article CAS Google Scholar
Author information
Author notes
- Current address: Department of Pulmonary Medicine, Duke Medical Center, Raleigh-Durham, NC 27710, U.S.A.
- urrent address: Inotek Corporation, Beverley, MA 01915, U.S.A.
Authors and Affiliations
- Department of Neonatology, Stokes Research Institute, Children's Hospital of Philadelphia, Philadelphia, 19104, Pennsylvania
Scott A Lorch, Raymond Foust III, Andrew Gow & Harry Ischiropoulos - Surgery Division and Children's Hospital Medical Center, Cincinnati, 45229, Ohio, U.S.A.
Mark Arkovitz - Critical Care Medicine, Children's Hospital Medical Center, Cincinnati, 45229, Ohio, U.S.A.
Andrew L Salzman & Csaba Szabo - Laboratoire PIOM/EPHE, ENSCPB, University of Bordeaux, France
Bernard Vayert & Michel Geffard
Authors
- Scott A Lorch
You can also search for this author inPubMed Google Scholar - Raymond Foust III
You can also search for this author inPubMed Google Scholar - Andrew Gow
You can also search for this author inPubMed Google Scholar - Mark Arkovitz
You can also search for this author inPubMed Google Scholar - Andrew L Salzman
You can also search for this author inPubMed Google Scholar - Csaba Szabo
You can also search for this author inPubMed Google Scholar - Bernard Vayert
You can also search for this author inPubMed Google Scholar - Michel Geffard
You can also search for this author inPubMed Google Scholar - Harry Ischiropoulos
You can also search for this author inPubMed Google Scholar
Additional information
Supported by grants HL 54926, HL 59664, and SCOR in Acute Lung Injury P50-HL 60290 (H.I.). A.G. and R.F. were supported by an NRSA award from NHLBI (HL 07748). H.I. is an Established Investigator of the American Heart Association.
Rights and permissions
About this article
Cite this article
Lorch, S., Foust, R., Gow, A. et al. Immunohistochemical Localization of Protein 3-Nitrotyrosine and _S_-nitrosocysteine in a Murine Model of Inhaled Nitric Oxide Therapy.Pediatr Res 47, 798–805 (2000). https://doi.org/10.1203/00006450-200006000-00020
- Received: 01 October 1999
- Accepted: 17 December 1999
- Issue Date: 01 June 2000
- DOI: https://doi.org/10.1203/00006450-200006000-00020