Alpha-Lactalbumin in Human Milk Alters the Proteolytic Degradation of Soluble CD14 by Forming a Complex (original) (raw)
Main
As the immunological analysis of human milk enters its sixth decade (1), considerable evidence supports a role for human milk, with its estimated 100,000 components, in regulating the early phase of gut colonization by microorganisms and in the development of the infant's innate immunity (2). It has been demonstrated that breast-fed newborns experience a lower incidence of gastrointestinal (GI) infections including inflammatory, respiratory, and allergic disease in comparison with formula-fed infants (3–6). Protection by milk has been attributed to maternal immunocompetent cells, immunoglobulins, immune reactive peptides, anti-infectious oligosaccharides, growth factors, lactoferrin, and complement components (7).
The bacterial pattern recognition receptor, cluster of differentiation 14 (CD14), plays a pivotal role in the immune recognition and reactivity to microbial cell wall components from Gram-negative and Gram-positive bacteria (8,9). Two soluble forms of CD14, 48 kD and 52 kD, are found in normal human plasma at a concentration of 2–3 μg/mL (9,10). Soluble CD14 (sCD14) binds to whole bacteria and bacterial cell wall components and mediates bacterial-induced proinflammatory cytokine production from cells that typically do not express membrane-bound CD14 (11). The biology of CD14 in the breast, in milk, and in the gut is emerging (7). The average concentration of sCD14 in human milk is 25–50 μg/mL, and consumption of ∼1 liter of milk per day by breast feeding infants suggests the GI tract is exposed to milligram quantities of this potent immunostimulatory protein. CD14 protein concentrations in bovine milk are less than that in human milk, only 5–11 μg/mL, but increase in response to bacterially induced mastitis (12,13). Recombinant CD14 also reduces intramammary infection by Escherichia coli (E. coli) in cows (14,15), suggesting that CD14 in bovine milk plays an important immunological role during mastitis. Conversely, mice transgenically altered to express concentrations of CD14 in milk similar to that in humans, 31–316 μg/mL, show no change in susceptibility to E. coli infection but show a considerable decrease in infection-related edema (16). This suggests sCD14 may reduce the inflammatory response to pathogenic bacteria.
The importance of sCD14 in the health and nutrition of breast-feeding infants including its role in the early colonization of microbes is poorly understood. We tested whether sCD14 forms a complex with other proteins present in human milk, and how this might influence CD14 proteolysis by GI tract proteases.
METHODS
Chemicals and reagents.
For immunoprecipitation, mouse anti-human CD14 IgG (Clone UCHM-1; Fisher Thermo Scientific, Ottawa, ON), goat anti-human alpha-lactalbumin IgG (Santa Cruz Biotechnology, Santa Cruz, CA), mouse and goat preimmune IgG (Sigma Chemical Co. Aldrich, St. Louis, MO), and Protein A agarose (Sigma Chemical Co. Aldrich) were purchased. For the proteolytic sensitivity assay, alpha-lactalbumin (Sigma Chemical Co. Aldrich), lysozyme (Sigma Chemical Co. Aldrich), and trypsin (Sigma Chemical Co. Aldrich) were also purchased. [14C]-Methyl iodide 250 μCi (10 mCi/mmol; Sigma Chemical Co. Aldrich) was used to label BSA (Sigma Chemical Co. Aldrich), and [14C]-methyl iodide 2 mCi (52.9 mCi/mmol; Perkin Elmer, Waltham, MA) was used to label recombinant human CD14 (rhCD14; R&D Systems, Minneapolis, MN). Other reagents purchased include hydrogen peroxide 30% (wt/vol) solution in water (Sigma Chemical Co. Aldrich), perchloric acid 70% (Sigma Chemical Co. Aldrich), Solvable (Perkin Elmer), and ScintiSafe Plus 50% LSC-Cocktail (Fisher Thermo Scientific).
14C-Labeling of proteins.
A protein solution (BSA or rhCD14 at 1 mg/mL and 0.01 mg/mL) was adjusted to pH 10.5 and lyophilized. In vacuo methylation was performed with 14C-methyl iodide (10 mCi/mmol) as previously described (17) with the following modifications. The in vacuo reaction was performed in a sealed glass test tube and incubated at 85°C for 48 h. Lyophilized and labeled proteins were recovered by resuspension in 500 μL of a 1% solution of NH4HCO3 and incubated at 37°C for 2 h to remove 14C-methyl esters formed with carboxyl groups on the protein. The sample was relyophilized to volatilize and remove 14C-methanol that may have formed during the reaction.
Animals.
The study was approved by the University of Ottawa's Animal Care Committee and performed in accordance with the University of Ottawa's Animal Care and Veterinary Services. Sprague Dawley rat pups (10 d old) weighing from 19 to 26 g were separated from their mother and housed in an incubator at 37°C, while being fasted for 2 h. The pups were returned to their mother and gavage fed a 250-μL bolus of either 25 μg/mL 14C-BSA (34,400 dpm/μg) or 14C-CD14 (163,000 dpm/μg). Animals were anesthetized at 20 min and 8 h postgavage, euthanized by cardiac puncture, and had organs harvested. Organs were stored at −70°C until needed. For determining the amount of degraded 14C-BSA or 14C-CD14 in the rat pups, stomach contents, normalized for volume, were resuspended in 500 μL of PBS, centrifuged, and the supernatant recovered. Supernatants were clarified using a 0.22-μm spin filter. After clarification, samples were applied to an Amicon (Thermo Scientific) centrifugal filter with a 30,000 molecular weight cutoff that captured degraded protein in the flow through and retained full-length protein in the retentate. Flow through and retentate were resuspended in 2-mL liquid scintillation cocktail and counted using a Tri-Carb liquid scintillation counter (Perkin Elmer).
Milk samples: Preparation and analysis.
This study was approved by the Research Ethics Board of the Children's Hospital of Eastern Ontario (Ottawa, Ontario) and the University of Ottawa Research Ethics Board. Human milk samples used in this study were collected from healthy nursing mothers and stored at −80°C. Before use, human milk samples were briefly centrifuged at 5000×g to separate milk fat and whole cells from milk plasma.
Immunoprecipitation of human milk.
Defatted human milk plasma was depleted of existing immunoglobulins with Protein A agarose beads (Sigma Chemical Co. Aldrich) as previously described (18). Immunodepleted milk plasma was incubated with either 10 μL (10 μg) of rabbit anti-mouse IgG for sCD14 immunoprecipitations or 10 μL (10 μg) of goat anti-mouse IgG for alpha-lactalbumin immunprecipitations and removed by treatment with Protein A agarose. Milk was then treated with either 30 μL (3 μg) of anti-sCD14 IgG or 10 μL (5 μg) of anti-alpha-lactalbumin IgG for 1 h at 4°C. After antibody incubation, 70 μL of Protein A agarose was added and incubated for an additional hour at 4°C. Protein A agarose beads were washed five times with 750 μL of PBS containing 0.1% Triton-×100 to remove any nonspecifically bound proteins. Proteins were eluted from agarose beads by treatment with 300-μL (pH 1.9) elution buffer with a formic acid:acetic acid:water ratio of 25:75:900 (vol/vol/v) for 1 h at 4°C. Samples were microcentrifuged to pellet agarose, and eluates were concentrated with an Amicon Ultra Filtration column with a molecular weight cutoff of 5 kD (Fisher Thermo Scientific). Columns were spun at 4200 rpm in a Beckman J-6B swinging bucket rotor for 25 min and repeated. After the second spin, filter membranes were washed with 500-μL distilled water, centrifuged, resuspended in 300 μL of neutralization buffer [100 mm Tris HCl pH 9.0], and centrifuged again. Samples were recovered in a final volume of 40 μL for SDS-PAGE and immunoblot analysis.
Mass spectrometry: Liquid chromatography-coupled mass spectrometry (LC-MS/MS).
Protein bands were excised from SDS PAGE gels and subjected to in-gel tryptic digestion as previously described (19,20). Tryptic digestions were performed for 8 h at 37°C with sequencing grade trypsin (Promega, Madison, WI). Peptides from each gel band were extracted, brought to a final volume of 10 μL with 5% formic acid, and analyzed by LC-MS/MS. Using an Agilent 1100 series HPLC system (Agilent Technologies, Palo Alto, CA), samples were loaded at 2 μL/min onto a 75 μm × 50 mm precolumn packed with 5 μm YMC ODS-A C18 beads (Waters, Milford, MA). After sample desalting, the flow was split, and peptides were eluted through a second 75 μm × 50 mm column packed with the same beads at ∼200 nL/min using a 5–80% gradient of acetonitrile with 0.1% formic acid for 1 h. The liquid chromatography effluent was electrosprayed into the sampling orifice of a LTQ linear ion trap mass spectrometer (ThermoFisher Scientific, Waltham, MA). MS/MS data were analyzed and matched to human protein sequences in the International Protein Index database using the Mascot search engine. Peptide and MS/MS mass tolerances were set at 2 Da and 0.8 Da, respectively (21).
Proteolysis assay.
Human milk CD14 was enriched by step gradient precipitation of milk plasma using ammonium sulfate (45–60%) (22). CD14 immunodot blots showed that CD14 was preferentially enriched at 60% ammonium sulfate concentration. After centrifugation the pellet was resolubilized in 10 mL of ddH2O overnight at 4°C. Buffer exchange and concentration were performed using an Amicon Filtron concentrator column to a final volume of 1.5 mL. SDS-PAGE separation of ammonium sulfate-enriched CD14 in combination with immunoblots and scanning densitometry (Sigma Plot, Systat Software, Inc. San Jose, CA) estimated a CD14 concentration of ∼450 ng/mL. For in vitro protease digestion assays, 10 μg of ammonium sulfate-precipitated CD14 in combination with either lysozyme or alpha-lactalbumin was digested at 37°C for either 60 min (trypsin) or 15, 30, 45, or 60 min (trypsin, chymotrypsin, and pancreatin multidigest).
RESULTS
Recombinant CD14 persists in the upper GI tract.
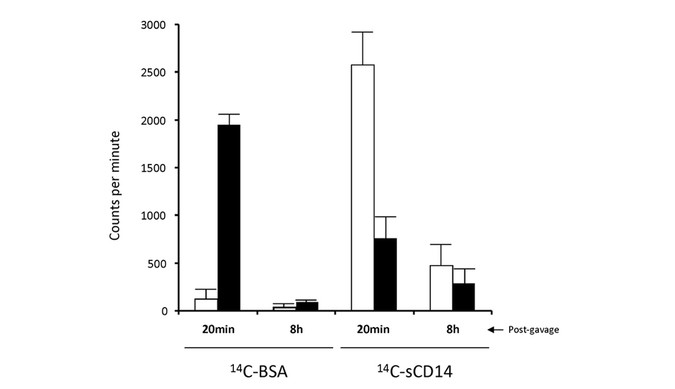
Ten-day-old rat pups were gavage fed a 25-μg/mL bolus of either 14C-labeled recombinant-CD14 or 14C-labeled BSA as a control. Molecular weight separation of intact and degraded radiolabeled proteins from stomach contents indicated that ∼90% of the BSA was not digested within the first 20 min after gavage feeding (Fig. 1) and was minimally detected in the stomach by 8 h postgavage. Conversely, ∼80% of sCD14 was degraded within 20 min of gavage feeding and ∼20% persisted in the stomach after 8 h. The findings suggest a pool of CD14 is sequestered in the stomach that does not undergo proteolytic degradation.
Figure 1
Fate of radio-labeled proteins in the neonate stomach. Ten-day-old Sprague Dawley rat pups (n = 12) were fed with a 250-μL bolus of 25 μg/mL 14C-CD14 or 14C-BSA. Animals were euthanized, and stomachs were collected at 20 min and 8 h postgavage. Stomach contents were separated using a 30,000 molecular weight cutoff membrane to fractionate intact (▪) proteins from 14C-labeled, degraded (□) proteins.
Alpha-lactalbumin and sCD14 form a complex in human milk.
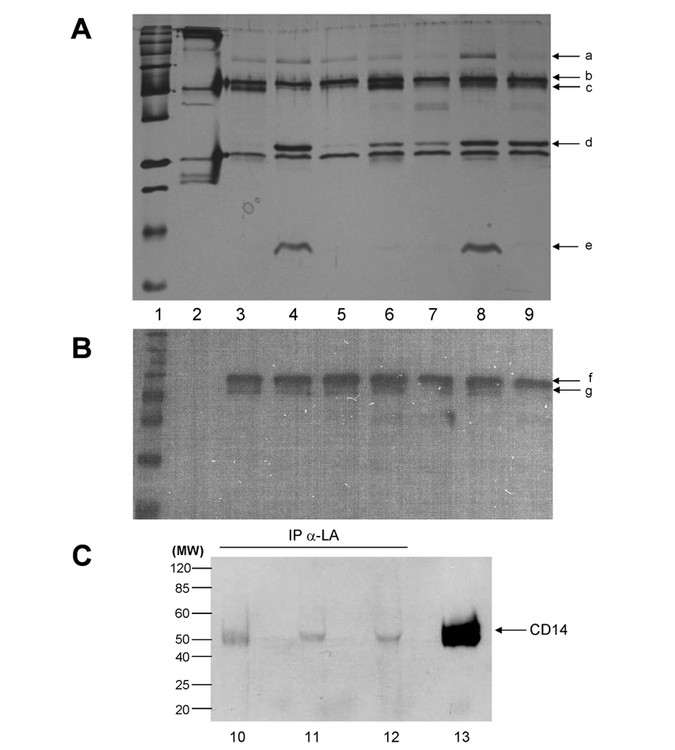
CD14 and associated proteins were immunoprecipitated from human milk samples from seven different mothers using a mouse monoclonal human-CD14 antiserum bound to a column. Of the seven samples, three novel bands on PAGE gels were identified at ∼80 kD, 27 kD, and 15 kD in comparison with the control IgG lane (Fig. 2A). The 80-kD band was similar in molecular mass to human lactoferrin (77 kD), an antimicrobial protein that has already been shown to interact with CD14 (23). We also identified a 27-kD protein that is under further investigation. Not all human milk samples tested were positive for these proteins, raising interesting questions about the heterogeneity in human milk and its putative impacts on infant nutrition and health.
Figure 2
Alpha-lactalbumin and CD14 form a complex in human milk. (A) Silver stain of immunoprecipitated CD14 proteins from seven human milk samples (Lanes 3–9) resolved by SDS PAGE. Lane 1: Molecular weight markers. Novel bands are marked by arrows a (∼75 kD), d (∼27 kD), and e (∼15 kD). Arrows b and c indicate the position of CD14 isomers. Lane 2 shows immunoprecipitation of human milk with a preimmune IgG antisera. (B) Western blot of CD14 immunoprecipitated human milk. Lanes are similar to (A) except that biotinylated molecular weight markers were used (Lane 1). CD14 isomers are highlighted by arrows f and g. (C) CD14 immunoblot of alpha-lactalbumin immunoprecipitated from three human milk samples (Lanes 10–12) and human milk as a positive control (Lane 13).
Mass spectrometric analysis of the 15-kD band identified alpha-lactalbumin, a major milk protein involved in lactose biosynthesis. To further characterize sCD14/alpha-lactalbumin complexes in milk, anti-alpha-lactalbumin IgG was used to reverse-immunoprecipitate sCD14 from human milk (Fig. 2C; Lanes 10–12). The sCD14 immunoblot analysis demonstrated that sCD14 was coimmunoprecipitated from human milk. Comparison of signal intensity of the immunoprecipitated sCD14 with the human milk control (Fig. 2C; Lane 13) suggests only a small pool of sCD14 is in the bound state. A unique characteristic of alpha-lactalbumin is that it forms a proteolytically resistant complex with lactose synthetase (24), suggesting that a complex of milk-derived sCD14 with alpha-lactalbumin may be equally resistant to proteolysis.
Alpha-lactalbumin alters the proteolytic degradation of human milk sCD14.
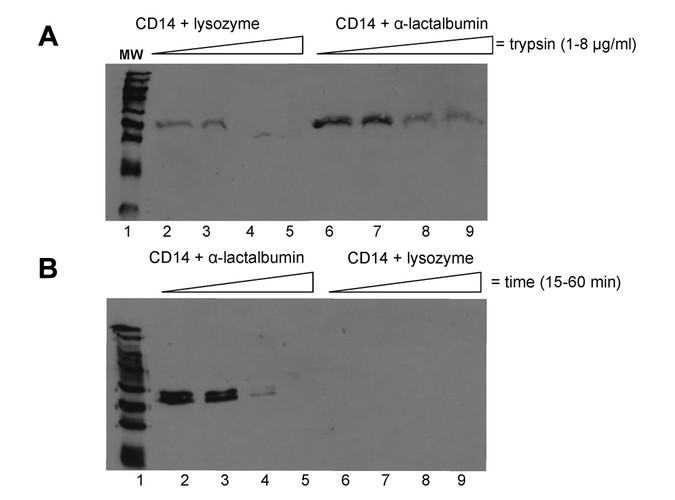
In vitro digests using trypsin alone or in combination with chymotrypsin and pancreatin were performed to investigate whether such a complex alters CD14 stability. The sCD14 from human milk is known to be proteolytically resistant to pepsin digestion and partially resistant to pancreatin digestion in vitro (25). The present results show that a higher concentration of trypsin is required to completely digest CD14 in the presence of alpha-lactalbumin than the control protein, lysozyme (Fig. 3A). Digestion of CD14 with a protease cocktail of trypsin, chymotrypsin, and pancreatin was also delayed in the presence of alpha-lactalbumin compared with lysozyme (Fig. 3B). The data demonstrate that CD14 is protected by alpha-lactalbumin and the consequence of this interaction in vivo may account for the persistence of CD14 in the upper GI tract, Fig. 1 (25).
Figure 3
Alpha-lactalbumin delays proteolysis of CD14 in vitro. (A) Western blot of in vitro trypsin digestion of breast milk CD14 performed at neutral pH for 1 h at 37°C with 0, 1, 2, 4, and 8 μg of trypsin in the presence of either alpha-lactalbumin (right) or lysozyme (left). (B) Western blot of in vitro digestion using trypsin, chymotrypsin, and pancreatin digest of breast milk CD14. Ten milligrams of CD14 was digested for 15, 30, 45, and 60 min in the presence of either alpha-lactalbumin (left) or lysozyme (right).
DISCUSSION
Paradoxically, both sCD14 and Gram-negative bacteria are found in human milk (7,26) and yet do not seem to trigger inflammation in the GI tract of breast-fed infants or in the mammary gland of the lactating mother. This suggests that the normal physiological response of sCD14 may be altered in these compartments.
Alpha-lactalbumin is a major protein found in human milk (20–25% of total protein) and has several physiological functions (27,28). Partial digestion of alpha-lactalbumin leads to the production of transient peptides having both antimicrobial and immunostimulatory properties to aid against infection (28). A novel folding variant, termed a molten globule state, of multimeric alpha-lactalbumin has been discovered, which has antimicrobial activity (29) and enhances apoptosis of tumors (30). The interaction of alpha-lactalbumin with sCD14 reported here extends the functionality of this dynamic milk protein. The persistence of recombinant CD14 in the neonate stomach (Fig. 1) suggests that sCD14 complexed with alpha-lactalbumin may permit sCD14 to migrate further along the GI tract than would be possible in the absence of this interaction. Therefore, sCD14 and alpha-lactalbumin occupy a specific temporal window during infant development, most notably after birth when the child begins feeding from the breast, and sCD14 levels are at their highest concentration in human milk (25,31,32). This suggests that early colonization of the gut and the presence of sCD14/alpha-lactalbumin complexes may be a coordinated process to ensure population by appropriate commensal microbes. The persistence of sCD14 in the upper GI tract suggests its biological role in limiting the colonization of microorganisms within this region as the upper GI tract houses seven orders of magnitude fewer bacteria than the colon (33).
Interestingly, sCD14/ alpha-lactalbumin did not occur consistently in all milk samples analyzed (Fig. 2). Milk samples used in this study were obtained from mothers who had been actively breast feeding from weeks to months postpartum, suggesting this protein complex may be temporally restricted, especially given that sCD14 is maximally expressed in colostrum and early milk (32). In human milk, sCD14 exists in a complex with at least three other proteins: a 15-kD protein, which is alpha-lactalbumin; a 77-kD protein, which is likely lactoferrin; and a yet uncharacterized 27-kD protein. Three of the four proteins are known to have antimicrobial activity. Recent work from Ohnishi et al. (34) suggests that His-tagged CD14 purified from Pichia pastoris seemed to inhibit E. coli growth. We have observed that human milk-derived sCD14 also reduces the viability of E. coli in vitro (unpublished observations). Together this demonstrates that sCD14 is part of a larger specialized complex whose combined antimicrobial activity would target a wide range of microorganisms in the upper GI tract. This so-called “gatekeeper complex” may serve two functions. The first is surveillance that may function to limit colonization of the upper GI tract. The second is planned obsolescence, whereby components of the complex, such as CD14, are degraded before reaching the colon thereby minimizing CD14-dependent inflammation. Alpha-lactalbumin also presents an interesting structural paradigm because of its ability to form nanotubes (35,36), which in the hydrolytic environment of the GI tract may function as a scaffold to shield sCD14 and other bioactive milk components from degradation.
Previous work from our laboratory has demonstrated that human milk-derived sCD14 is not detectable in the stool of newborn breast-fed infants and is adsorbed, sequestered, or degraded (25). In combination with the present results, sCD14 is still observed to be sensitive to proteolysis; however, the presence of a resistant pool in the stomach does not preclude the possibility of CD14 degradation further along the GI tract. Therefore, a more complete picture of the digestive fate of sCD14 in the GI tract of neonates including how this might influence gut colonization is warranted.
Abbreviations
CD14:
cluster of differentiation 14
sCD14:
soluble CD14
References
- Hanson LA 1959 Immunological analysis of human milk. Int Arch Allergy Appl Immunol 15: 245–256
Article CAS Google Scholar - Hanson LA, Silfverdal SA 2009 The mother's immune system is a balanced threat to the foetus, turning to protection of the neonate. Acta Paediatr 98: 221–228
Article Google Scholar - Bartick M, Reinhold A 2010 The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics 125: e1048–e1056
Article Google Scholar - César JA, Victora CG, Barros FC, Santos IS, Flores JA 1999 Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: nested case-control study. BMJ 318: 1316–1320
Article Google Scholar - Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD 1990 Protective effect of breast-feeding against infection. BMJ 300: 11–16
Article CAS Google Scholar - Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, Stanley FJ, Kendall GE, Burton PR 1999 Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ 319: 815–819
Article CAS Google Scholar - Vidal K, Donnet-Hughes A 2008 CD14: A soluble pattern recognition receptor in milk. Adv Exp Med Biol 606: 195–216
Article CAS Google Scholar - Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC 1990 CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding-protein. Science 249: 1431–1433
Article CAS Google Scholar - Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, Wright SD 1992 Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med 176: 1665–1671
Article CAS Google Scholar - Schütt C, Schilling T, Grunwald U, Schonfeld W, Kruger C 1992 Endotoxin-neutralizing capacity of soluble CD14. Res Immunol 143: 71–78
Article Google Scholar - Lødrup Carlsen KC, Granum B 2007 Soluble-CD14: Role in atopic disease and recurrent infections, including otitis media. Curr Allergy Asthma Rep 7: 436–443
Article Google Scholar - Wang Y, Zarlenga DS, Paape MJ, Dahl GE 2002 Recombinant bovine soluble CD14 sensitizes the mammary gland to lipopolysaccharide. Vet Immunol Immunopathol 86: 115–124
Article CAS Google Scholar - Lee JW, Paape MJ, Elsasser TH, Zhao X 2003 Elevated milk soluble CD14 in bovine mammary glands challenged with Escherichia coli lipopolysaccharide. J Dairy Sci 86: 2382–2389
Article CAS Google Scholar - Lee JW, Paape MJ, Elsasser TH, Zhao X 2003 Recombinant soluble CD14 reduces severity of intramammary infection by Escherichia coli. Infect Immun 71: 4034–4039
Article CAS Google Scholar - Lee JW, Paape MJ, Zhao X 2003 Recombinant bovine soluble CD14 reduces severity of experimental Escherichia coli mastitis in mice. Vet Res 34: 307–316
Article CAS Google Scholar - Wall R, Powell A, Sohn E, Foster-Frey J, Bannerman D, Paape M 2009 Enhanced host immune recognition of mastitis causing Escherichia coli in CD-14 transgenic mice. Anim Biotechnol 20: 1–14
Article CAS Google Scholar - Taralp A, Kaplan H 1997 Chemical modification of lyophilized proteins in nonaqueous environments. J Protein Chem 16: 183–193
Article CAS Google Scholar - Kline JB, Clevenger CV 2001 Identification and characterization of the prolactin-binding protein in human serum and milk. J Biol Chem 276: 24760–24766
Article CAS Google Scholar - Shevchenko A, Wilm M, Vorm O, Mann M 1996 Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal Chem 68: 850–858
Article CAS Google Scholar - Wall ML, Wheeler HL, Smith JC, Figeys D, Altosaar I 2010 Mass spectrometric analysis reveals remnants of host-pathogen molecular interactions at the starch granule surface in wheat endosperm. Phytopathology 100: 848–854
Article CAS Google Scholar - Perkins DN, Pappin DJ, Creasy DM, Cottrell JS 1999 Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567
Article CAS Google Scholar - Filipp D, Alizadeh-Khiavi K, Richardson C, Palma A, Paredes N, Takeuchi O, Akira S, Julius M 2001 Soluble CD14 enriched in colostrum and milk induces B cell growth and differentiation. Proc Natl Acad Sci U S A 98: 603–608
Article CAS Google Scholar - Baveye S, Elass E, Fernig DG, Blanquart C, Mazurier J, Legrand D 2000 Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect Immun 68: 6519–6525
Article CAS Google Scholar - Wehbi Z, Perez MD, Dalgalarrondo M, Sanchez L, Calvo M, Chobert JM, Haertle T 2006 Study of ethanol-induced conformational changes of holo and apo alpha-lactalbumin by spectroscopy and limited proteolysis. Mol Nutr Food Res 50: 34–43
Article CAS Google Scholar - Blais DR, Harrold J, Altosaar I 2006 Killing the messenger in the nick of time: persistence of breast milk sCD14 in the neonatal gastrointestinal tract. Pediatr Res 59: 371–376
Article Google Scholar - Martín R, Heilig HG, Zoetendal EG, Jiménez E, Fernández L, Smidt H, Rodríguez JM 2007 Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res Microbiol 158: 31–37
Article Google Scholar - Lönnerdal B, Forsum E, Gebremedhin M, Hambraeus L 1976 Breast-milk composition in Ethiopian and Swedish mothers. 2. Lactose, nitrogen, and protein contents. Am J Clin Nutr 29: 1134–1141
Article Google Scholar - Montagne P, Culliere ML, Mole C, Bene MC, Faure G 1999 Immunological and nutritional composition of human milk in relation to prematurity and mothers' parity during the first 2 weeks of lactation. J Pediatr Gastroenterol Nutr 29: 75–80
Article CAS Google Scholar - Håkansson A, Svensson M, Mossberg AK, Sabharwal H, Linse S, Lazou I, Lonnerdal B, Svanborg C 2000 A folding variant of alpha-lactalbumin with bactericidal activity against Streptococcus pneumoniae. Mol Microbiol 35: 589–600; Erratum in: Mol Microbiol 36:247
Article Google Scholar - Svensson M, Sabharwal H, Hakansson A, Mossberg AK, Lipniunas P, Leffler H, Svanborg C, Linse S 1999 Molecular characterization of alpha-lactalbumin folding variants that induce apoptosis in tumor cells. J Biol Chem 274: 6388–6396
Article CAS Google Scholar - Labéta MO, Vidal K, Nores JE, Arias M, Vita N, Morgan BP, Guillemot JC, Loyaux D, Ferrara P, Schmid D, Affolter M, Borysiewicz LK, Donnet-Hughes A, Schiffrin EJ 2000 Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J Exp Med 191: 1807–1812
Article Google Scholar - Vidal K, Labeta MO, Schiffrin EJ, Donnet-Hughes A 2001 Soluble CD14 in human breast milk and its role in innate immune responses. Acta Odontol Scand 59: 330–334
Article CAS Google Scholar - Adlerberth I, Wold AE 2009 Establishment of the gut microbiota in western infants. Acta Paediatr 98: 229–238
Article CAS Google Scholar - Ohnishi T, Muroi M, Tanamoto K 2010 Inhibitory effects of soluble MD-2 and soluble CD14 on bacterial growth. Microbiol Immunol 54: 74–80
Article CAS Google Scholar - Ipsen R, Otte J 2007 Self-assembly of partially hydrolysed alpha-lactalbumin. Biotechnol Adv 25: 602–605
Article CAS Google Scholar - Livney YD 2010 Milk proteins as vehicles for bioactives. Curr Opin Colloid Interface Sci 15: 73–83
Article CAS Google Scholar
Acknowledgements
We thank Lars Hanson for stimulating discussions; Van Thong Pham and Harvey Kaplan, Anhydrovac Inc, for providing radiolabeled CD14 and BSA proteins; Ottawa Institute of Systems Biology for providing the mass spectrometry services www.oisb.ca); Eve Õiglane for valuable perspectives on lactation and gut health; and members of the Altosaar Laboratory for critical review of the manuscript.
Author information
Authors and Affiliations
- Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, K1H 8M5, Ontario, Canada
William J Spencer, Andrew Binette, Tonya L Ward, Laura D R Davis, David R Mack & Illimar Altosaar - Steacie Institute for Molecular Sciences, National Research Council, Ottawa, K1A 0R6, Ontario, Canada
David R Blais - Department of Pediatrics, Children's Hospital of Eastern Ontario, Ottawa, K1H 8L1, Ontario, Canada
Joann Harrold & David R Mack
Authors
- William J Spencer
- Andrew Binette
- Tonya L Ward
- Laura D R Davis
- David R Blais
- Joann Harrold
- David R Mack
- Illimar Altosaar
Corresponding author
Correspondence toIllimar Altosaar.
Additional information
Supported by Grant (82816) from the Canadian Institutes of Health Research.
Rights and permissions
About this article
Cite this article
Spencer, W., Binette, A., Ward, T. et al. Alpha-Lactalbumin in Human Milk Alters the Proteolytic Degradation of Soluble CD14 by Forming a Complex.Pediatr Res 68, 490–493 (2010). https://doi.org/10.1203/PDR.0b013e3181f70f21
- Received: 27 May 2010
- Accepted: 14 July 2010
- Issue Date: December 2010
- DOI: https://doi.org/10.1203/PDR.0b013e3181f70f21