Pulmonary Arterial Contractility in Neonatal Lambs Increases with 100% Oxygen Resuscitation (original) (raw)
Main
The extent of oxygen supplementation during optimal resuscitation of a newborn infant remains controversial. Oxygen plays a crucial role in mediating pulmonary vascular transition at birth and even small increases in fetal oxygenation (similar to or less than that induced by room air ventilation) result in a dramatic decrease in pulmonary vascular resistance (1–3). However, the debate regarding the use of oxygen for neonatal resuscitation has intensified in recent years. New arguments have been added to the debate as more and more data indicate that not only is room air as good as 100% oxygen for resuscitation but that even a brief exposure to pure oxygen at birth might be detrimental (4). It has been suggested that breathing 100% oxygen dilates constricted pulmonary arteries more efficiently than room air. However, ultrasound and direct estimation of pulmonary arterial pressure in asphyxiated piglets demonstrated that pulmonary hypertension normalized as quickly with room air as with 100% oxygen (5,6).
NE constriction of pulmonary arteries isolated from fetal and neonatal animals after normal transition has been studied in detail (7–9). However, the effect of oxygen exposure during resuscitation and or ventilation on the contractile response to NE is not known.
We hypothesized that exposure to 100% oxygen during resuscitation would result in formation of reactive oxygen species and induce increased pulmonary arterial contractile response and that the increased contractility would be proportional to the duration of exposure to 100% oxygen. We also expected impaired relaxation responses to endothelium-dependent and -independent agents after exposure to 100% oxygen due to inactivation of nitric oxide by reactive oxygen species. We addressed these hypotheses using term lambs with varying levels of oxygen exposure.
METHODS
We studied the effect of resuscitation with 21% oxygen versus 100% oxygen for 30 min followed by normoxic ventilation for a total of 24 h on pulmonary arterial contraction to both receptor dependent and independent vasoconstrictors. Relaxations to endothelium dependent and independent vasodilators were also examined. We compared the constrictor responses to results obtained from pulmonary arteries isolated from normal 24-h-old lambs and newborn lambs ventilated with 100% oxygen for the entire 24 h.
The Laboratory Animal Care Committee at the State University of New York at Buffalo approved this study. Near term gestation pregnant ewes (139 d gestation, term approximately 145 d) were obtained from the Swartz family farm (Attica, NY). After 12 h of fasting, ewes were anesthetized with pentothal and halothane. Lambs were exteriorized by cesarean section. A small incision was made in the neck and systemic arterial and venous access was established through the carotid artery and jugular vein respectively. Lambs were intubated and then delivered. Lambs were sedated with fentanyl 2 μg/kg/dose every 2 h prn and received an initial dose of pancuronium bromide 0.1 mg/kg/dose at birth, which was repeated only if necessary for vigorous spontaneous movement despite adequate sedation. Repeat dose of pancuronium bromide was required in approximately two lambs in each group. Only one extra dose was required in these lambs at approximately 8–14 h of life. They were placed under servo-controlled radiant warmers and rectal temperature was maintained between 37.9 and 39°C. Intravenous fluids (dextrose 10% solution with 25 mEq of sodium chloride, 20 mEq of potassium chloride and 10 mEq of sodium bicarbonate per liter) were administered continuously at 100 mL/kg/d. Fluid composition and rate was adjusted based on serum electrolyte values. The lambs were ventilated with Servo 300 ventilators (Seimens, Mississauga, ON, Canada) with the following settings: positive end-expiratory pressure, 4; rate, 60/min; peak inspiratory pressure (PIP), approximately 25 cm of water (adjusted to deliver 10 mL/kg tidal volume using a BiCore CP-100 Monitor, BiCore Monitoring systems, Irvine, CA). Arterial blood gases were monitored frequently (every 5–15 min) during initial stabilization. Ventilator settings (PIP and rate) were adjusted to maintain arterial Pco2 between 35 and 50 mm Hg. A neonatologist, neonatal fellow, neonatal nurse practitioner, or neonatal nurses from the Women and Children's Hospital of Buffalo provided continuous care for the lambs.
The lambs were assigned to the following groups: i) newborn lambs (approximately 24 h after vaginal delivery, usually at 145–147 d gestation) obtained directly from the farm [room air spontaneous (RA Spont) group, n = 6]; ii) ventilated with 21% O2 for 30 min (arbitrarily called “resuscitation”) followed by adjustment of Fio2 for 24 h to maintain Pao2 between 45 and 65 mm Hg (21%Res group, n = 6), iii) ventilated with 100% O2 for 30 min, followed by adjustment of Fio2 to maintain Pao2 between 45 and 65 mm Hg (100%Res group, n = 6), iv) ventilated with 100% O2 for 24 h, without any adjustment of Fio2 (100%24h group, n = 5).
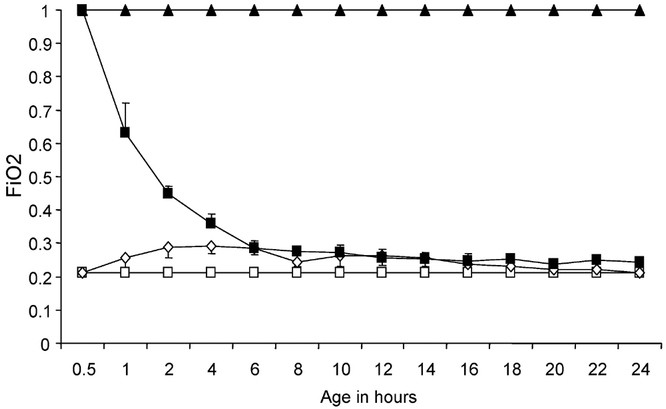
The Fio2 weaning protocol in the 100%Res group was as follows: After 30 min of ventilation with 100% oxygen, an arterial blood gas was obtained every 30 min. The difference in actual Pao2 and target Pao2 (65 mm Hg) was calculated. This number was divided by 7 (based on 100% oxygen − alveolar Po2 of approximately 700 mm Hg and that weaning by 1% should decrease Pao2 by 7 mm Hg in the absence of lung disease) and oxygen concentration was weaned by this number. For example, if Paco2 was 275 mm Hg with 70% oxygen, oxygen was weaned by 30% and blood gas repeated in 30 min. (275 − 65 = 210/7 = 30%). The actual trend in Fio2 wean is shown in Figure 1.
Figure 1
Four groups of lambs, based on oxygen exposure are shown in this figure where Fio2 is plotted on the Y-axis and X-axis shows age in hours. Term lambs were ventilated with 21% oxygen (21%Res: ⋄, n = 6), or 100% oxygen (100%Res: ▪, n = 6), for the first 30 min of life. Subsequently, oxygen was adjusted and both these groups were managed similarly with a target Pao2 of 45–65 mm Hg for 24 h. Lambs ventilated continuously with 100% oxygen for 24 h (100%24h: ▴, n = 5), and spontaneously breathing newborn lambs delivered at the farm (RA Spont: □, n = 6) were studied for comparison. All lambs were killed at 24 h of life.
After 24 h of ventilation, lambs were anesthetized with pentothal sodium and killed by rapid exsanguinations through a direct cardiac puncture. The heart and lungs were removed en bloc and fifth-generation pulmonary arteries (inner diameters of 500 μm) were dissected, isolated, and cut into rings as described previously (10). Rings were suspended in water-jacketed chambers filled with aerated (94% O2 − 6% CO2) modified Krebs-Ringer solution (118 mM sodium chloride, 4.7 mM potassium chloride, 2.5 mM calcium chloride, 1.2 mM magnesium sulfate, 1.2 mM potassium biphosphate, 25.5 mM sodium bicarbonate, and 5.6 mM glucose). A continuous recording of isometric force generation was obtained by tying each vessel ring to a force displacement transducer (model UC2, Statham Instruments, Hato Rey, PR) that was connected to a recorder (Gould Instrument Systems, Valley View, OH). After the arterial rings were mounted, they were allowed to equilibrate for 20 min in the bathing solution. A micrometer was used to stretch the tissues repeatedly in small increments over the following 45 min until resting tone remained stable at a passive tension of 0.8 g. Preliminary experiments determined that this procedure provided optimal length for generation of active tone to exogenous NE. Wet tissue weights were obtained at the end of each experiment, and contraction responses were normalized to tissue weight.
The following pharmacological agents were used: indomethacin, DL-propranolol, norepinephrine hydrochloride (NE), calcium ionophore A23187 and SNAP, and nitric oxide synthase antagonist, LNA. All drugs were obtained from Sigma-Aldrich (St. Louis, MO). SNAP was dissolved first in a small quantity of DMSO and then diluted in distilled water. Indomethacin was dissolved in ethanol and LNA was dissolved in warmed Kreb's solution. All other drugs were dissolved in distilled water. Ethanol and DMSO, at the concentrations used in these experiments, did not alter the preexisting tone of the pulmonary arteries.
Isolated pulmonary arteries were pretreated with indomethacin (10−5 M) to prevent the formation of vasoactive prostaglandins, and propranolol (10−6 M) to block β-adrenergic receptors. The arteries were first constricted with increasing doses of NE (10−8 to 10−6 M). The arteries were then washed, and when their tone returned to baseline, constricted with 118 mM of potassium chloride. Constriction responses were recorded as gram force and were normalized for wet arterial ring weight (expressed as grams of force/grams weight). Four to six rings were studied from each animal and the mean contraction from these rings was used for analysis. In some lambs, two additional arterial rings were used for relaxation studies: these vessels were constricted with an EC50 concentration of NE and relaxed with increasing concentrations of either A23187 (10−8 to 10−6 M) or SNAP (10−8 to 10−5 M). The vessels used in the SNAP relaxation studies were pretreated with LNA to block endogenous nitric oxide synthesis. These experiments were done in a dark room as LNA is sensitive to light.
All data are expressed as mean ± SEM, with n representing the number of animals studied. Statistical comparisons of the curves were performed with factorial or repeated measures ANOVA as appropriate. Student-Newman-Keuls posthoc testing was used as needed to compare multiple groups. All statistical analysis was performed with StatView software (Abacus Concepts, Berkley, CA). Significance was accepted at p < 0.05.
RESULTS
Oxygen exposure.
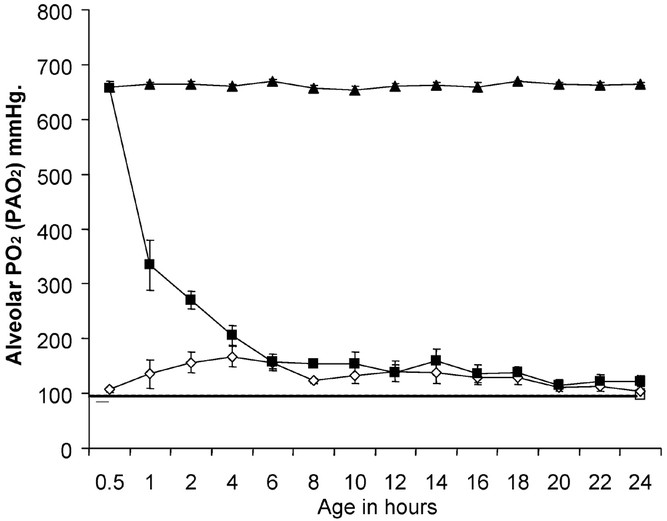
The Fio2 trend the in different groups of lambs is shown in Figure 1. Using the alveolar gas equation, the Po2 in the alveoli (PAo2) was calculated and is shown in Figure 2. The PAo2 in RA Spont group was estimated based on arterial blood gases obtained from two lambs before sacrifice. By design, the lambs in the 100%Res group had significantly higher Pao2 than the 21%Res group during the first 30 min of life. The Fio2 was gradually weaned over the next 3–4 h to approximately 0.25–0.30. The lambs in the 21%Res group had relatively normal Pao2 of 104–156 mm Hg throughout the study.
Figure 2
Calculated Po2 in the alveolar gas in the four groups of lambs depicted as mean (± SEM) over age in hours. In spontaneously breathing lambs (□, n = 2), one arterial gas was obtained before sacrifice and these results were extrapolated back over the 24-h period (dashed line). The 100%24h group (▴, n = 5) was exposed to significantly more oxygen than the 21% Res lambs (⋄, n = 6) throughout the 24-h period. The 100%Res group (▪, n = 6) was exposed to more oxygen than the 21%Res lambs for the first 4 h of life.
Arterial oxygenation.
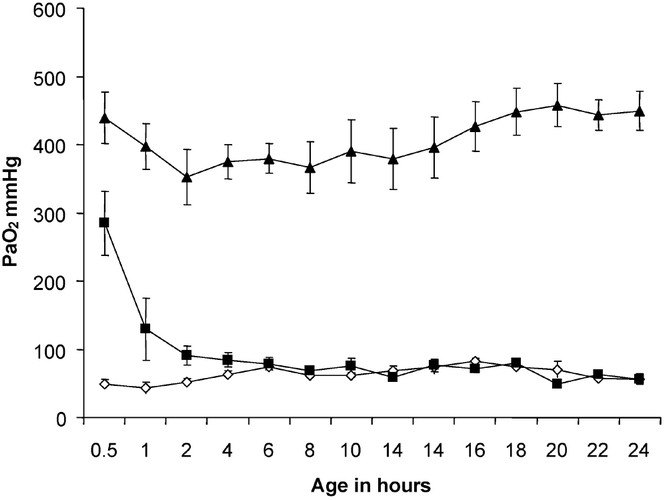
During the first 30 min, arterial oxygenation was significantly different between the groups (Fig. 3). The mean (± SEM) PAo2 during the first 30 min was 49 ± 7, 285 ± 47, and 449 ± 38 mm Hg in the 21%Res, 100%Res, and 100%24h groups, respectively. At the end of the 24-h period, the mean (± SEM) PAo2 was 56 ± 7, 56 ± 8, and 444 ± 29 mm Hg in 21%Res, 100%Res, and 100%24h groups, respectively. The mean Pao2 value measured in the two spontaneously breathing lambs before sacrifice (RA Spont group) was 55 mm Hg.
Figure 3
Arterial oxygenation (mean Pao2 ± SEM) changes over time in three groups of lambs. The 100%24h group (▴, n = 5) had significantly higher Pao2 levels than both the resuscitation groups throughout the period of study. The 100%Res group (▪, n = 6) had significantly higher Pao2 than the 21%Res group (⋄, n = 6) for the first 4 h of life.
Isolated vessel data.
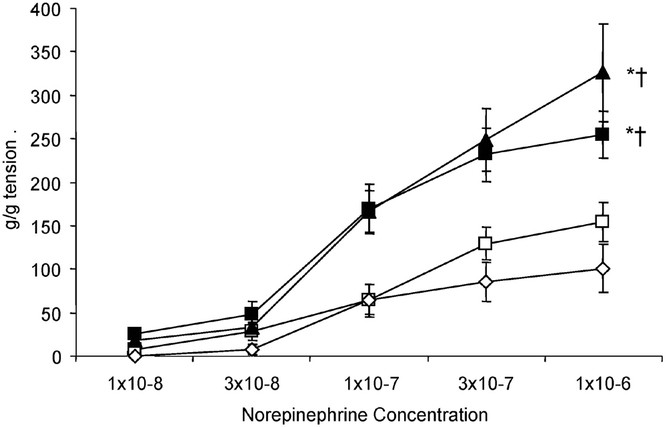
The wet pulmonary arterial ring weights were similar between the four groups. Cumulative NE concentration-response curves are shown in Figure 4. Pulmonary arteries isolated from 100%Res and 100%24h lambs showed significantly greater contraction to NE than 21%Res and RA Spont lambs.
Figure 4
Cumulative dose response curves to norepinephrine in pulmonary arteries isolated from 24-h-old lambs. Exposure to oxygen either during the first 30 min of life (100%Res: ▪, n = 5) or for 24 h (100%24h: ▴, n = 5) resulted in significantly increased contraction response compared with lambs resuscitated in room air (21%Res: ⋄, n = 5). Lambs spontaneously breathing room air (□, n = 6) did not significantly differ from the 21%Res group (*p < 0.05 compared with 21%Res, † p < 0.05 compared with RA Spont, by ANOVA repeated measures).
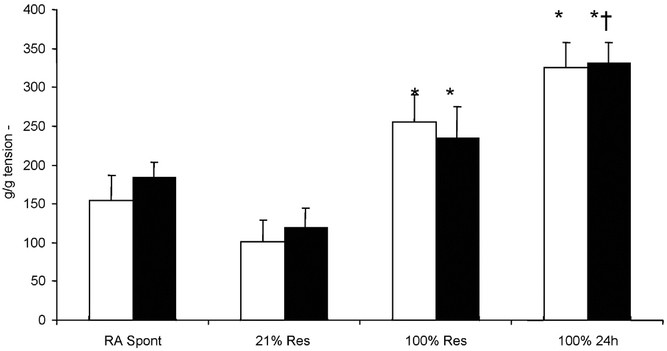
The pulmonary arterial contractions to 10−6 M of NE and 118 mM of potassium chloride from all the four groups of lambs are compared in Figure 5. Pulmonary arteries isolated from spontaneously room air breathing lambs (RA Spont) contracted similarly to 21%Res lambs in response to both NE and potassium chloride. Pulmonary arteries isolated from 100%Res lambs contracted significantly more than the 21%Res group. Pulmonary arteries isolated from 100%24h lambs contracted to a significantly higher degree to potassium chloride than the other three groups.
Figure 5
Contractions to 10−6 M of norepinephrine (▪) and 118 mM of potassium chloride (□) of isolated pulmonary arterial rings measured as grams of tension per gram arterial ring weight. (*p < 0.05 compared with 21%Res pulmonary arteries; † p < 0.05 compared with 100%Res pulmonary arteries by ANOVA Student-Newman-Keuls posthoc test). n = 6 lambs in RA Spont group; n = 5 each in 21%Res, 100%Res, and 100%24h groups).
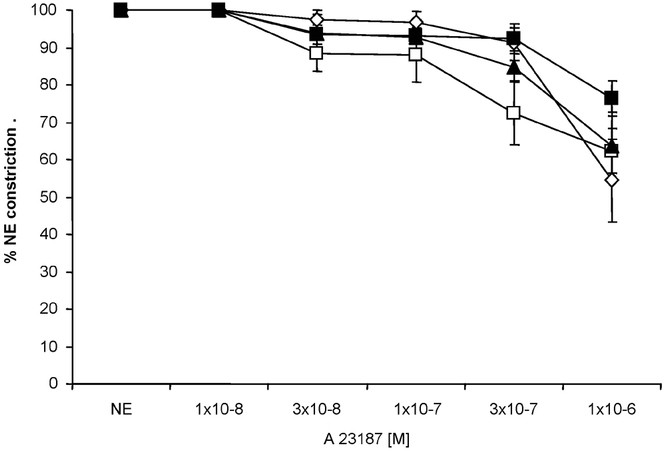
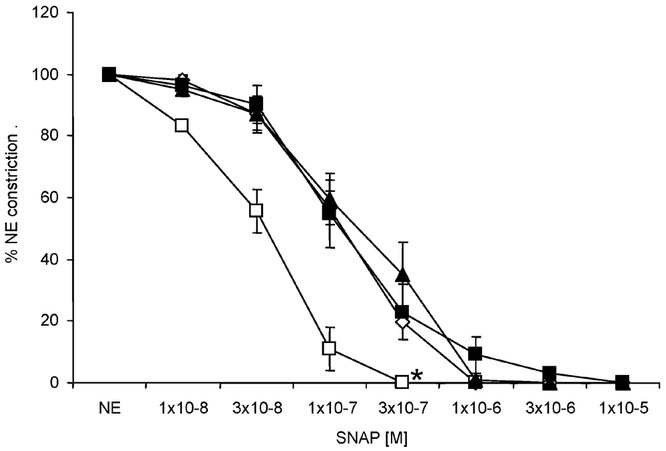
Relaxation responses to the calcium ionophore, A23187 and nitric oxide donor, SNAP were studied in the isolated pulmonary arteries. We and others have previously shown that A23187 stimulates endothelial nitric oxide synthase at low concentrations and relaxes pulmonary arteries (11,12). At 10−8 to 10−6 M concentrations, A23187 relaxed pulmonary arteries isolated from all groups in a similar manner (Fig. 6). Relaxation responses to SNAP were similar between the three ventilated groups (Fig. 7). In contrast, pulmonary arteries isolated from the RA Spont group relaxed significantly better to SNAP.
Figure 6
Cumulative concentration response curves to calcium ionophore and endothelial nitric oxide synthase agonist A23187 in pulmonary arteries isolated from 24-h-old lambs. Relaxation responses were similar in all four groups of lambs. RA Spont: □, n = 3; 21%Res: ⋄, n = 5; 100%Res: ▪, n = 4; 100%24h: ▴, n = 4; p > 0.05 by ANOVA repeated measures.
Figure 7
Cumulative concentration response curves to nitric oxide donor, SNAP in pulmonary arteries isolated from 24-h-old lambs. Pulmonary arteries isolated from 21%Res (⋄, n = 3), 100%Res (▪, n = 4), and 100%24h (▴, n = 4) groups of lambs relaxed in a similar fashion to SNAP. In contrast, pulmonary arteries isolated from RA Spont (□, n = 3) group relaxed significantly better to SNAP. (*p < 0.05 by ANOVA repeated measures compared with 100%Res).
DISCUSSION
We have demonstrated that even a brief exposure to 100% oxygen is adequate to significantly increase the contraction of fifth generation pulmonary arteries to NE and potassium chloride 24 h later. Our group and others have studied vascular reactivity of pulmonary arteries isolated from fetal, neonatal, juvenile, and adult animals extensively (7–9,13). However, to our knowledge, no data are available on changes in pulmonary vascular reactivity after conventional gas ventilation. By design, lambs in the 100%Res group were exposed to higher oxygen during the first few hours of life (Figs. 1 and 2). For the subsequent 19–20 h of the 24-h ventilation period, 100%Res and 21%Res groups were exposed to similar amounts of oxygen. As expected, the alveolar and arterial oxygen levels were similar in both groups during this later period (Figs. 2 and 3).
Considering spontaneous delivery and breathing room air as the gold standard, baseline NE and potassium chloride constriction data were obtained in pulmonary arteries isolated from 1-d-old lambs (Fig. 5). Pulmonary arteries obtained from lambs ventilated with 100% oxygen for 24 h (100%24h) generated significantly more tension with NE and potassium chloride. Even a brief 30-min exposure to 100% oxygen in the 100%Res group resulted in pulmonary arterial contractility that was greater than 21% Res lambs but less than 100%24h lambs. Because the 21%Res group did not differ significantly from spontaneously room air breathing lambs (RA Spont group), we suggest that oxygen exposure and not ventilation was the factor responsible for increasing pulmonary arterial contractility.
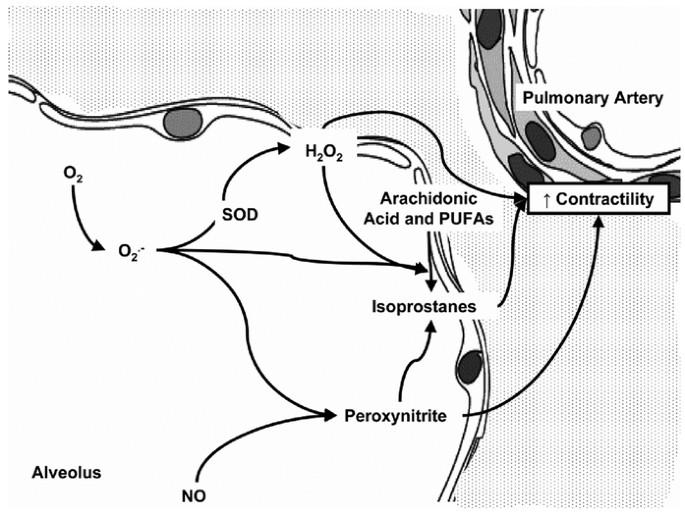
We speculate that the increased contractile response in lambs exposed to 100% oxygen is secondary to the formation of reactive oxygen species in the pulmonary arteries. Hyperoxic exposure can result in increased superoxide anion formation in the airway (Fig. 8). Excess superoxide may react directly with nitric oxide, disrupting its physiologic signaling. However, we did not observe a difference in responses to exogenous NO (delivered as SNAP) in the 21%Res versus 100% groups, indicating this direct interaction was not a likely explanation. In the presence of superoxide dismutase, hydrogen peroxide may be formed. Hydrogen peroxide is relatively stable and diffusible compared with many other reactive oxygen species, and has been reported to elicit pulmonary arterial constriction depending on the concentration and length of exposure (14).
Figure 8
Hypothesized role of reactive oxygen species and their metabolites in mediating increased pulmonary arterial contractility following exposure to 100% oxygen. Exposure to high oxygen concentrations results in formation of superoxide anions (O2·−). Superoxide anions combine with nitric oxide (NO) to form peroxynitrite, a potent pulmonary vasoconstrictor. Superoxide dismutase (SOD) enzyme converts superoxide anions to hydrogen peroxide (H2O2), also a pulmonary vasoconstrictor. When membrane lipids (arachidonic acid and poly unsaturated fatty acids, PUFA) are exposed to reactive oxygen species such as superoxide anions and hydrogen peroxide or peroxynitrite, a variety of isoprostanes are formed. Isoprostanes are known constrictors of pulmonary arteries.
Other possible agents mediating vasoconstriction include peroxynitrite and isoprostanes (Fig. 8). Superoxide anions combine with endogenous nitric oxide with high affinity to form toxic peroxynitrite (15). Peroxynitrite has been shown to be a potent vasoconstrictor of newborn but not adult pulmonary arteries from rats (16), possibly mediated by formation of isoprostanes (17). Elevated tracheal aspirate isoprostane levels have been reported in babies ventilated with high levels of oxygen compared with those ventilated with room air (18). Isoprostanes generated more contraction force in newborn rats exposed to oxygen (19).
Preliminary data from our laboratory indicate that one dose of intratracheal recombinant human superoxide dismutase (rhSOD) administered to lambs at birth partly prevents the increased pulmonary arterial contractile response to NE (10−6 M) seen following 24 h of ventilation with 100% oxygen (267 ± 26.3 g/g constriction with rhSOD + 100% 24 h compared with 326.6 ± 33.4 g/g in 100% 24 h group) (20). Dismutation of superoxide anions by intratracheal superoxide dismutase may decrease reactive oxygen species and/or prevent peroxynitrite formation and increase bioavailability of endogenous nitric oxide resulting in decreased pulmonary arterial contraction.
Endothelium dependent relaxations to A23187 were similar in pulmonary arteries isolated from all the groups (Fig. 6) contrary to our hypothesis. Therefore, we conclude that changes in endothelial nitric oxide synthase activity are unlikely to contribute to increased contractions noted in pulmonary arteries isolated from 100%Res lambs. Relaxations to the endothelium independent agent, SNAP were impaired in 21%Res, 100%Res, and 100%24h group pulmonary arteries compared with the RA Spont group (Fig. 7). Similar impairment in sodium nitroprusside–mediated relaxations has been observed following liquid ventilation in preterm lambs (13). These findings may indicate that mechanical ventilation, and not oxygen exposure, induces changes in smooth muscle reactivity. The increased gestational age of the RA Spont group of lambs at birth (145–147 d versus 139 d in ventilated groups) and the effect of spontaneous labor and vaginal delivery may also play a role in altered vasoreactivity.
What is the significance of these observations? Pulmonary arterial Po2 in fetal lambs is approximately 20 mm Hg (21). With room air ventilation at birth, Pao2 increases by 7- to 8-fold to 140–150 mm Hg and is adequate to mediate the significant decrease in pulmonary vascular resistance at birth. Ventilation with 100% oxygen results in Pao2 levels >600 mm Hg (>30-fold increase) and may induce oxidative damage. Human trials have shown that brief exposure to oxygen during resuscitation resulted in prolonged biochemical changes lasting 28 d (22). After resuscitation with 100% oxygen, the pulmonary arterial smooth muscle may be subject to oxidative injury resulting in higher contractile response to an additional vasoconstrictor stimulus. We are currently conducting in vivo studies to further explore this issue.
We acknowledge several drawbacks in this study. We designed the study to compare two groups of lambs that differed with respect to initial oxygen exposure for 30 min only. Ventilating lambs for 30 min in room air or oxygen is not “resuscitation” in the strict sense of this word in day-to-day pediatric practice. However, this study design gave us a controlled environment for first 24 h of life and is similar to other animal experiments studying oxygen resuscitation (6).
Many laboratories (including ours) that study isolated vessels in conventional tissue baths use a 94% oxygen and 6% CO2 gas mixture (8,23,24). The Po2 of the buffer solution bathing the tissue is close to 500 mm Hg and Pco2 is approximately 40 mm Hg. This approach was based on the assumption that the inner core of smooth muscle cells in the vessel may be relatively hypoxic because of lack of perfusion through the vasa vasorum. However, we considered the possibility that a high-buffer Po2 might affect our results. We found that using 94% room air and 6% CO2 still resulted in Po2 of 125 mm Hg in the vessel bath, higher than the arterial Po2 and considerably above the Po2 in the pulmonary arteries in vivo. Further, bubbling the tissue bath with 94% nitrogen and 6% CO2 resulted in a Po2 of 60–65 mm Hg, probably from equilibration with room air. Preliminary experiments in our isolated vessel systems using 94% nitrogen and 6% CO2 gas mixture did not reveal significant differences in contraction to NE or potassium chloride or relaxations to A23187 and SNAP. In fact, pulmonary arterial segments from two animals from each ventilated group were studied in baths bubbled with 94% nitrogen and 6% CO2. The mean g/g constriction response to 10−6 M concentration of NE was 139, 232, and 290 g/g from 21%Res, 100%Res, and 100%24h pulmonary arteries, respectively, in 94% nitrogen baths (similar to 101, 255, and 326 g/g, respectively, noted in 94% oxygen and 6% CO2 baths).
We conclude that even brief exposure to 100% oxygen at birth results in increased contractile responses of isolated pulmonary arteries to vasoconstrictor agents. Further experiments need to be conducted to understand the mechanisms for, and potential significance of, this important finding.
Abbreviations
LNA:
Nω nitro-l-arginine
NE:
norepinephrine
PAo2:
alveolar partial pressure of oxygen
PPHN:
persistent pulmonary hypertension of newborn
SNAP:
nitric oxide donor S-nitrosyl amino penicillamine
References
- Morin FC 3rd, Egan EA 1992 Pulmonary hemodynamics in fetal lambs during development at normal and increased oxygen tension. J Appl Physiol 73: 213–218
Article PubMed Google Scholar - Abman SH, Chatfield BA, Hall SL, McMurtry IF 1990 Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol 259: H1921–H1927
CAS PubMed Google Scholar - Tiktinsky MH, Morin FC 3rd 1993 Increasing oxygen tension dilates fetal pulmonary circulation via endothelium-derived relaxing factor. Am J Physiol 265: H376–H380
CAS PubMed Google Scholar - Saugstad OD 2004 The role of oxygen in neonatal resuscitation. Clin Perinatol 31: 431–443
Article PubMed Google Scholar - Fugelseth D, Borke WB, Lenes K, Saugstad OD 2004 Room air is as efficient as 100% oxygen in reversing hypoxemia-induced cardiovascular effects in newborn pigs. Pediatr Res 54: 618A
Google Scholar - Medbo S, Yu XQ, Asberg A, Saugstad OD 1998 Pulmonary hemodynamics and plasma endothelin-1 during hypoxemia and reoxygenation with room air or 100% oxygen in a piglet model. Pediatr Res 44: 843–849
Article CAS PubMed Google Scholar - Dunn JA, Lorch V, Sinha SN 1989 Responses of small intrapulmonary arteries to vasoactive compounds in the fetal and neonatal lamb: norepinephrine, epinephrine, serotonin, and potassium chloride. Pediatr Res 25: 360–363
Article CAS PubMed Google Scholar - Schindler MB, Hislop AA, Haworth SG 2004 Postnatal changes in response to norepinephrine in the normal and pulmonary hypertensive lung. Am J Respir Crit Care Med 170: 641–646
Article PubMed Google Scholar - Belik J 1995 The myogenic response of arterial vessels is increased in fetal pulmonary hypertension. Pediatr Res 37: 196–201
Article CAS PubMed Google Scholar - Steinhorn RH, Morin FC 3rd, Gugino SF, Giese EC, Russell JA 1993 Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol 264: H2162–H2167
CAS PubMed Google Scholar - Gao Y, Zhou H, Raj JU 1995 Endothelium-derived nitric oxide plays a larger role in pulmonary veins than in arteries of newborn lambs. Circ Res 76: 559–565
Article CAS PubMed Google Scholar - Steinhorn RH, Russell JA, Morin FC 3rd 1995 Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol 268: H1483–H1489
CAS PubMed Google Scholar - Villamor E, Degraeuwe PL, De Mey JG, Blanco CE 2003 Vascular reactivity of pulmonary arteries from premature lambs subjected to liquid ventilation. Biol Neonate 84: 172–178
Article CAS PubMed Google Scholar - Sheehan DW, Giese EC, Gugino SF, Russell JA 1993 Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol 264: H1542–H1547
Article CAS PubMed Google Scholar - Faraci FM, Didion SP 2004 Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24: 1367–1373
Article CAS PubMed Google Scholar - Belik J, Jankov RP, Pan J, Tanswell AK 2004 Peroxynitrite inhibits relaxation and induces pulmonary artery muscle contraction in the newborn rat. Free Radic Biol Med 37: 1384–1392
Article CAS PubMed Google Scholar - Janssen LJ, Catalli A, Helli P 2005 The pulmonary biology of isoprostanes. Antioxid Redox Signal 7: 244–255
Article CAS PubMed Google Scholar - Goil S, Truog WE, Barnes C, Norberg M, Rezaiekhaligh M, Thibeault D 1998 Eight-epi-PGF2alpha: a possible marker of lipid peroxidation in term infants with severe pulmonary disease. J Pediatr 132: 349–351
Article CAS PubMed Google Scholar - Belik J, Jankov RP, Pan J, Yi M, Chaudhry I, Tanswell AK 2004 Chronic O2 exposure in the newborn rat results in decreased pulmonary arterial nitric oxide release and altered smooth muscle response to isoprostane. J Appl Physiol 96: 725–730
Article CAS PubMed Google Scholar - Lakshminrusimha S, Gugino SF, Mathew B, Nielsen LC, Goldsby T, Morin FC 3rd, Davis J, Russell JA, Steinhorn RH 2005 Intratracheal recombinant human superoxide dismutase (IT-rhSOD) prevents increased pulmonary arterial contractility following ventilation with 100% O2 in neonatal control and persistent pulmonary hypertension (PPHN) lambs. Pediatr Acad Soc 57: 2536
Google Scholar - Morin FC 3rd, Egan EA, Norfleet WT 1988 Indomethacin does not diminish the pulmonary vascular response of the fetus to increased oxygen tension. Pediatr Res 24: 696–700
Article CAS PubMed Google Scholar - Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J 2001 Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 107: 642–647
Article CAS PubMed Google Scholar - Villamor E, Kessels CG, Fischer MA, Bast A, de Mey JG, Blanco CE 2003 Role of superoxide anion on basal and stimulated nitric oxide activity in neonatal piglet pulmonary vessels. Pediatr Res 54: 372–381
Article CAS PubMed Google Scholar - Steinhorn RH, Morin FC 3rd, Gugino SF, Giese EC, Russell JA 1993 Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol 264: H2162–H2167
CAS PubMed Google Scholar
Acknowledgements
The authors thank Karen Wynn, NNP, Huamei Wang, M.D., Nasir Rashid, M.D., Margaret Brick, Jan Capell, RN, and Sharon Baumgartner for their expert technical assistance during ventilation studies.
Author information
Authors and Affiliations
- Department of Pediatrics, University at Buffalo, Buffalo, 14222, New York
Satyan Lakshminrusimha, James A Russell, Rita M Ryan, Sylvia F Gugino, Frederick C Morin, Daniel D Swartz & Vasanth H Kumar - Department of Physiology and Biophysics, University at Buffalo, Buffalo, 14222, New York
James A Russell - Department of Pediatrics, Northwestern University, Chicago, 60614, Illinois
Robin H Steinhorn
Authors
- Satyan Lakshminrusimha
You can also search for this author inPubMed Google Scholar - James A Russell
You can also search for this author inPubMed Google Scholar - Robin H Steinhorn
You can also search for this author inPubMed Google Scholar - Rita M Ryan
You can also search for this author inPubMed Google Scholar - Sylvia F Gugino
You can also search for this author inPubMed Google Scholar - Frederick C Morin
You can also search for this author inPubMed Google Scholar - Daniel D Swartz
You can also search for this author inPubMed Google Scholar - Vasanth H Kumar
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toSatyan Lakshminrusimha.
Additional information
Funded by American Academy of Pediatrics/Neonatal Resuscitation Program Grant # 1040244 (V.H.K.) and HL#54705 (R.H.S.).
Rights and permissions
About this article
Cite this article
Lakshminrusimha, S., Russell, J., Steinhorn, R. et al. Pulmonary Arterial Contractility in Neonatal Lambs Increases with 100% Oxygen Resuscitation.Pediatr Res 59, 137–141 (2006). https://doi.org/10.1203/01.pdr.0000191136.69142.8c
- Received: 25 April 2005
- Accepted: 15 June 2005
- Issue Date: 01 January 2006
- DOI: https://doi.org/10.1203/01.pdr.0000191136.69142.8c