Selective Losses of Brainstem Catecholamine Neurons After Hypoxia-Ischemia in the Immature Rat Pup (original) (raw)
Main
Prematurity and hypoxia-ischemia (HI) in newborn infants are critical risk factors contributing to perinatal mortality and neurologic morbidity. Perinatal HI in the preterm neonate can lead to long-term brain injury and a broad range of neurologic deficits that include motor disabilities, autonomic dysfunction, epilepsy, memory, and attention disorders (1,2). Premature neonates are particularly susceptible to white matter injury in the brain. Neuronal injury in the brain also ensues and damage to certain areas of the brain may be associated with neurologic impairments in adults or children who have experienced HI as a neonate. However, very little is known about how perinatal HI affects neurochemically-defined groups of neurons in the immature preterm brain. It is therefore difficult to ascertain which specific brain nuclei and neuronal networks might be responsible for, or at least contribute to, the long-term functional deficits observed and how different neurons vary in their susceptibility to HI injury.
Injury to the brainstem after HI may be a critical contributor to autonomic and cardiorespiratory dysfunction in premature infants and neonatal animals (3–5). The brainstem consists of well-defined neurochemical groups of neurons, in particular the catecholamine neurons, serving as critical integratory sites in the brain. Distinct populations of brainstem catecholamine neurons reside in the locus coeruleus (A6 noradrenergic neurons), nucleus tractus solitarius (NTS) (A2 noradrenergic and C2 adrenergic neurons), and ventrolateral medulla (VLM) (A1 noradrenergic and C1 adrenergic neurons). These neuronal populations constitute the major sources of noradrenergic and adrenergic networks in the brain. The NTS and VLM catecholamine neurons, located in the medulla oblongata, have major roles in cardiovascular and respiratory control mechanisms, gastrointestinal, vasomotor tone, and neuroendocrine regulation (6,7). The locus coeruleus A6 noradrenergic neurons in the pontine tegmentum have significant roles in central processing of attention, arousal, learning, cognitive behaviors, and have also been implicated in respiratory control (8,9). Thus, the brainstem catecholamine neurons represent key candidates that might contribute to numerous HI-induced functional deficits.
Brainstem injury occurs in the dorsal medulla and pontine structures in neonates with HI encephalopathy (4,10–13). However, the effects of perinatal HI on specific neurochemical populations in the brainstem, such as the locus coeruleus, NTS and VLM catecholamine neurons, have not been investigated. In the adult rat, the noradrenaline concentration in the locus coeruleus and ipsilateral cortex is reduced after middle cerebral artery occlusion (14,15). Although the brainstem catecholamine populations are the major sources of noradrenaline in the brain, it is not known if these findings reflect losses of brainstem noradrenergic neurons. Necrotic and apoptotic markers have been demonstrated in the brainstem of postnatal day (P)-7 (P7) rat pups subjected to HI (16) but others, using prenatal hypoxia models, have found conflicting effects (17–19). The different findings may reflect the type of hypoxia and/or ischemia model, species differences, the region of the brainstem investigated, or the developmental stage at which the perturbation was applied.
In the present study, we hypothesized that HI in the immature preterm brain can affect brainstem catecholamine neurons. We examined whether HI in P3 rat pups can alter numbers of brainstem noradrenergic and adrenergic neurons in the locus coeruleus, NTS, and VLM 18 d after HI. We also investigated whether there are changes in forebrain catecholamine fibers after P3 HI.
MATERIALS AND METHODS
Subjects.
Experiments were carried out on Wistar rats. Dams and their pups were housed under standard laboratory conditions in temperature-controlled rooms (22°C) maintained on a 12-h light/dark cycle (lights on 6.00 am). Food and water were available ad libitum. To minimize possible influences of circadian rhythms, experiments were completed between 8.00 am and 11.00 am. All the procedures were conducted in accord with ethical approvals and experiment guidelines stipulated by the University of Auckland Animal Ethics Committee. All efforts were made to minimize the number of animals used and any suffering.
HI insult.
On P3, pups of mixed sexes were randomly assigned to a control (n = 10) or a HI group (n = 11). Pups subjected to HI were anesthetized using halothane administered via tubing placed over the pup's snout. The right common carotid artery was isolated from surrounding tissue, cauterized, and cut. Pups were then allowed to recover for 30 min at 37°C in 85% ± 5% humidity then subjected to 30 min of 6% O2 at 37°C in the humidified chamber. After hypoxia, pups were returned to the dam. The control group did not undergo surgery but were placed in the chamber and breathed room air for 30 min before being returned to the dam. This is an established preterm HI model in the immature rat pup that produces typical behavioral and pathologic features seen in human premature neonates who have experienced a HI event (20–24). The P3 brain development stage is analogous to the preterm human neonate brain at approximately 24–28 wk gestation in terms of number of synapses, neurochemical development, and cortical organization (22,25).
Immunohistochemistry.
On P21, animals were killed by pentobarbitone overdose (80 mg/kg, i.p.) and perfused transcardially with 4% formaldehyde (in 0.1 M PBS, pH 7.4). The brain was removed and postfixed in formaldehyde. The brainstem was cryoprotected overnight in 10% sucrose (in 0.1 M PBS, pH 7.4, 4°C) after which serial 50 μm sections were collected using a sliding microtome. The forebrain was subjected to an increasing percentage of ethanol then embedded in paraffin. Consecutive 6 μm forebrain sections were collected at 100 μm intervals onto Menzel Superplus adhesive slides and dried. Sections were then dewaxed using xylene and rehydrated in preparation for immunohistochemistry.
For brainstem immunohistochemistry, two 1-in-5 series of brainstem sections were processed for cytoplasmic antigen detection of the catecholamine synthesis enzymes tyrosine hydroxylase (TH) (1:15,000; Diasorin, Stillwater) and phenyl-_N_-methyl transferase (PNMT) (1:30,000; Diasorin, Stillwater). For forebrain immunohistochemistry, two series of sections were immunolabeled; one for myelin basic protein (MBP) (1:10,000; Chemicon International, CA) and one for the catecholamine marker dopamine-beta-hydroxylase (DβH) (1:5,000; Chemicon International, CA). Each series was incubated for 36 h in the respective primary antibody followed by 2 h incubation in secondary antibody biotinylated antimouse (TH, MBP, and DβH; 1:400, Jackson ImmunoResearch, PA) or biotinylated antirabbit (PNMT; 1:400, Jackson ImmunoResearch, PA). Sections were then immersed in an avidin-biotin-horseradish peroxidase complex solution (Vector Elite Kit) for 2 h. Horseradish peroxidase activity was visualized with diaminobenzidine. To minimize possible variations in immunocytochemistry, sections from the control and experiment groups were processed simultaneously. Sections were mounted on chrome-alum slides, dehydrated in a series of alcohols, cleared in xylene, and coverslipped.
Data analysis.
In the medulla oblongata, numbers of TH- and PNMT-positive cells were counted at 250 μm intervals over 17 sections from 2.5 mm caudal to 1.5 mm rostral to obex along the VLM and NTS cell columns. The VLM and NTS contain partially overlapped populations of noradrenergic cells (the A1 and A2 cells groups) situated in caudal aspects and adrenergic cells (the C1 and C2 cell groups) situated in rostral aspects. Because PNMT only occurs in adrenergic cells, counts of PNMT-immunolabeled cells were VLM C1 or NTS C2 adrenergic cells. The number of noradrenergic A1 or A2 cells was estimated by subtracting the number of PNMT cells in one section from the number of TH cells in the adjacent section (26). For the pontine locus coeruleus, numbers of TH-positive cells were counted in two sections.
Forebrain sections were immunolabeled to visualize MBP- and DβH-immunoreactivity. The density of MBP- or DβH-immunolabeling was determined in four consecutive sections using phase analysis software (analysis Life Science Research) and the percentage change ipsilateral to the carotid ligation relative to the left hemisphere was calculated. To determine the effect of P3 HI on cerebral hemisphere size, the outlines of the left and right cerebral hemispheres from four consecutive sections (100 μm intervals) were traced using the software program analysis Life Science Research. In the brainstem, area was determined in the same way in sections from −0.5 to 0.25 mm. The percentage change ipsilateral to ligation relative to the contralateral side was calculated. The effects of P3 on HI were determined by comparing numbers in control and HI animals using an analysis of variance followed by t test post hoc tests. Data are expressed as mean ± SEM. Statistical significance was set at p < 0.05.
RESULTS
Effects of P3 HI on brain area and myelin.
In animals subjected to HI, there was an 18.0% reduction in cerebral hemisphere size ipsilateral to the ligation compared with the nonligated side, however, there was no change in brainstem area size ipsilateral to the ligation (−0.003% ± 0.005%). We observed a 17.2% reduction in MBP-positive immunolabeling ipsilateral to the carotid ligation compared with the nonligated side. Compared with control animals, there was no significant difference in the cerebral hemisphere size, brainstem area, or MBP-immunolabeling on the nonligated side. These findings extend and confirm that the P3 HI insult produced outcomes comparable with previous studies (20,21).
Effects of HI on NTS catecholamine neurons.
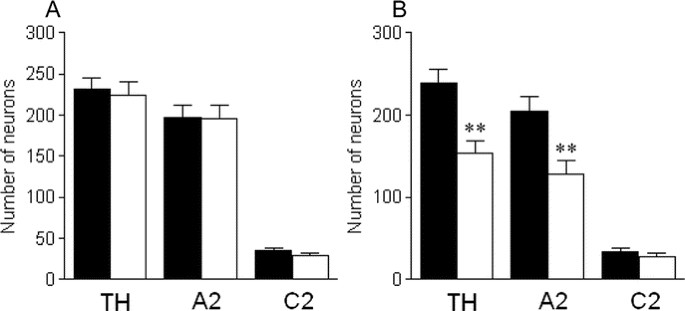
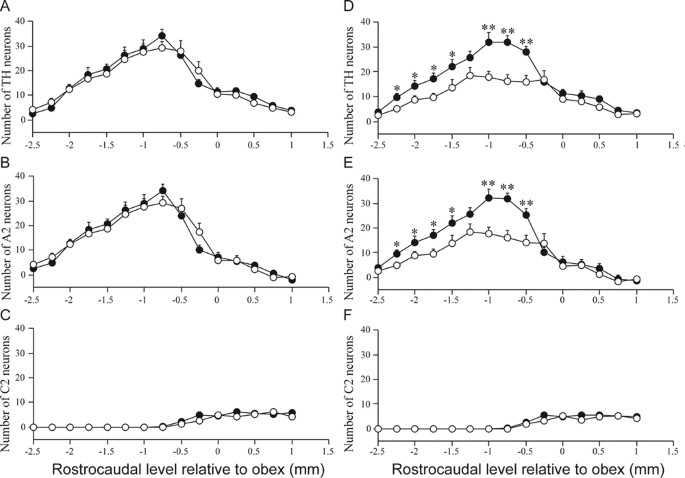
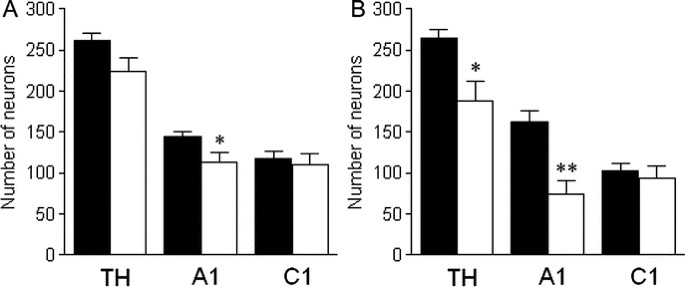
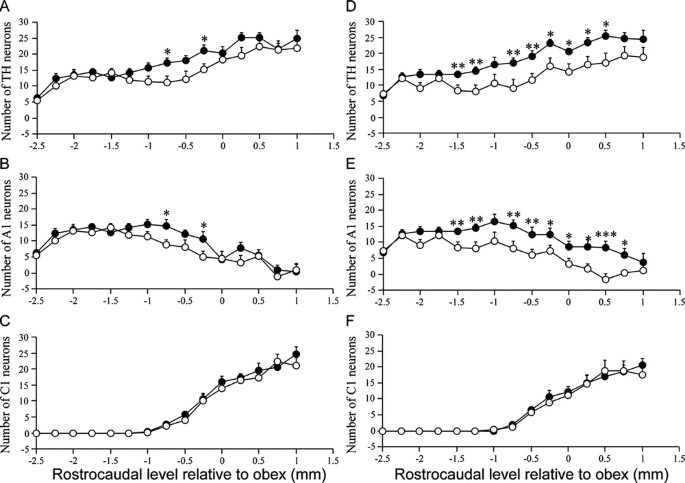
In animals subjected to P3 HI, there was a significant reduction in the total number of NTS TH-positive neurons compared with the total NTS TH counts in control animals. After P3 HI, there was a significant 35.7% reduction in numbers of NTS catecholamine neurons and a significant 35.3% NTS A2 noradrenergic neurons ipsilateral to the carotid ligation compared with counts in control animals on the corresponding right side (Figs. 1 and 2). The majority were lost from −2.25 to −0.5 mm relative to obex (Fig. 3). In contrast there was no effect of P3 HI on total NTS C2 adrenergic neuron counts ipsilateral to the ligation (Fig. 2) and there was no difference in C2 adrenergic counts from control and P3 HI along the entire rostrocaudal extent of the NTS (Fig. 3). For all NTS catecholamine counts, there were no significant differences between control and P3 HI counts on the nonligated side.
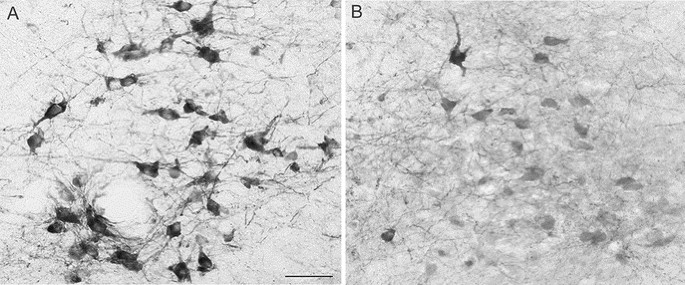
Figure 1
Cross sections through the NTS immunolabeled for TH (A and B) in a rat subjected to P3 HI on the nonligated (A) and ligated side (B). Scale bar = 50 μm.
Figure 2
Counts of NTS catecholamine neurons, A2 noradrenergic neurons and C2 adrenergic neurons on the nonligated (A) and ligated side (B) in control (▪) and P3 HI animals (□). **p < 0.01.
Figure 3
Rostrocaudal distributions of NTS catecholamine neurons (A, D), A2 noradenergic neurons (B, E) and C2 adrenergic neurons (C, F) on the nonligated (A_–_C) and ligated side (D_–_F) in control (•) and P3 HI animals (○). *p < 0.05; **p < 0.01.
Effects of HI on VLM catecholamine neurons.
In animals subjected to P3 HI, there was significant reduction in the total number of VLM TH-positive neurons compared with control animals. After P3 HI, there was a significant, 57.1%, reduction in numbers of VLM catecholamine neurons ipsilateral to the ligation compared with counts in control animals on the corresponding right side (Figs. 4 and 5). There was also a reduction in the number of TH-positive neurons on the nonligated side. Compared with control animals there was a significant reduction in the number of A1 noradrenergic neurons on both the ligated (58.6% reduction) as well as the nonligated side (35.0% reduction) in the VLM of P3 HI animals (Figs. 4 and 5). On the nonligated side, VLM A1 noradrenergic neurons were predominantly lost between −1.0 and −0.75 mm relative to obex (Fig. 6B). Ipsilateral to the ligation, VLM A1 noradrenergic neurons were predominantly lost from −1.5 to 0.5 mm relative to obex (Fig. 6E). In contrast, there was no effect of P3 HI on numbers of C1 adrenergic neurons compared with control counts on either side of the VLM (Figs. 5 and 6).
Figure 4
Cross sections through the VLM immunolabeled for TH (A, B) in a rat subjected to P3 HI on the nonligated (A) and ligated side (B). Scale bar = 50 μm.
Figure 5
Counts of VLM catecholamine neurons, A1 noradrenergic neurons and C1 adrenergic neurons on the nonligated (A) and ligated side (B) in control (▪) and P3 HI animals (□). *p < 0.05; **p < 0.01.
Figure 6
Rostrocaudal distributions of VLM catecholamine neurons (A, D), A1 noradenergic neurons (B, E) and C1 adrenergic neurons (C, F) on the nonligated (A_–_C) and ligated side (D_–_F) in control (•) and P3 HI animals (○). *p < 0.05; **p < 0.01; †p < 0.001.
Effects of HI on locus coeruleus catecholamine neurons.
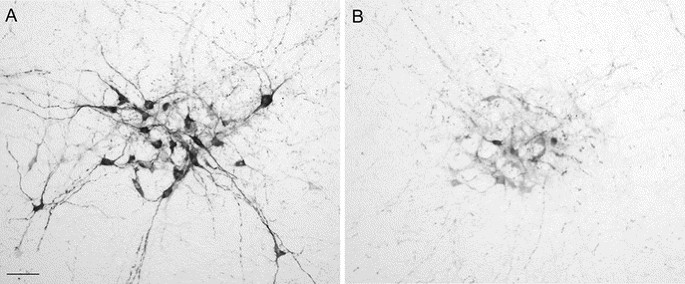
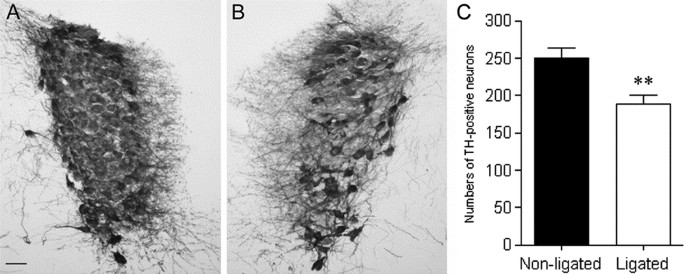
After P3 HI, TH neurons were lost in the locus coeruleus (Fig. 7). Ipsilateral to the ligation in P3 HI animals, we observed a significant 24% reduction in the total number of TH-positive neurons compared with the nonligated side. There was no significant difference between control and P3 HI TH-positive neuron counts on the nonligated side.
Figure 7
Cross sections through the locus coeruleus immunolabeled for TH on the nonligated (A) and ligated side (B) in a rat subjected to P3 HI. Counts of TH-positive neurons (C) on the nonligated (▪) and ligated side (□). **p < 0.05. Scale bar = 50 μm.
Effects of HI on DβH-positive immunolabeling in the forebrain.
Because the major effect of P3 HI was on noradrenergic neurons in the brainstem, we investigated whether forebrain DβH-positive immunolabeling was also affected by P3 HI. After P3 HI, there was a significant 27.5% loss of DβH-immunolabeling ipsilateral to the ligation compared with control animals on the corresponding right side. There was no difference in DβH-immunolabeling between control and P3 HI groups on the nonligated side.
DISCUSSION
An outstanding finding of the present study was that damage occurs in the brainstem after P3 HI. This is commensurate with reports in human neonates describing neuronal losses in the brainstem after fetal asphyxia, HI, or acute perinatal asphyxia (4,10–13). We present novel evidence that brainstem neurons are lost and that numbers of catecholamine neurons in the locus coeruleus, NTS, and VLM are decreased 18 d after P3 HI. We found that only noradrenergic, but not adrenergic, neurons were affected by P3 HI. In addition, we report an associated reduction in forebrain DβH-immunolabeling. Together these findings suggest that P3 HI disrupts catecholaminergic networks in the immature rat brain and that specific neurochemically-defined populations in the brainstem, the noradrenergic neurons, are lost after P3 HI.
Effects of P3 HI on NTS catecholamine neurons.
We observed a significant loss of noradrenergic neurons in the NTS ipsilateral to the ligation 18 d after P3 HI but no change in NTS C2 adrenergic neuron counts, suggesting that the C2 adrenergic population is less vulnerable to HI-induced injury than NTS A2 noradrenergic neurons. Consistent with our findings, it has been reported that neurons localized in cranial nerve nuclei, including the NTS, are severely damaged in premature infants with HI encephalopathy (10,13). The selective loss of A2 noradrenergic neurons could influence a number of functions including cardiovascular, respiratory, and autonomic regulatory mechanisms and thus may provide a neuroanatomical basis for certain deficits observed in preterm infants who have previously experienced a HI episode.
VLM catecholamine neurons.
The VLM exhibited the greatest reduction in numbers of A1 noradrenergic neurons after P3 HI. In the VLM, effects were predominantly observed ipsilateral to the ligation; however, the nonligated side also showed a significant reduction in numbers of VLM A1 noradrenergic neurons. There was no effect of P3 HI on VLM C1 adrenergic neuron counts suggesting that adrenergic populations are less vulnerable to HI-induced injury than noradrenergic neurons, as seen in the NTS.
The VLM has major roles in the regulation of the cardiovascular system, vasomotor tone, neuroendocrine control, and sympathetic outflow. The VLM A1 noradrenergic neurons in particular are critical in the control of neuroendocrine systems involving the hypothalamus (26–28) and disruption of neural inputs from the A1 neurons could have considerable effects on oxytocin, vasopressin, and ACTH secretion. Disruption of hypothalamic-pituitary-adrenal (HPA) axis function occurs after HI injury (29–31) and A1 and A2 neurons contribute to HPA axis responses (26,32). Thus, the observed losses of noradrenergic neurons might contribute to such neuroendocrine imbalances after a perinatal insult.
Locus coeruleus noradrenergic neurons.
In the locus, coeruleus neuronal losses were observed ipsilateral to the ligation. Noradrenaline concentration is reduced in the adult locus coeruleus after middle cerebral artery occlusion (14) and thus a loss of locus coeruleus neurons, as seen in the present study, may contribute to such an effect.
The locus coeruleus provides the major source of noradrenergic inputs to the brain innervating widespread brain areas including the cortex, hippocampus, thalamus, and hypothalamus and has a significant role in the central processing of alertness, learning, adaptive behaviors, arousal, and sleep (8,9). Many of these functions are disrupted after perinatal HI injury and thus loss of locus coeruleus neurons may contribute to such deficits.
Forebrain DβH immunolabeling.
After P3 HI, we observed a significant reduction in DβH-immunolabeling in the forebrain ipsilateral to the ligation suggesting that losses of noradrenergic and adrenergic labeling occurred. The reduction in DβH-immunolabeling was greater than the cerebral hemisphere area change and thus it is unlikely that this effect was solely a reflection of hemisphere size. However, given the selective loss of brainstem noradrenergic neurons it is conceivable that the reduction in DβH-immunolabeling reflects the loss of noradrenergic and not adrenergic fiber networks in the brain. This is consistent with findings in the adult rat that cerebral ischemia produces a 30–40% reduction in noradrenaline concentration in the ipsilateral cortex (15) and that prenatal hypoxia significantly alters DβH-immunolabeling in the guinea pig forebrain (33). Interestingly, after prenatal exposure to hypoxia, brain regions that exhibit changes in in vivo TH activity at P21 are noradrenergic areas of the brainstem (17) and regions innervated by noradrenergic fibers such as the motor cortex area and hippocampus (18); further emphasizing the vulnerability of the noradrenergic system to perinatal insults.
Putative mechanisms affecting noradrenergic neuron survival.
The mechanisms by which locus coeruleus, NTS, and VLM noradrenergic neurons are affected by P3 HI are not known. The brainstem itself is not believed to endure ischemia in models of HI that involve occlusion of the middle cerebral artery or the common carotid artery, particularly because the brainstem lies outside the vascular fields of these vessels. Instead blood flow to the brainstem increases during HI (34). In addition, unlike the forebrain, there was no change in brainstem area after P3 HI. Thus, the brainstem can be considered remote from primary HI injury sites in the forebrain and brainstem injury may evolve via secondary injury mechanisms. In this context, several postulated mechanisms may be responsible for the observed noradrenergic neuronal losses in the brainstem after P3 HI. Although our results indicate that there was not a global loss of neurons in the brainstem, it is also conceivable that other noncatecholamine populations of neurons could also be affected by P3 HI.
Factors that may have contributed to the degeneration of neural connections include excitotoxic mechanisms affecting axonal and cell body survival, neuroinflammation, loss of synaptic inputs, and target deprivation (35,36). The reduction in DβH-immunolabeling may be directly attributed to the degeneration of noradrenergic neurons in the brainstem. Alternatively, retrograde degeneration of noradrenergic projections may also have led to the loss of noradrenergic cell bodies in the locus coeruleus, NTS, and VLM. A further possibility is that higher brain centers that sustain significant neuronal injury after HI, and with descending inputs to the brainstem noradrenergic neurons, might be responsible for the observed neuronal losses through loss of synaptic input and stimulation of brainstem neurons. For example, the cerebral cortex has direct projections to the NTS and the VLM (37) and in the case of the VLM there are bilateral neural pathways (38). The bilateral loss of VLM neurons may also result from NTS neuron losses because bilateral connections exist between the NTS and VLM (39,40) including direct, excitatory NTS inputs to VLM A1 noradrenergic cells (40). The precise sequence of events and potential brain regions involved in the loss of brainstem neurons are not known and future studies are required to at least clarify the temporal relationships of the observed changes.
The caudal distribution of NTS and VLM noradrenergic neurons may dictate their vulnerability to HI-induced injury. Based on functional divisioning in the NTS, caudally distributed A2 cells are influenced primarily by peripheral chemosensory inputs (41). After transient focal ischemia in the adult rat Nnos expression in the caudal VLM increases but decreases in the rostral VLM (42). Using nuclear Fos as a marker of neuronal activation, hypoxia primarily activates A2 noradrenergic neurons (43) and middle cerebral artery occlusion in adult rats induces Fos in TH-positive neurons of the NTS and VLM; the majority of Fos-positive TH neurons apparent in the VLM (44,45). These distribution patterns are similar to the selective injury patterns in the present study. The significance of these putative associations may be relevant to the long-term synaptic and metabolic status of the A1 and A2 neurons caused by secondary injury mechanisms after the P3 HI insult. Future studies may elucidate whether there are regional and/or inherent differences in cellular or molecular mechanisms pertaining to noradrenergic neurons compared with adrenergic neurons.
Concluding remarks.
We provide evidence that brainstem catecholamine neurons in the locus coeruleus, NTS, and VLM are lost because of perinatal HI and that there are associated changes in forebrain DβH-immunolabeling. This study pinpoints changes in brainstem noradrenergic neuron populations suggesting that noradrenergic networks in the brain are particularly susceptible to injury after P3 HI. Autonomic, cardiorespiratory, movement, and attention disorders may occur in children previously exposed to perinatal HI and these functions involve brainstem catecholamine neurons. The locus coeruleus, NTS, and VLM noradrenergic neurons provide extensive neural inputs to many forebrain structures fulfilling their important integratory roles in the CNS and thus disruptions to noradrenergic neurocircuitry could potentially contribute to HI-induced deficits.
Abbreviations
ACTH:
adrenocorticotropin hormone
DβH:
dopamine-beta-hydroxylase
HI:
hypoxia-ischemia
HPA:
hypothalamic-pituitary-adrenal
MBP:
myelin basic protein
NTS:
nucleus tractus solitarius
P:
postnatal day
PNMT:
phenyl-_N_-methyl transferase
TH:
tyrosine hydroxylase
VLM:
ventrolateral medulla
References
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR 2003 Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111: 939–948
Article PubMed Google Scholar - Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor HG, Flannery D, Klein N, Borawski E 2004 Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics 114: 932–940
Article PubMed Google Scholar - Chen RV, Perlman JM 2006 Sudden cardiac arrest in an intubated premature infant with cerebellar and brainstem injury: is there a link?. Pediatrics 117: 1814–1817
Article PubMed Google Scholar - Tu YF, Chen CY, Lin YJ, Chang YC, Huang CC 2005 Neonatal neurological disorders involving the brainstem: neurosonographic approaches through the squamous suture and the foramen magnum. Eur Radiol 15: 1927–1933
Article PubMed Google Scholar - Antier D, Zhang BL, Poisson D, Pourcelot L, Sannajust F 1998 Influence of neonatal focal cerebral hypoxia-ischemia on cardiovascular and neurobehavioral functions in adult Wistar rats. Neurosci Lett 250: 57–60
Article CAS PubMed Google Scholar - Dampney RA 1994 Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364
Article CAS PubMed Google Scholar - Saper CB 1995 Central autonomic system. In: Paxinos G (ed) The Rat Nervous System. Academic Press, San Diego, CA, pp 107–135
Google Scholar - Aston-Jones G, Rajkowski J, Cohen J 1999 Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry 46: 1309–1320
Article CAS PubMed Google Scholar - Berridge CW, Waterhouse BD 2003 The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84
Article PubMed Google Scholar - Sugama S, Eto Y 2003 Brainstem lesions in children with perinatal brain injury. Pediatr Neurol 28: 212–215
Article PubMed Google Scholar - Pasternak JF, Gorey MT 1998 The syndrome of acute near-total intrauterine asphyxia in the term infant. Pediatr Neurol 18: 391–398
Article CAS PubMed Google Scholar - Leech RW, Brumback RA 1988 Massive brain stem necrosis in the human neonate: presentation of three cases with review of the literature. J Child Neurol 3: 258–262
Article CAS PubMed Google Scholar - Dambska M, Laure-Kamionowska M, Liebhart M 1987 Brainstem lesions in the course of chronic fetal asphyxia. Clin Neuropathol 6: 110–115
CAS PubMed Google Scholar - Robinson RG, Shoemaker WJ, Schlumpf M 1980 Time course of changes in catecholamines following right hemispheric cerebral infarction in the rat. Brain Res 181: 202–208
Article CAS PubMed Google Scholar - Robinson RG, Shoemaker WJ, Schlumpf M, Valk T, Bloom FE 1975 Effect of experimental cerebral infarction in rat brain on catecholamines and behavior. Nature 255: 332–334
Article CAS PubMed Google Scholar - Peng JH, Feng Y, LeBlanc MH, Rhodes PG, Parker JC Jr 2005 Apoptosis and necrosis in developing cerebellum and brainstem induced after focal cerebral hypoxic-ischemic injury. Brain Res Dev Brain Res 156: 87–92
Article CAS PubMed Google Scholar - Peyronnet J, Roux JC, Geloen A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y 2000 Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol 524: 525–537
Article CAS PubMed PubMed Central Google Scholar - Perrin D, Mamet J, Scarna H, Roux JC, Bérod A, Dalmaz J 2004 Long-term prenatal hypoxia alters maturation of brain catecholaminergic systems and motor behavior in rats. Synapse 54: 92–101
Article CAS PubMed Google Scholar - Tolcos M, Harding R, Loeliger M, Breen S, Cock M, Duncan J, Rees S 2003 The fetal brainstem is relatively spared from injury following intrauterine hypoxemia. Brain Res Dev Brain Res 143: 73–81
Article CAS PubMed Google Scholar - Sizonenko SV, Sirimanne E, Mayall Y, Gluckman PD, Inder TE, Williams CE 2003 Selective cortical alteration after hypoxic-ischemic injury in the very immature rat brain. Pediatr Res 54: 263–269
Article PubMed Google Scholar - Sizonenko SV, Kiss JZ, Inder TE, Gluckman PD, Williams CE 2005 Distinctive neuropathologic alterations in the deep layers of the parietal cortex after moderate ischemic-hypoxic injury in the P3 immature rat brain. Pediatr Res 57: 865–872
Article CAS PubMed Google Scholar - Stadlin A, James A, Fiscus R, Wong YF, Rogers M, Haines C 2003 Development of a postnatal 3-day-old rat model of mild hypoxic-ischemic brain injury. Brain Res 993: 101–110
Article CAS PubMed Google Scholar - Perlman JM 1998 White matter injury in the preterm infant: an important determination of abnormal neurodevelopment outcome. Early Hum Dev 53: 99–120
Article CAS PubMed Google Scholar - Vannucci RC 1990 Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res 27: 317–326
Article CAS PubMed Google Scholar - Hagberg H, Peebles D, Mallard C 2002 Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev 8: 30–38
Article PubMed Google Scholar - Buller K, Xu Y, Dayas C, Day T 2001 Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1beta-induced hypothalamic pituitary adrenal axis responses. Neuroendocrinology 73: 129–138
Article CAS PubMed Google Scholar - Buller KM, Smith DW, Day TA 1999 Differential recruitment of hypothalamic neuroendocrine and ventrolateral medulla catecholamine cells by non-hypotensive and hypotensive hemorrhages. Brain Res 834: 42–54
Article CAS PubMed Google Scholar - Cunningham ET Jr, Bohn MC, Sawchenko PE 1990 Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 292: 651–667
Article PubMed Google Scholar - Salchner P, Engidawork E, Hoeger H, Lubec B, Singewald N 2003 Perinatal asphyxia exerts lifelong effects on neuronal responsiveness to stress in specific brain regions in the rat. J Investig Med 51: 288–292
Article PubMed Google Scholar - Krugers HJ, Knollema S, Kemper RH, Ter Horst GJ, Korf J 1995 Down-regulation of the hypothalamo-pituitary-adrenal axis reduces brain damage and number of seizures following hypoxia/ischaemia in rats. Brain Res 690: 41–47
Article CAS PubMed Google Scholar - Olsson T, Marklund N, Gustafson Y, Näsman B 1992 Abnormalities at different levels of the hypothalamic-pituitary-adrenocortical axis early after stroke. Stroke 23: 1573–1576
Article CAS PubMed Google Scholar - Dayas CV, Buller KM, Day TA 2001 Medullary neurons regulate hypothalamic corticotrophin releasing factor cell responses to an emotional stressor. Neuroscience 105: 707–719
Article CAS PubMed Google Scholar - Bernert G, Hoeger H, Mosgoeller W, Stolzlechner D, Lubec B 2003 Neurodegeneration, neuronal loss, and neurotransmitter changes in the adult guinea pig with perinatal asphyxia. Pediatr Res 54: 523–528
Article PubMed Google Scholar - Vannucci RC, Lyons DT, Vasta F 1988 Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke 19: 245–250
Article CAS PubMed Google Scholar - Block F, Dihne M, Loos M 2005 Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol 75: 342–365
Article CAS PubMed Google Scholar - Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C 1998 Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull 46: 281–309
Article CAS PubMed Google Scholar - Sequeira H, Viltart O, Ba-M'Hamed S, Poulain P 2000 Cortical control of somato-cardiovascular integration: neuroanatomical studies. Brain Res Bull 53: 87–93
Article CAS PubMed Google Scholar - Yasui Y, Breder CD, Saper CB, Cechetto DF 1991 Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303: 355–374
Article CAS PubMed Google Scholar - Buller KM, Dayas CV, Day TA 2003 Descending pathways from the paraventricular nucleus contribute to the recruitment of brainstem nuclei following a systemic immune challenge. Neuroscience 118: 189–203
Article CAS PubMed Google Scholar - Chan RK, Peto CA, Sawchenko PE 1995 A1 catecholamine cell group: fine structure and synaptic input from the nucleus of the solitary tract. J Comp Neurol 351: 62–80
Article CAS PubMed Google Scholar - Ciriello J 1983 Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci Lett 36: 37–42
Article CAS PubMed Google Scholar - Ally A, Nauli SM, Maher TJ 2005 Molecular changes in nNOS protein expression within the ventrolateral medulla following transient focal ischemia affect cardiovascular functions. Brain Res 1055: 73–82
Article CAS PubMed Google Scholar - Buller KM, Smith DW, Day TA 1999 NTS catecholamine cell recruitment by hemorrhage and hypoxia. Neuroreport 10: 3853–3856
Article CAS PubMed Google Scholar - Wessel TC, Joh TH, Volpe BT 1991 In situ hybridization analysis of c-fos and c-jun expression in the rat brain following transient forebrain ischemia. Brain Res 567: 231–240
Article CAS PubMed Google Scholar - Wu YP, Ling EA 1998 Induction of Fos-like immunoreactivity in the hypothalamic, medullary and thoracic spinal cord neurons following middle cerebral artery occlusion in rats. Neurosci Res 30: 145–153
Article CAS PubMed Google Scholar
Acknowledgements
The authors thank Clay Winterford, Queensland Institute for Medical Research for assistance with histology.
Author information
Authors and Affiliations
- Perinatal Research Centre, University of Queensland, Queensland, 4029, Australia
Kathryn M Buller, Julie A Wixey, Michelle Carty & Paul B Colditz - Liggins Institute and the National Research Centre for Growth and Development, University of Auckland, Auckland, New Zealand
Praneeti Pathipati & Christopher E Williams - Hortresearch, Auckland, New Zealand
Arjan Scheepens
Authors
- Kathryn M Buller
You can also search for this author inPubMed Google Scholar - Julie A Wixey
You can also search for this author inPubMed Google Scholar - Praneeti Pathipati
You can also search for this author inPubMed Google Scholar - Michelle Carty
You can also search for this author inPubMed Google Scholar - Paul B Colditz
You can also search for this author inPubMed Google Scholar - Christopher E Williams
You can also search for this author inPubMed Google Scholar - Arjan Scheepens
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toKathryn M Buller.
Additional information
This work is supported by a Royal Brisbane Women's Hospital Foundation Strategic Initiative Grant (KMB).
Rights and permissions
About this article
Cite this article
Buller, K., Wixey, J., Pathipati, P. et al. Selective Losses of Brainstem Catecholamine Neurons After Hypoxia-Ischemia in the Immature Rat Pup.Pediatr Res 63, 364–369 (2008). https://doi.org/10.1203/PDR.0b013e3181659774
- Received: 29 August 2007
- Accepted: 18 November 2007
- Issue Date: April 2008
- DOI: https://doi.org/10.1203/PDR.0b013e3181659774