Perinatal Respiratory Control and Its Modulation by Adenosine and Caffeine in the Rat (original) (raw)
Main
Fetal respiratory movements are episodic and progressively more inhibited toward the end of pregnancy when periods without respiratory movements dominate (1). Respiratory activity must be generated continuously from birth. New tactile stimuli, such as light, cooling, removal of the umbilical circulation, arousal, air in the upper and lower airways, increased pulmonary blood flow, increased oxygen consumption, vagal input from mechanoreceptors, are all involved in the initiation and maintenance of breathing (2). This transition of respiratory control is probably related to modulatory factors affecting the CPG for respiration located in the brain stem (rostral ventrolateral medulla oblongata).
Adenosine is one of the neuromodulators involved in respiratory control and may be especially important around birth (3–5). Centrally applied adenosine analogs depress respiratory rate and depth (6–8) via adenosine A1 receptors in the brain stem (5, 9). Circulating levels of adenosine decrease immediately after birth (3), correlating to decreased extracellular levels in the brain (10), and a decrease of adenosinergic inhibition may contribute to the establishment of postnatal breathing.
Furthermore, the effect of adenosine is antagonized by caffeine in doses resembling those daily consumed by humans (11). High doses of caffeine intake during gestation or the early postnatal period have been shown to alter adenosine receptor development (12), affect postnatal behavior, and increase the incidence of apnea in rats (11, 13–16). Po2 during fetal life is lower than after birth. This will increase adenosine levels (17) and also the activity of pontine centers (18), both of which have been shown to depress respiratory centers in the medulla oblongata (8, 19). The possible roles of a decreased adenosinergic and pontine inhibition in the change of respiratory control after birth are not known.
The present study was undertaken to investigate the perinatal transition of the respiratory control and its modulation by adenosine. We were particularly interested in studying the effects of adenosine on medullary (CPG) and pontine structures involved in respiratory control. To study the effects of long-term adenosine receptor modulation (blocking), the fetuses and neonates were exposed to caffeine. This is also interesting as many human fetuses and neonates are exposed to methylxanthines in doses that may cause both short- and long-term effects on adenosine receptor expression and behavior. To compare central respiratory control before and after birth an in vitro brain stem-spinal cord preparation from fetal (20, 21) and neonatal rats was used. To examine a structural correlate to these functional studies of adenosine in the developing respiratory system, we also studied the ontogeny of adenosine A1 receptors and their functional coupling to G-proteins and A1-recepter mRNA in pons and medulla.
We show in the present paper that even though the isolated respiratory CPG does not undergo major changes at birth, the ability of adenosine A1 receptors to inhibit the respiratory rhythm decreases within 24 h after birth. Furthermore, in pups exposed to a low dose of caffeine, the pontine inhibition of brain stem respiratory rhythm is increased.
METHODS
Experimental Animals
Timed-pregnant Wistar rats and their litters were used. Dams were given caffeine (Sigma Chemical Co., St. Louis, MO, U.S.A.; 0.3 g/L;n = 23) in the drinking water, exchanged every third day to fresh solutions, from E 2 throughout gestation and postnatal life. Twenty-one dams received ordinary tap water. The day when a vaginal plug was found was designated E0. The regional animal ethics committee approved the experiments, which followed the European Community regulations.
Brain Stem-Spinal Cord Preparation
The brain stem and spinal cord of E18 and E21 fetal rats and 2-h, 24-h, and 72-h (P3) postnatal Wistar rats (n = 76) were dissected and isolated as previously described (5, 22). One fetal rat from each litter was killed immediately (<60 s) after decapitation and cesarean section had been performed on the mother. The preparation was continuously perfused at a rate of 3.0–3.5 mL/min in a 2-mL chamber with the following aCSF (mM): NaCl, 124; KCl, 5.0; KH2PO4, 1.2; CaCl2, 2.4; MgSO4, 1.3; NaHCO3, 26; glucose, 30; equilibrated with 95% O2 and 5% CO2; at 27 ± 0.5°C, pH 7.4. Forty-four preparations were decerebrated rostral to cranial nerve V, and recordings of respiratory activity were performed with the pons remaining during perfusion with normal aCSF. A pontomedullary transection was then performed in all preparations (n = 76), rostrally decerebrating the brain stem between cranial nerve root VI and the lower border of the trapezoid body.
Respiratory activity of the preparation was recorded from the C4 or C5 ventral roots (C4) using suction electrodes. In some experiments (n = 20) the unit activity of respiratory-related neurons in the ventrolateral medulla was also recorded extracellularly using a glass microelectrode filled with 2% pontamine sky blue in 0.5% sodium chloride (resistance, 2–6 MΩ). The electrode was inserted through the ventral surface into the rostral ventrolateral medulla with a micromanipulator (MW-4, Narishige, Tokyo, Japan). Respiration-related neuronal activity was found 50–300 μm below the surface (23).
Signals were amplified and band-pass filtered (10 Hz to 5 kHz, differential AC amplifier model 1700, A-M Systems, Everett, WA, U.S.A.). The C4 activity was rectified and integrated with a time constant of 100 ms. These activities were continuously monitored, registered with a strip-chart recorder, digitized continuously or in 2- to 3-min intervals/10 min, at 1–5 kHz, and stored in a computer for off-line analysis. _f_R (bursts/min) and Ti (ms) were calculated from the mean C4 burst interval and C4 burst width, respectively, during 2–5 min of recording.
Drugs
The metabolically stable adenosine A1 receptor agonist R-PIA (RBI, Natick, MA, U.S.A.) was dissolved in 5% DMSO and then further diluted in standard solution to 1.0 μM. The adenosine receptor antagonist theophylline (Sigma Chemical Co.) was diluted in standard solution to a final concentration of 20 mg/L from a 1 mM stock solution in ethanol. The final concentrations of DMSO or ethanol in the solutions were <0.01%, and control experiments with only DMSO or ethanol added to the standard solution did not change the respiratory activity. Drug solutions were prepared freshly to ensure full potency of drug effect. All drugs were added to the aCSF, and the pH was equilibrated to 7.4 with 95% O2 and 5% CO2 before application to the preparation, which was started by switching perfusion from normal to drug-containing aCSF. Sufficient time to reach steady-state drug response (R-PIA, 20 min, and theophylline, 15 min), as previously determined (5, 9), was allowed before evaluation of drug effect was performed.
Receptor Autoradiography and In Situ Hybridization
Rat brains were examined at E14, E18, E21, exactly 2 h and 24 h after vaginal delivery, and P3, P7, and P14. From each treatment group six animals (from two litters) were used for in situ hybridization and receptor binding studies. From embryos and pups until P3 the whole head was frozen whereas in older animals the brain was rapidly dissected out. Heads and brain were then frozen on dry ice and stored at −20°C. Sagittal sections from the left hemisphere were cut using a Leitz cryostat. Sections were collected from the lateral part of the olfactory bulb toward the midline. For in situ hybridization, 14-μm-thick sections were thaw-mounted on poly-l-lysine (50 μg/mL) -coated slides, and for autoradiography 10-μm-thick sections were thaw-mounted on gelatin-coated slides. Specimens from different ages were processed in the same in situ hybridization and receptor binding runs to allow comparison of signals and binding density.
Adenosine A1-receptor autoradiography.
Receptor density was determined using receptor autoradiography with the adenosine A1 receptor antagonist [3H]DPCPX (0.5 nM), and nonspecific binding was determined using R-PIA (100 μM). Ten-micrometer sections were preincubated in 170 mM Tris-HCl buffer containing 1 mM EDTA, 2 U/mL adenosine deaminase, and 1 mM MgCl2 at 37°C for 30 min. Sections were then washed twice for 10 min at 23°C in 170 mM Tris-HCl buffer. Incubations were performed for 2 h at 23°C in 170 mM Tris-HCl buffer containing [3H]DPCPX (120 Ci/mmol, 0.5 nM), 2 U/mL adenosine deaminase, and 100 μM GTP. The incubation with DPCPX was performed in the presence of GTP to convert all of the receptors to the low-affinity state for agonists and thereby remove all endogenous adenosine (24). Sections were then washed twice for 5 min each in ice-cold Tris-HCl, dipped three times in ice-cold distilled water, and dried at 4°C using a strong fan. Slides were exposed to 3H film (Amersham Pharmacia Biotech, Uppsala, Sweden) with 3H microscales for 4 wk.
Adenosine A1 receptor agonist-stimulated GTPγ[35S] autoradiography.
G-protein coupling of adenosine A1 receptors during development was determined using GTPγ[35S] autoradiography (25) with the adenosine A1 receptor agonist CPA (30 nM). Ten-micrometer-thick sections mounted on gelatin-coated slides were incubated in 50 mM Tris-HCl buffer (pH 7.7) containing 3 mM MgCl2, 0.2 mM EGTA, and 100 mM NaCl at 25°C for 10 min. Slides were then incubated in Tris-HCl buffer containing GDP (1 mM) at 25°C for 25 min. Incubations were performed for 2 h at 25°C in 50 mM Tris-HCl buffer containing GTPγ[35S] (1250 Ci/mmol, purchased from PerkinElmer Life Science Products, Boston, MA, U.S.A., 0.04 nM), GDP (30 mM), and (CPA, 30 mM). The basal activity was assessed with GDP in the absence of A1 receptor agonist, and nonspecific binding was assessed in the presence of the GTP analog Gpp(NH)p (100 μM). Sections were then washed twice for 10 min each in ice-cold Tris-HCl, dipped three times in ice-cold distilled water, and dried at 4°C using a strong fan. Slides were exposed to Hyperfilm β-max (Amersham, Pharmacia Biotech, Uppsala, Sweden) for 24 h.
In situ hybridization.
The mRNA for the A1 receptor was identified with an antisense oligonucleotide probe. The 48-mer A1 adenosine receptor probe was complementary to nucleotides 985–1032 of the rat A1 receptor (26). The probe has been tested for specificity (27). The oligodeoxyribonucleotides were radiolabeled using terminal deoxyribonucleotidyl transferase and 35S-dATP to a specific activity of about 109 cpm/μg. Slide-mounted sections were hybridized in a cocktail containing 50% formamide, 4× saline-sodium citrate, 1× Denhardt's solution, 1% sarcosyl, 0.02 M NaPO4 (pH 7.0), 10% dextran sulfate, 0.06 M dithiothreitol, 0.5 mg/mL sheared salmon sperm DNA, and 107 cpm/mL of probe. After hybridization for 15 h at 42°C, the sections were washed four times in 1× saline-sodium citrate at 55°C, then dipped briefly in water, 70%, 95%, and 99.5% ethanol, and air-dried. Finally, the sections were apposed to Hyperfilm β-max for 4 wk.
Image analysis.
Analysis of receptor expression and binding was performed using a computerized image-analysis system (Imaging Systems, St. Catherines, Ontario, Canada). Relative OD of expression or binding was measured in autoradiographs, and amounts of receptor-bound radioactivity of the specific brain regions were determined using 3H microscale standards (Amersham Pharmacia Biotech). Specific binding was calculated by subtraction of the OD values in sections in which nonspecific binding was determined. Different regions of the brain in the prenatal rats were identified using the atlas by Altman and Bayer (28) and in postnatal rats using atlases by Sherwood and Timiras (29) and Paxinos et al.(30).
Statistical Analysis
Off-line analysis was performed using a personal computer and the commercially available programs Axoscope and Axotape (Axon Inc., Foster, CA, U.S.A.), Origin (Microcal Software Inc., Northampton, MA, U.S.A.), and JMP (SAS Institute Inc., Cary, NC, U.S.A.). The results are presented as mean ± SEM. After ANOVA by the F test, statistical analysis was performed by multivariate analysis of variance (MANOVA) repeated-measure design, two-tailed paired t test, or Wilcoxon signed rank test when variance was unequal. Tukey-Kramer's HSD test was used to compare multiple means. Spearman rank nonparametric correlation was performed on variables with respect to postnatal age, to evaluate a possible age-dependency of the results. Quantitative receptor autoradiography and in situ hybridization results were analyzed by MANOVA using the procedures in the SYSTAT program (HALLOGRAM Publishing, Aurora, CO, U.S.A.). All these measurements were performed on sections from six animals. A p ≤ 0.05 was considered to be statistically significant.
RESULTS
Development of brain stem respiratory rhythm.
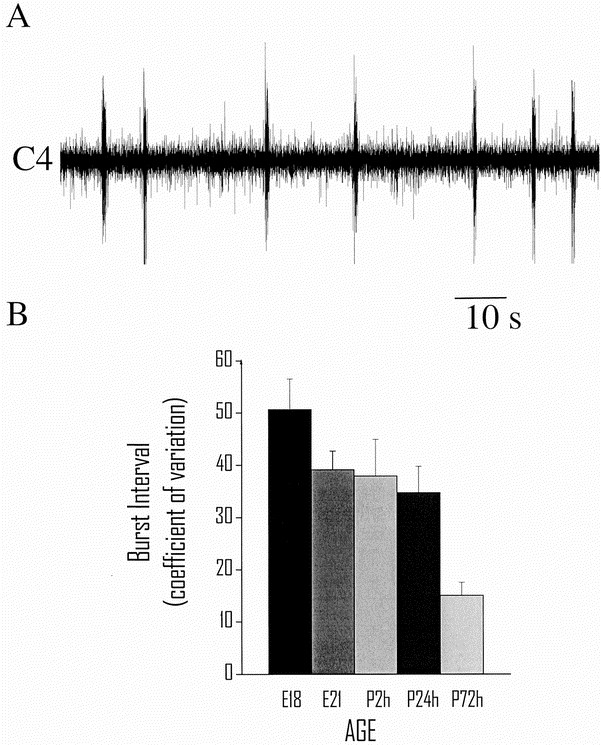
An irregular rhythmic respiratory activity was established in all preparations at E18 (Fig. 1_A_). The _f_R at E18 was slower than at all other ages (p < 0.05, Tukey-Kramer's HSD test) The regularity of respiratory activity, measured as a decrease in the CV of the interval between C4 burst discharges, increased with embryonic and postnatal age (p < 0.0001, age versus CV Spearman rank correlation; Fig. 1_B_). Ti in each C4 burst increased with embryonic age (E18, 414 ± 90 ms; E21, 641 ± 135 ms; P2h, 819 ± 81 ms; P24h, 860 ± 57 ms; P72h, 812 ± 91 ms;p < 0.05, ANOVA). At E21 a respiratory pattern resembling that after birth had been established with regard to pontine inhibition, control _f_R, and Ti. When comparing the central respiratory rhythm generated before birth (E21) with that generated 2 and 24 h after birth, no significant differences were found.
Figure 1
Development of brain stem respiratory rhythm generation. A, continuous but irregular C4 respiratory frequency and burst width from a fetal (E18) brain stem-spinal cord preparation without the pons. B, regularity of respiratory rhythm quantified as CV of C4 burst interval (mean ± SEM). Regularity of rhythmic respiratory activity increased with postnatal age (age vs CV, Spearman rank correlation p < 0.0001).
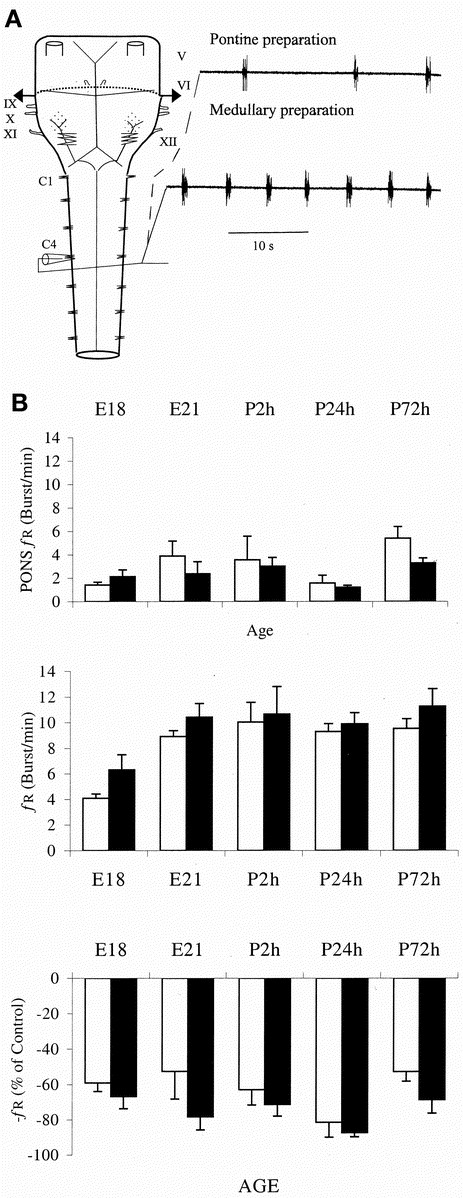
In preparations in which recordings initially were performed with the pons remaining (n = 44, E18–P3), removal of the pons resulted in a significant increase in _f_R (from 2.3 ± 0.3 to 9.1 ± 0.3 bursts/min;p < 0.0001, paired t test; Fig. 2_A_). Mean _f_R did not differ between age groups when the pons remained (Fig. 2_B_, top), but was significantly slower at E18 after pontomedullary transection compared with all other age groups (p < 0.01, Tukey-Kramer's HSD test; Fig. 2_B_, middle).
Figure 2
Development of respiratory rhythm generation and its modulation by pons. A, schematic illustration of the brain stem-spinal cord preparation is shown on the left side. Dashed line indicates division when removing the pons. Cranial nerves V–XII and ventral roots C1–C4 are shown. On the right side the respiratory activity in a preparation from a 1-d-old postnatal rat before (upper trace) and after removal of the pons (lower trace). B, mean ± SEM control C4 _f_R (bursts/min) in brain stem-spinal cord preparation with pons (top) and with pons removed (middle), and inhibition exerted by pons on respiratory rhythm generated in medulla oblongata (bottom, % of control _f_R with pons intact compared with _f_R after removal of pons) plotted against age. Both pontine inhibition of respiratory activity and basal _f_R were increased in the caffeine-treated group (p < 0.05, MANOVA, repeated-measures design). White columns represent control group (n = 5–8) and black columns represent caffeine-treated group (n = 4–6).
Effect of R-PIA on medullary respiratory rhythm.
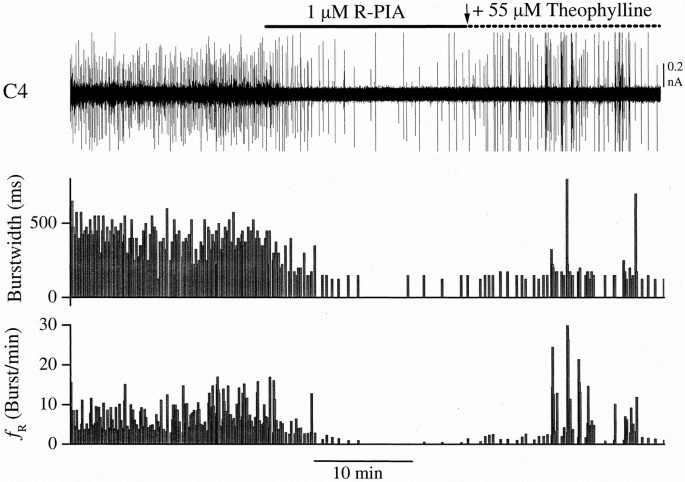
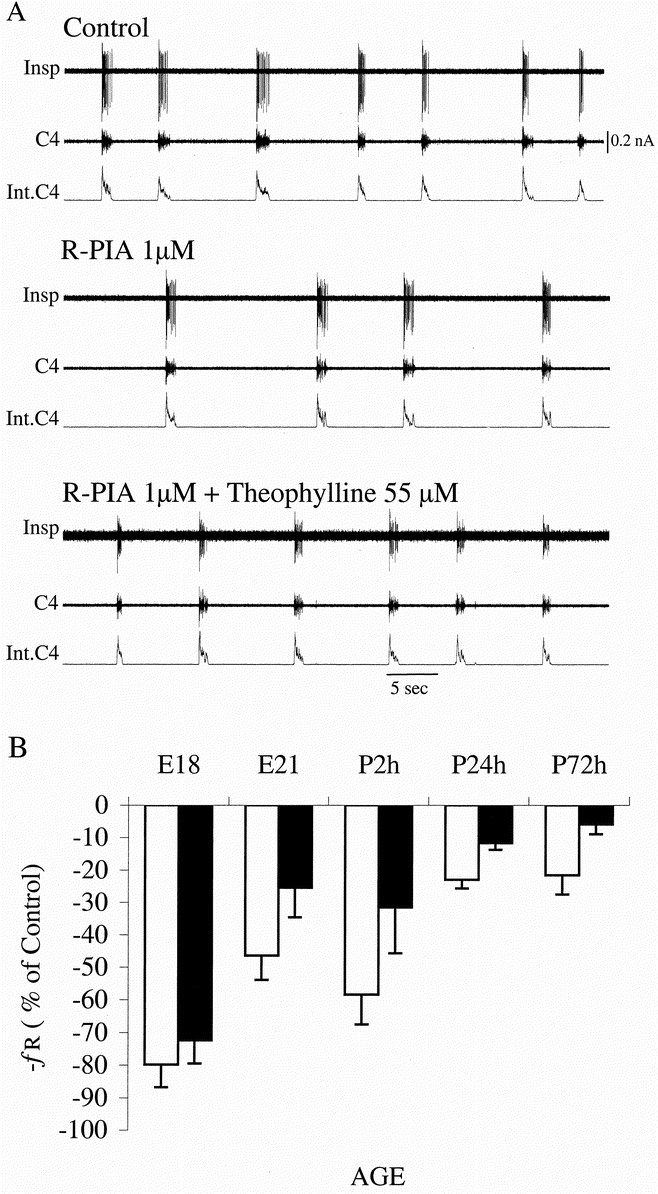
In the experiments in which brain stem respiratory-related neuronal activity was measured, it decreased after administration of R-PIA (1 μM) in parallel with C4 activity, as evident in Figures 3 and 4. These depressant effects on respiratory activity were reversed by theophylline (55 μM). The effect of R-PIA was age-dependent (p < 0.0001, MANOVA, repeated-measures design) with less _f_R reduction with increasing fetal and postnatal age. The effect of R-PIA was significantly reduced 24 h after birth compared with E21 and 2h (p < 0.01, Tukey-Kramer's HSD test).
Figure 3
Effects of R-PIA on fetal respiratory activity. Tracings depict respiratory activity recorded from E18 preparation. Note that the effect of R-PIA is pronounced and reversible by theophylline. Upper trace, C4 respiratory activity;middle trace, burst width (Ti) in ms; and lower trace, _f_R (bursts/min).
Figure 4
Age-related effects of R-PIA on respiratory activity. A, recording from E21 rat and effects of R-PIA (1 μM) and theophylline (55 μM). Insp, inspiratory neuron;C4, C4 ventral root activity;Int.C4, integrated C4. B, mean ± SEM response (% of control) of C4 _f_R to R-PIA plotted against age. The effect of R-PIA was related to perinatal age (p < 0.0001, MANOVA, repeated-measures design). Within 24 h after birth the respiratory depressant effect of R-PIA is decreased (p < 0.05) compared with the effect at E21 and P2 h. No significant difference in the response to R-PIA was found between the control (white columns) and caffeine-exposed groups (black columns).
Caffeine exposure and development of respiratory rhythm.
Caffeine-exposed pups did not differ from controls with respect to litter size or weight. The pontine inhibition of brain stem-generated C4 activity was more pronounced in the caffeine group (p < 0.05, MANOVA, repeated-measures design; Fig. 2_B_). With the pons removed, brain stem-spinal cord preparations from caffeine-exposed pups had a higher _f_R than controls (p <0.05 t test; Fig. 2_B_, middle). There was no significant difference in the response to R-PIA between preparations from caffeine-treated and control groups (Fig. 4_B_).
A1 mRNA, DPCPX-binding, and A1-stimulated GTPγ[35S] binding.
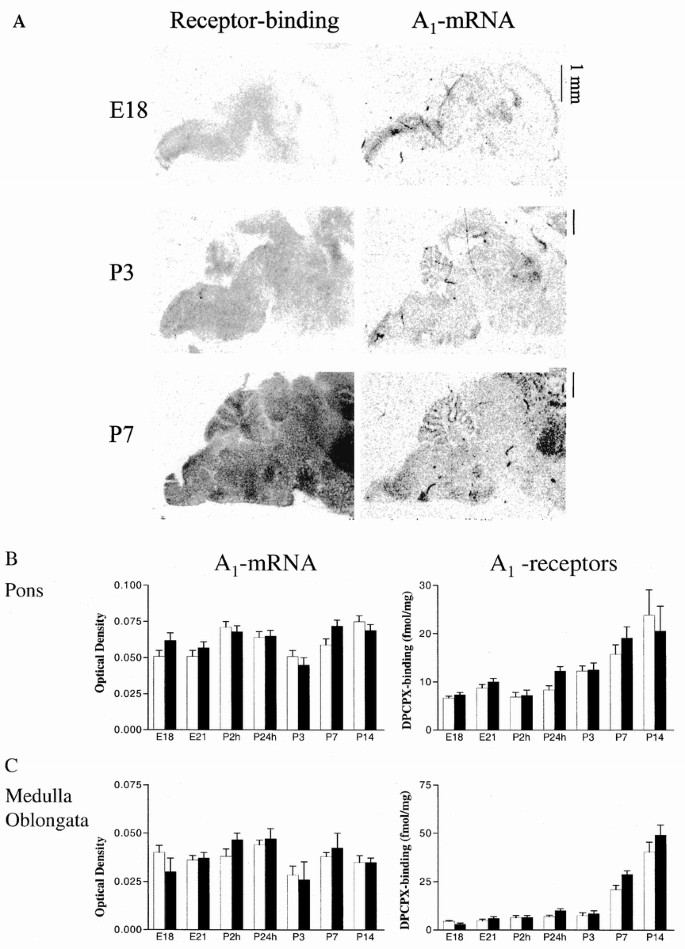
Adenosine A1 receptor mRNA was present on E14 in low levels in the pontine neuroepithelium. On E21, specific A1 mRNA expression was identified in the pontine gray nucleus (not shown). In pups decapitated shortly after vaginal delivery (2 and 24 h), there were no dramatic changes in adenosine receptors in the brain compared with levels just before birth, but at P3 there was a tendency to a down-regulation of A1 mRNA expression. At P7 A1 mRNA was selectively expressed and DPCPX-binding was enriched in several brain stem nuclei, including the pontine gray nucleus (Fig. 5_A_). The facial nucleus, lateral reticular nucleus, and the periambiguous area also seemed to have increased binding.
Figure 5
A1-receptor mRNA expression and [3H]DPCPX binding. A, film autoradiograms generated from receptor autoradiography (left side) and in situ hybridization (right side) using sagittal sections. Adenosine A1 mRNA expression and A1 receptor binding are present in the brain at E18 (whole brain is shown) and are enriched in several brain stem nuclei at P3 and P7 (caudal parts of the brain are shown). Receptor binding was determined using [3H]DPCPX binding (0.5 nM), and in situ hybridization was performed using an oligonucleotide probe. Nonspecific binding was determined and was equal to background. B, pre- and early postnatal time course of adenosine A1 mRNA and [3H]DPCPX binding in pons in controls (white bars) and low-dose (0.3 g/L) caffeine-treated (black bars) rat fetuses and pups. Mean ± SEM of groups with n = 6. C, pre- and early postnatal time course of adenosine A1 mRNA and [3H]DPCPX binding in medulla in controls (white bars) and low-dose caffeine-treated (black bars) rat fetuses and pups. Note that A1 mRNA is generally flat and A1 receptors tend to increase with postnatal age. Mean ± SEM of groups with n = 6.
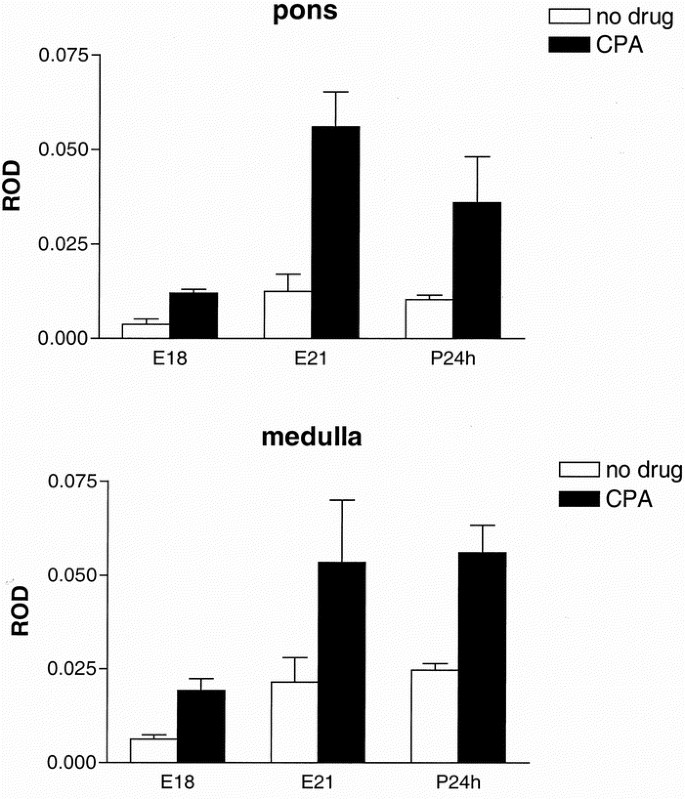
When adenosine A1 receptors were stimulated with CPA, basal GTPγ[35S] binding in the pons and medulla was more than doubled at E18, E21, and P24h (p < 0.001, two-way ANOVA), indicating a functional coupling to G-proteins of these receptors already at E18 (Fig. 6). Caffeine-exposed animals had the same levels of A1 mRNA in the pons and medulla as controls, but [3H]DPCPX binding was slightly higher in the caffeine-treated animals than in controls; however, data were only significant when pooled from different age groups. There was an increased [3H]DPCPX binding in the pons when comparing E18–P7 (2–47%, p = 0.019) and in the medulla when comparing P24h–P14 (12–37%, p = 0.018).
Figure 6
A1 receptors and functional G-protein coupling. Basal GTPγ[35S] binding (white bars) and GTPγ[35S] binding after stimulation with the adenosine A1 receptor agonist CPA (black bars) at E18, E21, and P24h. The increased GTPγ[35S] binding after agonist stimulation indicates a functional coupling of the receptor to the G-protein already at E18 in pons (top) and medulla oblongata (bottom). Nonspecific binding was determined and was equal to background. ROD, relative optical density. Mean ± SEM of groups with n = 6.
DISCUSSION
The present study shows that whereas there seems to be no dramatic change of basic respiratory rhythm control at birth, an adenosine A1 receptor agonist inhibits the respiratory rhythm generation more markedly before and around birth than 24 h postnatally. Furthermore, in pups chronically exposed to a low dose of caffeine via maternal intake, the pontine inhibition of brain stem respiratory rhythm is more pronounced compared with that of controls.
Perinatal development of respiratory rhythm.
We demonstrate that even though the isolated CPG for breathing continuously matures during perinatal life, no major change in pontine inhibition, _f_R, TI, or stability of pattern occurs between E21 and 2 and 24 h after birth. These finding s indicate that it is mainly afferent, suprapontine input and changes in the local environment (i.e. increased Po2) that alter breathing from a discontinuous to a continuous behavior at birth.
We also show that the pontine inhibition of respiratory centers in the medulla is already present at E18 and does not change between E21 and P3. This inhibition is mediated by noradrenergic activity originating from A5 pontine structures (19). The A5 nucleus appears in the rat on fetal day 17 (31) and our new findings suggest that already at E18, the A5 nucleus modulates medullary respiratory activity. Thus, the pons may be involved in the formation of the early CPG for respiration. This would be in accordance with previous data demonstrating an important role of the pons in the development of medullary respiratory networks and control of breathing (32). We could not demonstrate a change in the pontine inhibition around birth. However, our studies were performed using similar in vitro conditions for preparations taken before and after birth. A postnatal change in Po2 would probably decrease the activity of the A5 nucleus in vivo, causing less pontine inhibition and thus increasing CPG activity in the medulla oblongata after birth.
Adenosinergic respiratory depression decrease after birth.
A reduced ability of adenosine A1 agonist to decrease respiratory activity occurred already within 24 h postnatally (Fig. 4). This new finding narrows the window when a switch in adenosine's ability to depress breathing occurs. Previous studies in rats (5, 9), rabbits (4), and piglets (33), have reported a similar reduction of adenosinergic effect to occur within the first days or weeks after birth. Immediately after birth, as the partial pressure for oxygen increases, circulating levels of adenosine decrease (3, 34), correlating with decreased extracellular levels in the brain (10), and thus the adenosinergic inhibition of breathing is reduced. Taken together with the present findings, this will contribute to an increased activity of the CPG and establishment of continuous breathing. The changes in _f_R were accompanied by a simultaneous change in brain stem respiration-related neuronal activity in all preparations in which recordings were performed. This suggests that adenosine also during the fetal period affects _f_R by acting on brain stem respiratory neurons via adenosine A1 receptors in the medulla oblongata (9).
Caffeine exposure and development of respiratory rhythm.
Here we demonstrate that long-term caffeine intake during gestation and the early postnatal period increases the baseline frequency of C4 respiratory output. Adenosine depresses and adenosine antagonist increases respiration during control conditions (5, 35), suggesting the occurrence of an endogenous adenosinergic tonic activity during the perinatal period. In addition, long-term caffeine intake seems to increase the inhibition exerted by pontine structures in vitro. Methylxanthines increase the turnover of noradrenaline (36) and spontaneous electrical activity of noradrenergic neurons. Thus a possible explanation for our findings could be that caffeine, by inducing an increased activity of pontine noradrenergic neurons such as those situated in the A5 area, increases the inhibition from pontine structures to the respiratory centers in the medulla oblongata. This finding is of interest because the pons is involved in respiratory depression during hypoxia (18). We have preliminary in vivo data indicating a change in hypoxic respiratory response in caffeine-treated pups, which could agree with the present in vitro findings of a change in pontine control of brain stem activity. However, further studies will be needed to elucidate eventual detrimental effects on respiratory control in vivo.
A1 mRNA, DPCPX-binding, and A1-stimulated GTPγ[35S] binding.
A new finding is that A1 receptor coupling to G-protein occurred prenatally in the brain stem as indicated by A1-stimulated GTPγ[35S] binding (Fig. 6). This is in contrast to previous studies indicating that the major development in terms of density and coupling to second messenger-forming systems in the cerebral cortex and hippocampus occurs postnatally (37, 38). However, the phylogenetically old brain stem matures earlier than the phylogenetically younger parts of the brain examined in the previous studies. Thus, A1-receptor coupling to G-proteins occurs prenatally in the brain stem. We found robust A1 mRNA labeling and DPCPX binding in the pons-medulla toward the end of gestation (E18–E21) and thereafter in accordance with previous studies (39–41). The pontine gray nucleus is connected to cerebellum, and we are not aware of reports indicating a relation to respiratory control. Our finding of increased binding in brain stem nuclei, including the periambiguous area, agrees with a previous study in immature sheep (42), which reported that the highest density of A1 receptors are found in the rostral ventrolateral medulla, the area that contains the CPG for respiration (43). However, in the present study receptor binding was examined in sagittal slices, and a more precise determination of adenosine A1 mRNA and A1 receptor distribution within the brain stem was not performed. In pons and medulla there are no major changes in A1 receptor development in caffeine-exposed fetuses and pups, in agreement with our previous report concerning effects of perinatal caffeine exposure to other parts of the brain (44).
One might explain the increased control _f_R by a decrease in functional adenosine receptors in the caffeine-treated group. However, if A1 receptors were affected at all by long-term exposure to low-dose caffeine, they were rather up-regulated. These findings suggest that caffeine affects the adenosine receptors at another level rather than by altering the receptor number. Even though the in vitro preparations were superfused with caffeine-free aCSF for a substantial time (104 ± 7 min) before control measurement was performed, remaining caffeine could explain both the increased _f_R and the tendency toward decreased R-PIA effect in the caffeine group. Alternatively, caffeine affects A1 receptor signaling downstream of the receptor or induces other types of adaptive changes, as occur in the adult animal in which agonist binding has been shown to increase without detectable changes in A1 mRNA or antagonist binding (45, 46).
The effect of the adenosine A1 receptor agonist R-PIA decreased within 24 h after birth. However, there was no corresponding decrease in A1 receptor binding or A1-stimulated G-protein coupling 24 h after birth. A decreased binding affinity of A1 receptor to A1 agonist has previously been reported to occur with increasing postnatal age (4, 47). This could explain the decreased sensitivity for adenosine A1 receptor-mediated respiratory depression after birth; however, this was not determined in the present study.
We conclude that respiration is already strongly modulated by adenosinergic action at the level of the medulla oblongata at E18 and that this modulatory action is reduced 24 h after birth. Furthermore, long-term maternal caffeine intake during gestation increases the pontine inhibition of and the activity of medulla oblongata respiratory centers in offspring without changing A1 receptor mRNA and protein expression.
Abbreviations
CPA:
_N_6-cyclopentyladenosine
CPG:
Central Pattern Generator
DPCPX:
8-cyclopentyl-1, 3-dipropylxanthine
f R :
frequency of respiratory activity (bursts/min)
Ti:
inspiratory time
R-PIA:
_N_6-(2-phenylisopropyl) adenosine, R (−) isomer
aCSF:
artificial cerebrospinal fluid
E:
embryonic day
P:
postnatal day
GTPγ[35S]:
guanylyl-5′-O-(γ-[35S]thio)-triphosphate
CV:
coefficient of variation
References
- Maloney JE, Adamson TM, Brodecky V, Dowling MH, Ritchie BC 1975 Modification of respiratory center output in the unanesthetized fetal sheep “in utero.”. J Appl Physiol 39: 552–558
Article CAS Google Scholar - Blanco C 1991 Role of the brainstem in the changes at birth; initiation of continuous breathing and its maintenance. In: Hanson MA (ed) The Fetal and Neonatal Brainstem: Developmental and Clinical Issues. University Press, Cambridge, pp 106–126.
Google Scholar - Irestedt L, Dahlin I, Hertzberg T, Sollevi A, Lagercrantz H 1989 Adenosine concentration in umbilical cord blood of newborn infants after vaginal delivery and cesarean section. Pediatr Res 26: 106–108
Article CAS Google Scholar - Runold M, Lagercrantz H, Fredholm BB 1986 Ventilatory effect of an adenosine analogue in unanesthetized rabbits during development. J Appl Physiol 61: 255–259
Article CAS Google Scholar - Herlenius E, Lagercrantz H, Yamamoto Y 1997 Adenosine modulates inspiratory neurons and the respiratory pattern in the brainstem of neonatal rats. Pediatr Res 42: 46–53
Article CAS Google Scholar - Lagercrantz H, Yamamoto Y, Fredholm BB, Prabhakar NR, Euler C 1984 Adenosine analogues depress ventilation in rabbit neonates: theophylline stimulation of respiration via adenosine receptors?. Pediatr Res 18: 387–390.
Article CAS Google Scholar - Barraco RA, Ridi MR, Parizon M 1990 The adenosine analog, 5′-_N_-ethylcarboxamidoadenosine, exerts mixed agonist action on cardiorespiratory parameters in the intact but not decerebrate rat following microinjections into the nucleus tractus solitarius. Brain Res 530: 54–72
Article CAS Google Scholar - Bissonnette JM, Hohimer AR, Knopp SJ 1991 The effect of centrally administered adenosine on fetal breathing movements. Respir Physiol 84: 273–285
Article CAS Google Scholar - Herlenius E, Lagercrantz H 1999 Adenosinergic modulation of respiratory neurones in the neonatal rat brainstem in vitro. J Physiol 518: 159–172
Article CAS Google Scholar - Koos BJ, Mason BA, Punla O, Adinolfi AM 1994 Hypoxic inhibition of breathing in fetal sheep: relationship to brain adenosine concentrations. J Appl Physiol 77: 2734–2739.
Article CAS Google Scholar - Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE 1999 Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83–133
CAS Google Scholar - Guillet R, Kellogg CK 1991 Neonatal exposure to therapeutic caffeine alters the ontogeny of adenosine A1 receptor in brain of rats. Neuropharmacol 30: 489–496
Article CAS Google Scholar - Guillet R 1990 Neonatal caffeine exposure alters adenosine receptor control of locomotor activity in the developing rat. Dev Pharmacol Ther 15: 94–100
Article CAS Google Scholar - Tye K, Pollard I, Karlsson L, Scheibner V, Tye G 1993 Caffeine exposure in utero increases the incidence of apnea in adult rats. Reprod Toxicol 7: 449–452
Article CAS Google Scholar - Guillet R, Dunham L 1995 Neonatal caffeine exposure and seizure susceptibility in adult rats. Epilepsia 36: 743–749
Article CAS Google Scholar - Holloway WRJ, Thor DH 1982 Caffeine sensitivity in the neonatal rat. Neurobehav Toxicol Teratol 4: 331–333
CAS PubMed Google Scholar - Yoneyama Y, Shin S, Iwasaki T, Power GG, Araki T 1994 Relationship between plasma adenosine concentration and breathing movements in growth-retarded fetuses. Am J Obstet Gynecol 171: 701–706
Article CAS Google Scholar - Okada Y, Kawai A, Muckenhoff K, Scheid P 1998 Role of the pons in hypoxic respiratory depression in the neonatal rat. Respir Physiol 111: 55–63
Article CAS Google Scholar - Hilaire G, Monteau R, Errchidi S 1989 Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res 485: 325–332
Article CAS Google Scholar - Di Pasquale E, Monteau R, Hilaire G 1992 In vitro study of central respiratory-like activity in the fetal rat. Exp Brain Res 89: 459–464
Article CAS Google Scholar - Greer JJ, Smith JC, Feldman JL 1992 Respiratory and locomotor patterns generated in the fetal rat brain stem-spinal cord in vitro. J Neurophysiol 67: 996–999
Article CAS Google Scholar - Suzue T 1984 Respiratory rhythm generation in the in vitro brainstem-spinal cord preparation of the neonatal rat. J Physiol Lond 354: 173–183
Article CAS Google Scholar - Arata A, Onimaru H, Homma I 1990 Respiration-related neurons in the ventral medulla of newborn rats in vitro. Brain Res Bull 24: 599–604
Article CAS Google Scholar - Fastbom J, Fredholm B 1990 Effects of longterm theophylline treatment on adenosine A1-receptors in rat brain: autoradiographic evidence for increased receptor number and altered coupling to G-proteins. Brain Res 507: 195–199
Article CAS Google Scholar - Sim LJ, Selley DE, Childers SR 1995 In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA 92: 7242–7246
Article CAS Google Scholar - Mahan LC, McVittie LD, Smyk-Randall EM, Nakata H, Monsma FJ, Gerfen CR, Sibley DR 1991 Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol 40: 1–7
CAS Google Scholar - Johansson B, Ahlberg S, van der Ploeg I, Brene S, Lindefors N, Persson H, Fredholm BB 1993 Effect of long term caffeine treatment on A1 and A2 adenosine receptor binding and on mRNA levels in rat brain. Naunyn Schmiedebergs Arch Pharmacol 347: 407–414
Article CAS Google Scholar - Altman J, Bayer SA 1995 Atlas of Prenatal Rat Brain Development. CRC Press, Boca Raton, FL, p 589
- Sherwood SN, Timiras PS 1970 A Stereotaxic Atlas of the Developing Brain. Univ of California Press, California Press, Berkeley, CA, U.S.A.
- Paxinos G, Törk I, Tecott LH, Valentino KL 1991 Atlas of the Developing Rat Brain. Academic Press, San Diego, p 271
- Amaral DG, Sinnamon HM 1977 The locus coeruleus: neurobiology of a central noradrenergic nucleus. Prog Neurobiol 9: 147–196
Article CAS Google Scholar - Jacquin TD, Borday V, Schneider-Maunoury S, Topilko P, Ghilini G, Kato F, Charnay P, Champagnat J 1996 Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron 17: 747–758
Article CAS Google Scholar - Elnazir B, Marshall JM, Kumar P 1996 Postnatal development of the pattern of respiratory and cardiovascular response to systemic hypoxia in the piglet: the roles of adenosine. J Physiol Lond 492: 573–585
Article CAS Google Scholar - Fukuda S, Katoh S, Yamamoto K, Hashimoto M, Kitao M 1990 Correlation between levels of plasma adenosine triphosphate and stress to the fetus at delivery. Biol Neonate 57: 150–154
Article CAS Google Scholar - Schmidt C, Bellingham MC, Richter DW 1995 Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J Physiol Lond 483: 769–781
Article CAS Google Scholar - Nehlig A, Daval JL, Debry G 1992 Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Rev 17: 139–170
Article CAS Google Scholar - Johansson B, Georgiev V, Fredholm BB 1997 Distribution and postnatal ontogeny of adenosine A2A receptors in rat brain: comparison with dopamine receptors. Neuroscience 80: 1187–1207
Article CAS Google Scholar - Marangos PJ, Patel J, Stivers J 1982 Ontogeny of adenosine binding sites in rat forebrain and cerebellum. J Neurochem 39: 267–270
Article CAS Google Scholar - Rivkees SA 1995 The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Dev Brain Res 89: 202–213
Article CAS Google Scholar - Weaver DA 1996 A1-adenosine receptor gene expression in fetal rat brain. Dev Brain Res 94: 205–223
Article CAS Google Scholar - Reppert SM, Weaver DR, Stehle JH, Rivkees SA 1991 Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol 5: 1037–1048
Article CAS Google Scholar - Bissonnette JM, Reddington M 1991 Autoradiographic localization of adenosine A1 receptors in brainstem of fetal sheep. Dev Brain Res 61: 111–115
Article CAS Google Scholar - Rekling JC, Feldman JL 1998 Prebötzinger complex and pacemaker neurons—hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60: 385–405
Article CAS Google Scholar - Åden U, Herlenius E, Tang L-Q, Fredholm B 2000 Minor effects of maternal caffeine intake on adenosine ontogeny in the rat brain. Pediatr Res 48: 177–183
Article Google Scholar - Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB 1996 Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci 17: 108–113
Article CAS Google Scholar - Johansson B, Georgiev V, Lindstrom K, Fredholm BB 1997 A1 and A2A adenosine receptors and A1 mRNA in mouse brain: effect of long-term caffeine treatment. Brain Res 762: 153–164
Article CAS Google Scholar - Guillet R, Kellogg CK 1991 Neonatal caffeine exposure alters developmental sensitivity to adenosine receptor ligands. Pharmacol Biochem Behav 40: 811–817
Article CAS Google Scholar
Acknowledgements
The authors thank Dr. Michael Runold and Prof. Bertil Fredholm for valuable comments on the manuscript and Assoc. Prof. R.A. Harris for English corrections.
Author information
Authors and Affiliations
- Department of Woman and Child Health, Karolinska Hospital, Stockholm, 171 76, Sweden
Eric Herlenius, Ulrika Ådén, Lie Qi Tang & Hugo Lagercrantz - Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, 171 76, Sweden
Ulrika Ådén
Authors
- Eric Herlenius
You can also search for this author inPubMed Google Scholar - Ulrika Ådén
You can also search for this author inPubMed Google Scholar - Lie Qi Tang
You can also search for this author inPubMed Google Scholar - Hugo Lagercrantz
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toEric Herlenius.
Additional information
Supported by grants from the Swedish Medical Research Council (SMFR Nos. 5234 and 2553), the Swedish Society for Medical Research, Society for Childcare, and the Jeanssonska and the Fraenckel Foundations for Medical Research.
Rights and permissions
About this article
Cite this article
Herlenius, E., Ådén, U., Tang, L. et al. Perinatal Respiratory Control and Its Modulation by Adenosine and Caffeine in the Rat.Pediatr Res 51, 4–12 (2002). https://doi.org/10.1203/00006450-200201000-00004
- Received: 28 August 2000
- Accepted: 29 June 2001
- Issue Date: 01 January 2002
- DOI: https://doi.org/10.1203/00006450-200201000-00004