CD95-Induced Apoptosis Contributes to Loss of Primed/Memory but Not Resting/Naive T Cells in Children Infected with Human Immunodeficiency Virus Type 1 (original) (raw)
Main
Disease progression in HIV-1-infected individuals toward AIDS is characterized by progressive CD4+ T helper cell depletion(1). Although compensatory mechanisms may maintain total T cell numbers at a constant level for some time, development of AIDS has been shown to be preceded by rapid decline in CD4+ and CD8+ T cell numbers(2).
Increased apoptosis in infected as well as noninfected cells has previously been invoked in the process of T cell loss(3, 4). The CD95/APO-1/Fas receptor/ligand system is a key regulator of apoptosis in normal and malignant T cells(5–8). Upon activation, T cells express the CD95 cell surface R, a member of the tumor necrosis factor-R/nerve growth factor-R super-family, that induces apoptosis upon oligomerization(9–11). We and others have demonstrated a strong increase in CD95 expression on freshly isolated T cells from children(12) and adults(13–16) with HIV-1 infection. In addition, spontaneous apoptosis as well as specific CD95-mediated apoptosis was enhanced in both CD4+ and CD8+ T cells from HIV-1+ children(17).
Regeneration of a depleted T cell compartment requires either redistribution of primed and unprimed T cells from lymphatic organs, peripheral expansion due to proliferation of primed T cells, or development and selection of new unprimed T cells from progenitor cells in the thymus(thymopoiesis)(18). Thus, increased thymopoiesis and T cell recovery in children but not in adults was observed after intensive chemotherapy(19). Recent thymic emigrants(i.e. “naive” or “virgin” T cells) are thought to be characterized by expression of CD45RA, the high molecular weight form of the leukocyte common antigen CD45, found on more than 90% of cord blood T cells(20). On the other hand, previously activated T cells with the ability to react more quickly and more intensely upon second antigen exposure (i.e. “primed” or“memory” T cells) express high levels of CD45RO (the low molecular weight isoform of CD45) and increased levels of surface molecules involved in T cell activation and adhesion(21).
Because the population of CD45RA+ CD8+ T cells may also contain primed cells(22), additional surface markers are used to identify T cells that have not yet been stimulated by antigen,i.e., resting/naive T cells. Thus, the lymphocyte homing receptor L-selectin (CD62L) is expressed on most naive T cells and a subpopulation of memory T cells(23). Activation of lymphocytes in vitro leads to loss of L-selectin within hours due to endoproteolytic release of the receptor from the cell surface(24). Using the simultaneous expression of CD45RA and L-selectin to identify resting/naive T cells, a progressive loss of resting/naive T cells has been shown to occur during the asymptomatic stage of HIV infection both in children and adults(25–28). Whether or not loss of unprimed T cells is the result of an increased sensitivity of these cells toward apoptosis or of an activation-induced shift from naive to memory T cells prone to apoptotic cell death in HIV-1-infected individuals(29) is not known.
In normal human peripheral blood lymphocytes, CD95 is preferentially expressed on CD45RO+ memory T cells but not on naive CD45RA+ T cells(9, 11). Neonatal cord blood T cells, which are predominantly CD45RA+, express only low levels of CD95(11). Activation-induced cell death, which is mediated via the CD95 receptor/ligand system(30), can be induced in activated but not in resting peripheral blood T cells from healthy individuals(31). We have previously shown that CD95 expression in HIV-1+ children is not restricted to CD45RO+ memory T cells but also increased on naive CD45RA+ T cells(17). To elucidate the role of the CD95 receptor/ligand system in depletion of naive T cells during pediatric HIV-1 infection we studied sensitivity toward CD95-triggered apoptosis in naive and memory T cell subsets defined by the expression of high and low molecular weight isoforms of the leukocyte common antigen CD45 and the lymphocyte homing receptor L-selectin. Resting/naive (L-selectinbright CD45RA+) T cells from HIV-1-infected children as well as from healthy controls did not express high levels of CD95 and were not primed to undergo CD95-mediated apoptosis. Loss of naive (CD45RA+ CD45RO-) peripheral blood T cells in 14 patients in vivo was found to be paralleled by a strong increase in the percentage of CD95high T cells in vivo and anti-CD95-induced apoptosis of CD4+ and CD8+ T cells in vitro.
Despite the fact that only a relatively small number of patients was studied, we conclude that the loss of naive unprimed T cells during the asymptomatic phase of HIV-1 infection may be caused by a shift from apoptosis-resistant resting/naive T cells to cells with a primed/memory phenotype and increased sensitivity toward CD95-mediated apoptotic cell death. Development of overt immunodeficiency in HIV-infected children may thus critically depend on the equilibrium of accelerated programmed cell death and thymic regenerative capacity (i.e. the ability to continuously create naive unprimed T cells).
METHODS
Patients. Heparinized venous blood was obtained from 14 HIV-1+ children and adolescents during routine blood sampling. All patients were seen at monthly intervals at the University Children's Hospital, Heidelberg, Germany, as part of the “German Multicenter Study on HIV Infection in Children,” conducted according to the Declaration of Helsinki and approved by the ethical committee of the Childrens' Hospital of Heidelberg. CD4+ cell counts were determined at 3-mo intervals according to published guidelines(32), and the arithmetic mean of two measurements was calculated for classification according to criteria of the Centers for Disease Control (CDC)(33).
Clinical data of the patients are shown in Table 1. Eleven patients had aquired HIV-1 due to vertical transmission, in three patients the modus of infection was unknown. Five patients had been on antiretroviral therapy with zidovudine for more than 2 y, one patient had taken zidovudine and didanosine for more than 6 mo. Ten patients received monthly immunoglobulins i.v., and 10 patients were on oral TMP/SMX as primary prophylaxis of Pneumocystis carinii pneumonia. Three patients received fluconazole as secondary prophylaxis against fungal infections. None of the patients showed clinical or laboratory signs of concomitant opportunistic or other infectious diseases at the time of the study.
Table 1 Patients studied
Control blood samples were obtained from four healthy children (1, 2, 3, and 8 y of age), who had HIV-1+ mothers. These children were followed in our outpatient clinic and were repeatedly shown to be HIV-1- by Western blot, HIV-p24 antigen assay, and viral culture. Patients or control subjects and/or their relatives gave informed consent before venipuncture.
Immunophenotyping of peripheral blood lymphocytes. Multiparameter immunophenotyping was done with a FACS-can flow cytometer interfaced to an Apple-Macintosh Quadra 650 microcomputer (Becton Dickinson, Heidelberg, Germany). Fluorescence compensation was adjusted using samples of peripheral blood lymphocytes labeled individually with anti-CD8 MAb conjugated to each fluorochrome used. For each cell, data for forward scatter, side scatter, and the fluorescence of fluorescein in FL-1 (FITC, 520 nm emission maximum), PE (576 nm) in FL-2, and PerCP (677 nm) in FL-3 were collected. Lymphocytes were gated by a forward (FSC) versus right angle (90°) light scatter plot (SSC). Lymphocyte recovery and purity was controlled by backgating using CD45-FITC/CD14-PE dual labeling. For each staining, data from 20 000 events in the lymphocyte gate were collected and subsequently analyzed using the CELLQuest software (Becton Dickinson). Analysis cursors were set to read no more than 2% of the matched isotype-negative control cells as positive.
The following mouse MAb were used: anti-CD3 (UCHT-1, IgG1, FITC, or PE), anti-CD4 (13B8.2, IgG1, FITC, PE), anti-CD8 (B9.11, IgG1, FITC, PE), anti-CD45(ALB12, IgG1, FITC), anti-CD14 (RM052, IgG2a, PE), anti-CD45RA (ALB11, IgG1, FITC), anti-CD45RO (UCHL-1, IgG2a, PE) (all obtained from Immunotech, Hamburg, Germany); anti-CD3 (SK7, IgG1, PerCP), anti-CD4 (clone SK3, IgG1, PerCP), and anti-CD8 (clone SK1, IgG1, PerCP) (Becton Dickinson). The following isotype-matched negative control MAb were used: IgG1 FITC or PE (clone 679.1MC7, Immunotech), IgG2a FITC or PE (clone U7.27, Immunotech), and IgG1 PerCP (clone X40, Becton Dickinson).
Determination of CD95+ T cells in peripheral blood from HIV-1+ children. PBMC were isolated by Ficoll gradient centrifugation as described previously(12). Cells were washed twice with PBS containing 1% FCS(Biochrom, Berlin, Germany) and 0.1% NaN3 and resuspended at a concentration of 2 × 106 cells/mL. Immunophenotyping was performed using biotinylated anti-APO-1 MAb (IgG3) and Streptavidin-PE (Becton Dickinson) in FL-2 or streptavidin-Quantum Red (PE-Cy5-conjugate, Sigma Chemical Co., Deisenhofen, Germany) in FL-3, in conjunction with mouse MAb necessary for identification of the lymphocyte subset under investigation as described elsewhere(12, 17). For isotype control the nonbinding, biotinylated mouse MAb FII23 (IgG3) was used.
For estimation of fluorescence intensity as a measure of CD95 antigen density, fluorochrome-conjugated beads displaying different fluorescence intensities (Linear Flow Orange/Deep Red Flow Cytometry Intensity Calibration Kits, Molecular Probes Europe, Leiden, The Netherlands) were mixed and measured at the end of the experiments without changing the instrument settings. The marker for cells that expressed CD95 at high levels(CD95high cells) was set according to peak 2 of the bead mixture.
Determination of CD95 expression on naive and memory T cell subsets. For further analysis of CD95 expression on naive and memory T cells, isolated PBMC from five HIV-1+ patients representative of the spectrum of different disease stages and four age-matched HIV-1- controls representative of the age distribution in the patient cohort were resuspended in RPMI 1640 supplemented with 10% FCS, penicillin/streptomycin, L-glutamine, and N- 2-hydroxyethylpiperazine-_N'-_2-ethanesulfonic acid buffer(supplemented medium), and adherent cells (monocytes and macrophages) were removed by plastic adherence. For this purpose, the suspended PBMCs were incubated for 45-60 min at 37 °C in a cell culture flask (Falcon no. 3028, Becton Dickinson). Nonadherent cells (i.e. B, T, and natural killer cells) were carefully discarded. T cells were then further purified by incubation of the nonadherent cells with the mouse MAb anti-CD19 (HD37, generous gift of G. Moldenhauer, DKFZ), anti-CD16 (3G8, IgG1, Immunotech), and anti-CD14 (RM052, IgG2a, Immunotech) for 30 min at 4 °C. Sufficient goat anti-mouse antibody-coated magnetic beads (Dynabeads M-450, Dynal, Hamburg, Germany) were added to achieve a 5:1 bead:target cell ratio, and the suspension was rotated for additional 30 min at 4 °C. Beads bearing the CD19+, CD14+, and CD16+ cells were displaced with a magnet, and the unbound negatively selected CD3+ T cells were removed by pipetting. Over 95% pure T cells were obtained as determined by FACScan analysis. This cell suspension was further depleted of either CD4+ or CD8+ T cells using the appropriate mouse MAb (clones 13B8.2 and B9.11, Immunotech) and goat anti-mouse antibody-coated magnetic beads. Purified CD4+ and CD8+ T cells were then used to characterize CD95 expression on subsets identified by differential expression of CD45RA and CD45RO(34). Unprimed resting/naive T cells(L-selectinbright CD45RA+) were further identified using the mouse MAb anti-CD45RA and anti-CD62L (DREG-56, IgG1, PE) (Dianova, Hamburg, Germany) according to Rabin et al.(27).
Determination of naive and memory T cells in peripheral blood from HIV-1+ children. The percentage of peripheral blood T cells expressing different isoforms of the leukocyte common antigen CD45 was determined using a whole blood lysis method. Briefly, 100 μL of anticoagulated whole blood were incubated with 20 μL of each fluorochrome-conjugated MAb for 30 min at 4 °C in the dark. Then lysing solution (FACSLyse, Becton Dickinson) was added, and samples were incubated for an additional 10 min at room temperature in the dark and subsequently washed twice with PBS containing 1% FCS and 0.1% NaN3. Finally, 200μL of 2% paraformaldehyde in PBS were added to the cell pellets, and the samples were stored at 4 °C until measurement. T lymphocyte subsets were characterized by differential expression of CD45RA and CD45RO on CD3+ T cells as described previously(34). A gate was set on CD3+ T cells (SSC versus FL-3), and the percentage of naive(CD45RAbright CD45RO-) T cells and primed/memory(CD45RObright CD45RA-) T cells was calculated using region statistics.
Determination of spontaneous and CD95-mediated apoptotic cell death of naive and memory T cell subsets during short-term culture in vitro. Unseparated, freshly isolated PBMC (5 × 105 cells/well) were distributed in 96-well flat-bottom plates with supplemented medium or cultured in plates that were precoated with anti-CD95 (10 μg mL-1) MAb as described elsewhere(30). Cells were collected after 24 h of culture, and cell death was determined on gated CD4+ and CD8+ cells as described previously(35). Briefly, apoptotic cells were identified by the characteristic morphology of“shrinking death” (decreased FSC and increased SSC). Percentage of specific CD95-triggered apoptosis (specific cell death) was calculated according to the formula: 100 × [anti-CD95-induced cell death (%) - spontaneous cell death (%)]/[100 - spontaneous cell death (%)].
In eight patients apoptotic cells were additionally detected using the binding of FITC-labeled recombinant human annexin V (Bender MedSystems, Vienna, Austria) as described elsewhere(36). Because of a linear correlation (r = 0.88; p < 0.001) between the percentage of annexin V+ cells and the percentage of cells displaying morphologic features of apoptosis, the percentage of apoptotic cells was determined by morphologic criteria only.
In 7 HIV-1+ patients sensitivity toward spontaneous and anti-CD95-triggered apoptosis of resting/naive, naive, and memory T cells in vitro was measured after 48 h of culture. T cell subsets were identified by electronic gating using differential expression of either CD4 or CD8 in combination with CD45RA and CD45RO as well as CD45RA and CD62L. The percentage of apoptotic cells in each gated population was calculated by FSC-SSC analysis. Furthermore, the representation of each subset in the population of viable lymphocyte (as identified by their characteristic features in the FSC-SSC plot) after 48 h of culture was calculated. Because unstimulated peripheral blood T cells from healthy individuals do not undergo spontaneous or CD95-mediated apoptosis(9, 17, 31), control samples from healthy children could not be included in these experiments.
Statistical analysis. Data are reported by their arithmetic mean ± SEM unless otherwise stated. Correlation analysis was performed by calculating Pearson's correlation coefficient. A p value < 0.05 was assumed to indicate statistical significance.
RESULTS
CD95 expression in resting/naive, naive, and primed/memory T cells. First we investigated whether the increase in CD95 expression observed in T cells from HIV-1+ children(12) was due to a global increase in CD95 antigen density on all different T cell subsets or was caused by an increased representation of T cell subsets displaying a CD95high phenotype, e.g. CD45RO+ (memory) T cells. Previous experiments had shown a high level of CD95 expression not only on CD45RO+ T cells but also on naive CD45RA+ T cells from HIV-1-infected children(17). This may indicate a global increase in CD95 expression even on resting/naive, unprimed T cells or could be due to the presence of an increased percentage of recently activated T cells in the peripheral blood of HIV-1+ children. Although these cells are still CD45RA+, they express activation markers, such as CD45RO and CD95 (“transitional cells”)(34). In addition, expression of the lymphocyte homing receptor L-selectin is lost after T cell activation(24). Simultaneous determination of the surface expression of CD45RA and CD45RO or L-selectin allows for the precise identification of T cells engaged in the naive-to-memory transition process. We therefore isolated T cells from five HIV-1+ children and four healthy control subjects by negative selection to identify resting/naive, activated/naive, and primed/memory CD4+ and CD8+ T cell subsets by differential expression of the relevant surface antigens and determined the expression of CD95 on these subsets.
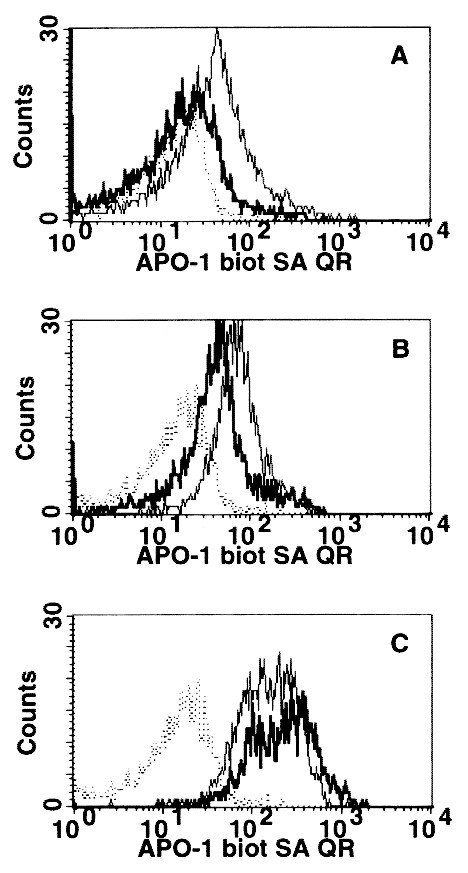
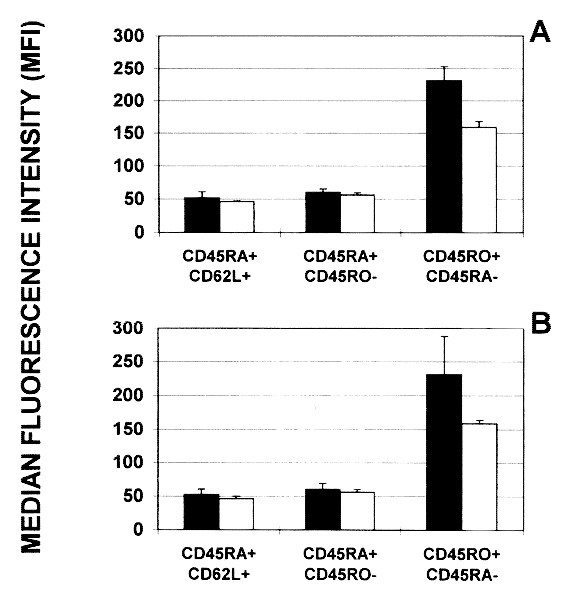
We found that CD95 expression on peripheral blood T cells from HIV-1+ patients and in healthy controls increased as these cells acquired a primed/memory phenotype. The result of a typical measurement in one patient with oligosymptomatic disease (CDC stage B) is shown in Fig. 1. CD95 expression on freshly isolated T cells was low in resting/naive (L-selectinbright CD45RA+) cells (Fig. 1A), increased slightly in naive (CD45RA+ CD45RO-) cells (Fig. 1B), and was highest on primed/memory (CD45RA- CD45RO+) T cells of either the CD4+ and the CD8+ subset (Fig. 1C). In all HIV-1+ children and healthy control subjects studied, a low percentage of resting/naive T cells in the CD4+ and the CD8+ subset expressed low levels of CD95 (Fig. 2,A and B). The majority of these cells were CD95 negative (Table 2). In contrast, high expression of CD95 and an increased percentage of CD95+ and CD95high cells were found in primed/memory (CD45RA- CD45RO+) T cells. No significant difference between HIV-1+ children and healthy control subjects regarding CD95 expression on resting/naive, naive, and primed/memory CD4+ and CD8+ T cell subsets was found.
Figure 1
CD95/APO-1 expression in negatively selected CD4+ (□) and CD8+ (―) T cell subsets from an HIV-1-infected child. The CD95 expression pattern was determined after gating on resting/naive L-selectinbright CD45RA+ (A), naive CD45RA+ CD45RO- (B), and primed/memory CD45RO+ CD45RA- (C) T cell subpopulations. The dotted line (----) indicates the staining pattern of lymphocytes with the mouse MAb FII23 (IgG3) used as an isotype-matched negative control.
Figure 2
CD95-expression (median fluorescence intensity) in resting/naive (L-selectinbright CD45RA+), naive (CD45RA+ CD45RO-), and primed/memory (CD45RO+ CD45RA-) CD4+(A) and CD8+ (B) T cell subsets from five HIV-1-infected children (black bars) compared with four healthy controls(white bars).
Table 2 CD95 expression on resting/naive(CD45RA+ CD62L+), naive (CD45RA+CD45RO_-), and primed/memory (CD45RO_+CD45RA_-) T cells from five HIV-1_+patients and four healthy control subjects
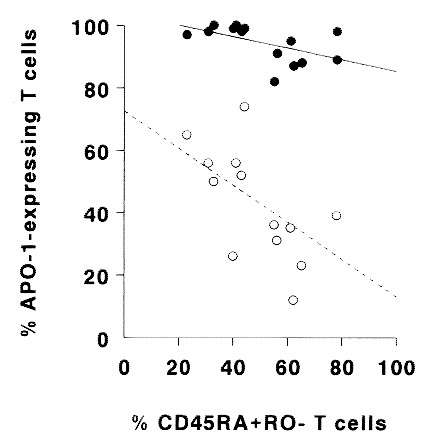
Increase of CD95 high T cells in HIV-1+ children with loss of naive T cells in vivo. Because naive T cells are lost during disease progression in HIV-1 infection, we measured CD95 expression on CD3+ T cells relative to the actual frequency of naive (CD45RA+ CD45RO-) CD3+ T cells in the peripheral blood of 14 HIV-1+ children. No significant correlation between the overall percentage of CD95+ and CD45RO+ or CD45RA+ T cells was found in these patients (Fig. 3). From 80 to 100% of T cells from all patients were CD95+. However, when the percentage of CD95high T cells was calculated, a clear negative correlation with the percentage of naive T cells in the peripheral blood was found (r = -0.59, p < 0.05;Fig. 3). Thus, increased expression of CD95 on peripheral blood T cells from HIV-1+ children compared with age-matched healthy control subjects is due to a decreased frequency of CD95low naive T cells in the peripheral blood of these patients rather than to aberrant expression of CD95 on naive and unprimed T cells.
Figure 3
The percentage of CD95high peripheral blood T cells (○) from HIV-1-infected children increases with a decreasing percentage of circulating naive (CD45RA+ CD45RO-) T cells(r = -0.59; p < 0.05). The percentage of CD95+ T cells (•) shows no significant correlation (r = -0.18;p = NS).
Resistance of resting/naive T cells toward spontaneous and CD95-triggered apoptosis in HIV-1+ children in vitro. Next, we wanted to know whether resting/naive, naive, and primed/memory CD4+ and CD8+ T cell subsets from HIV-1+ children differ in susceptibility toward spontaneous and anti-CD95-triggered apoptosis in vitro. Because unstimulated peripheral blood T cells from healthy individuals do not undergo spontaneous or CD95-mediated apoptosis(9, 17, 31), experiments were performed on samples from seven HIV-1+ children only. Sensitivity of different T cell subsets toward spontaneous and anti-CD95-induced apoptosis was measured during in vitro culture of PBMCs for 48 h in the presence or absence of plate-bound anti-CD95 MAb.
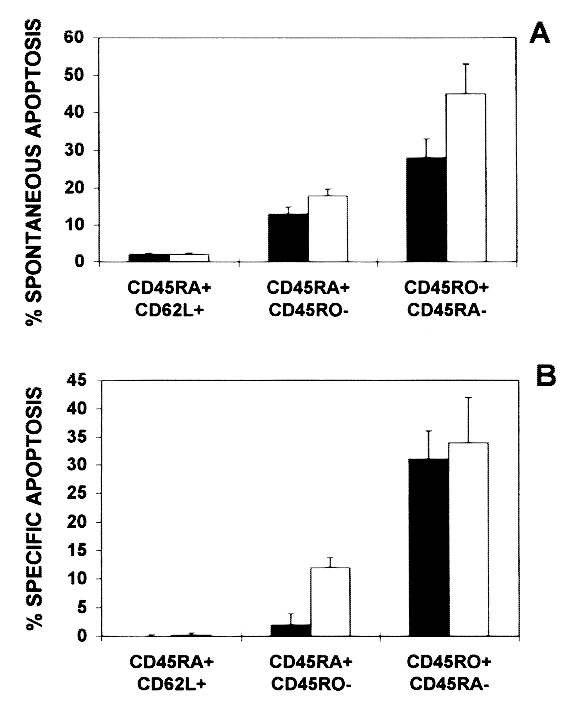
Apoptotic cell death was quantified by counting the percentage of apoptotic cells using FSC-SSC analysis. Resting/naive, naive, and primed/memory T cell subsets were identified by three-color immunophenotyping as described in“Methods.” CD4+ and CD8+ resting/naive(L-selectinbright CD45RA+) T cells did not undergo apoptosis after incubation with medium (Fig. 4A) or plate-bound anti-CD95 MAb (Fig. 4B). Apoptotic T cells in culture expressed L-selectin only at low levels or were L-selectin negative. Naive(CD45RA+ CD45RO-) CD8+ T cells showed an increased sensitivity toward anti-CD95-induced apoptosis when compared with resting naive CD8+ T cells (Fig. 4B). This difference in sensitivity of naive versus resting naive cells toward CD95-triggering was not seen in CD4+ T cells (Fig. 4B).
Figure 4
Apoptosis of resting/naive (L-selectinbright CD45RA+), naive (CD45RA+ CD45RO-), and primed/memory(CD45RO+ CD45RA-) T cells of CD4+ (black bars) and CD8+ (white bars) subsets after in vitro culture of PBMC from seven HIV-1+ children for 48 h. Spontaneous apoptosis (A) and specific anti-CD95 MAb-triggered apoptosis (B) was calculated by counting the percentage of cells with decreased forward light scatter and increased right-angle light scatter as described in “Methods.” The percentage of specific CD95-triggered apoptosis was calculated using the formula: 100 × [anti-CD95-induced cell death (%) - spontaneous cell death (%)]/[100 - spontaneous cell death (%)].
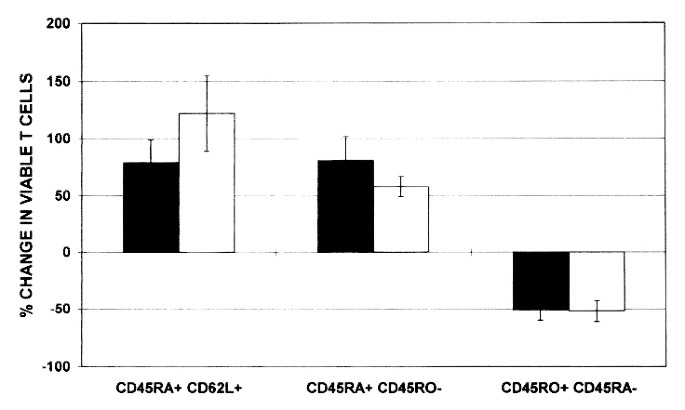
Due to preferential apoptosis of primed/memory cells (and independently from the age of the patients), the proportion of both resting naive and naive CD4+ and CD8+ T cells increased in the viable cell population either spontaneously or after CD95 triggering with a cross-linking MAb for 48 h (Fig. 5). After 48 h of in vitro culture in the presence of cross-linking anti-CD95 antibodies, viable T cells (as judged by FSC-SSC characteristics) contained 80-100% more resting/naive, 50-100% more naive, and 40-60% less primed/memory cells than at the beginning of the experiment.
Figure 5
Changes in the distribution of resting/naive(L-selectinbright CD45RA+), naive (CD45RA+ CD45RO-), and primed/memory (CD45RO+ CD45RA-) T cell subsets among viable CD4+ (black bars) and CD8+ (white bars) lymphocytes after in vitro culture of PBMCs from seven HIV-1+ children for 48 h in the presence of a cross-linking anti-CD95 antibody. The percent change in viable T cells was calculated by subtracting the percentage of a given T cell subset at 0 h from the value obtained after 48 h of in vitro culture, divided by the value at 0 h.
Anti-CD95-induced T cell apoptosis in vitro increased with a decreasing percentage of naive (CD45RA+ CD45RO-) peripheral blood T cells in 14 HIV-1+ patients (data not shown). Thus, increased apoptotic cell death of both CD4+ and CD8+ T cells observed during short-term in vitro culture of PBMC from HIV-1+ children is due to priming for apoptosis in T cells with a memory phenotype and-at least in the CD8+ T cell subset-also in previously activated naive cells.
DISCUSSION
The data in this study demonstrate that increased expression of CD95 on peripheral blood T cells from HIV-1+ children is caused by an increased representation of T cell subsets displaying a CD95high phenotype and a decreased frequency of CD95low naive T cells. No evidence was found for HIV-1-specific aberrant expression of CD95 on resting/naive and unprimed CD4+ and CD8+ T cells. These data indicate that the regulation of CD95 expression in peripheral blood T cells from HIV-1-infected children follows general patterns similar to healthy individuals(5, 11).
In normal T cells the CD95 system is tightly controlled to maintain peripheral T cell homeostasis(8). Up-regulation of the CD95 receptor on peripheral blood T cells parallels expression of the activation marker CD45RO and occurs gradually during in vitro culture in the presence of T cell mitogens and IL-2(5, 9, 31, 37). In the present study, high expression levels of CD95 were found in freshly isolated primed/memory(CD45RO+ CD45RA-) T cells but not in naive (CD45RA+ CD45RO-) T cells from both HIV-1+ children and healthy controls. In HIV-1-infected children the percentage of CD95high T cells increased significantly with a decreasing percentage of naive T cells. These data further corroborate the important role of T cell activation in the up-regulation of CD95 receptor on peripheral blood T cells during HIV-1 infection.
Increased sensitivity of CD4+ and CD8+ T cells toward spontaneous as well as CD95-mediated apoptosis was found in HIV-1+ children and not in age-matched healthy control subjects(17). The data presented in this study provide evidence that both CD95 expression on peripheral blood T cells and their sensitivity toward anti-CD95-induced apoptosis increase as these cells acquire a primed/memory phenotype. In lymphocytes from healthy adults, other cellular conditions in addition to surface expression of the CD95 receptor are required for anti-CD95-induced cell death, because viability of freshly isolated CD45RO+ T cells from healthy adult donors is not changed by incubation with anti-CD95 antibodies(11). Sensitivity toward apoptosis-inducing effects of anti-CD95 antibodies was aquired after prolonged activation in vitro for at least 4-6 d(9, 38).
Increased CD95 expression and sensitivity of freshly isolated T cells toward CD95-triggered apoptosis during short-term culture in vitro may reflect increased “physiologic aging” and accelerated differentiation due to continuous stimulation of CD8+ T cells in vivo by HIV-1 or other viral antigens(39). On the other hand, immunomodulating HIV gene products such as HIV-1 Tat and gp120(40) may presensitize primed/memory CD4+ T cells in HIV-1-infected individuals in vivo for CD95-mediated apoptosis. Thus, mechanisms that may drive the continuous shift from apoptosis-resistant, resting naive to apoptosis-sensitive, primed/memory T cells in HIV-infected individuals may include specific activation signals induced by viral gene products that have previously been shown to change T cell function and to deregulate the CD95-receptor/ligand system in vitro.
An important finding of our study is that both CD4+ and CD8+ resting/naive (L-selectinbright CD45RA+) T cells of HIV-1+ children did not undergo spontaneous or anti-CD95-induced apoptosis in vitro. These data show that newly formed resting/naive T cells in HIV-1+ children are spared from the apoptosis-promoting effect of HIV-1 infection. The percentage of L-selectinbright CD45RA+ resting/naive T cells increased during short-term culture in vitro. All apoptotic cells were L-selectin dim or negative. Thus, in HIV-1+ patients, the signal that initiates the apoptotic process either spontaneously or after CD95 triggering causes concomitant shedding of L-selectin. Activation of lymphocytes in vitro leads to loss of L-selectin expression within hours due to endoproteolytic release of the receptor from the cell surface(23, 24). Shedding of L-selectin from the surface of T cells undergoing apoptosis may be caused by aberrant activation signals leading to programmed cell death instead of proliferation and/or gain of function (e.g. cytokine production).
In conclusion, the progressive loss of resting naive T cells during the course of HIV-1 infection in children and adults(18, 25–28) is neither due to up-regulation of CD95 receptor density on resting/naive T cells nor to increased propensity of these cells for apoptotic cell death. The increased percentage of CD95high T cells, as well as the increased sensitivity toward spontaneous and CD95-mediated apoptosis of both CD4+ and CD8+ primed/memory and CD8+ naive T cells, appears to be part of a persistent quasi-activated state of T cells recently described in a cross-sectional study of 154 HIV-infected adults(41). The finding that the physiologic resistance of resting/naive T cells toward CD95-induced apoptosis is preserved during HIV infection suggests that attempts to slow down CD95-mediated apoptosis of primed/memory T cells may be able to stabilize the T cell pool in the patients.
Abbreviations
CD:
cluster of differentiation
FL:
fluorescence channel
FSC:
forward light scatter
IVIG:
i.v. immunoglobulin substitution
PBMC:
peripheral blood mononuclear cells
PE:
phycoerythrin
PerCP:
Peridin-chlorophyll
R:
receptor
SSC:
rectangular light scatter
SMX:
sulfmethoxazole
TMP:
trimethoprim
References
- Levy J 1993 Pathogenesis of human immunodeficiency virus infection. Microbiol Rev 57: 183–289.
CAS PubMed PubMed Central Google Scholar - Margolick JB, Munoz A, Donnenberg AD, Park LP, Galai N, Giorgi JV, O'Gorman MRG, Ferbas J 1995 Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. Nat Med 1: 674–680.
Article CAS PubMed Google Scholar - Gougeon ML 1995 Does apoptosis contribute to CD4 cell depletion in human immunodeficiency virus infection?. Cell Death Differ 2: 1–8.
CAS PubMed Google Scholar - Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A 1995 Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med 1: 129–134.
Article CAS PubMed Google Scholar - Trauth BC, Klas C, Peters AMJ, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH 1989 Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245: 301–305.
Article CAS PubMed Google Scholar - Debatin KM, Goldman CK, Bamford R, Waldmann TA, Krammer PH 1990 Monoclonal antibody-mediated apoptosis in adult T cell leukemia. Lancet 335: 497–500.
Article CAS PubMed Google Scholar - Debatin KM, Goldman CK, Waldmann TA, Krammer PH 1993 APO-1 induced apoptosis of leukemia cells from patients with adult T cell leukemia. Blood 81: 2972–2977.
Article CAS PubMed Google Scholar - Krammer PH, Dhein J, Walczak H, Behrmann I, Mariani S, Matiba B, Fath M, Daniel PT, Knipping E, Westendorp MO, Stricker K, Bäumler C, Hellbardt S, Germer M, Peter ME, Debatin KM 1994 The role of APO-1-mediated apoptosis in the immune system. Immunol Rev 142: 175–191.
Article CAS PubMed Google Scholar - Klas C, Debatin KM, Jonker RR, Krammer PH 1993 Activation interferes with the apoptotic pathway in mature human T cells. Int Immunol 5: 625–631.
Article CAS PubMed Google Scholar - Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME 1995 Cytotoxicity-dependent APO-1(Fas/CD95)-associated proteins (CAP) form a death-inducing signalling complex(DISC) with the receptor. EMBO J 14: 5579–5588.
Article CAS PubMed PubMed Central Google Scholar - Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Taniguchi N 1992 Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol 149: 3753–3758.
CAS PubMed Google Scholar - Debatin KM, Fahrig-Faissner A, Enenkel-Stoodt S, Kreuz W, Benner A, Krammer PH 1994 High expression of APO-1 (CD95) on T-lymphocytes from human immunodeficiency virus infected children. Blood 83: 3101–3103.
Article CAS PubMed Google Scholar - Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA 1995 Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med 181: 2029–2036.
Article CAS PubMed Google Scholar - Estaquier J, Idziorek T, Zou W, Emilie D, Farber C-M, Bourez J-M, Ameisen JC 1995 T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95(FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med 182: 1759–1767.
Article CAS PubMed Google Scholar - Gehri R, Hahn S, Rothen M, Steuerwald M, Nuesch R, Erb P 1996 The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS 10: 9–16.
Article CAS PubMed Google Scholar - Silvestris F, Cafforio P, Frassanito MA, Tucci M, Romito A, Nagata S, Dammacco F 1996 Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS 10: 131–141.
Article CAS PubMed Google Scholar - Bäumler CB, Böhler T, Herr I, Benner A, Krammer PH, Debatin K-M 1996 Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1 infected children. Blood 88: 1741–1746.
Article PubMed Google Scholar - Roederer M 1995 T-cell dynamics of immunodeficiency. Nat Med 1: 621–622.
Article CAS PubMed Google Scholar - Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, Wexler LH, Gress RE 1995 Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 332: 143–149.
Article CAS PubMed Google Scholar - Fujii Y, Okumura M, Inada K, Nakahara K, Matsuda H 1992 CD45 isoform expression during T cell development in the thymus. Eur J Immunol 22: 1843–1850.
Article CAS PubMed Google Scholar - Cerottini J-C, MacDonald HR 1989 The cellular basis of T-cell memory. Annu Rev Immunol 7: 77–89.
Article CAS PubMed Google Scholar - Okumura M, Fujii Y, Inada K, Nakahara K, Matsuda H 1993 Both CD45RA+ and CD45RA- subpopulations of CD8+ T cells contain cells with high levels of lymphocyte function-associated antigen-1 expression, a phenotype of primed T cells. J Immunol 150: 429–437.
CAS PubMed Google Scholar - Tedder TF, Steeber DA, Chen A, Engel P 1995 The selectins: vascular adhesion molecules. FASEB J 9: 866–873.
Article CAS PubMed Google Scholar - Kishimoto TK, Jutila MA, Butcher EC 1990 Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA 87: 2244–2248.
Article CAS PubMed PubMed Central Google Scholar - Plaeger-Marshall S, Hultin P, Bertolli J, O'Rourke S, Kobayashi R, Kobayashi AL, Giorgi JV, Bryson Y, Stiehm ER 1993 Activation and differentiation antigens on T cells of healthy, at-risk, and HIV-infected children. J Acquir Immune Defic Syndr 6: 984–993.
CAS PubMed Google Scholar - Ibegbu C, Spira TJ, Nesheim S, Mendez H, Lee F, Polliotti B, Caba J, Nahmias A 1994 Subpopulations of T and B cells in perinatally HIV-infected and noninfected age-matched children compared with those in adults. Clin Immunol Immunopathol 71: 27–32.
Article CAS PubMed Google Scholar - Rabin RL, Roederer M, Maldonado Y, Petru A, Herzenberg LA, Herzenberg LA 1995 Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J Clin Invest 95: 2054–2060.
Article CAS PubMed PubMed Central Google Scholar - Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA 1995 CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest 95: 2061–2066.
Article CAS PubMed PubMed Central Google Scholar - Janossi G, Borthwick N, Lomnitzer R, Medina E, Bertel Squire S, Philipps AN, Lipman M, Johnson MA, Lee C, Bofill M 1993 Lymphocyte activation in HIV-1 infection. I. Predominant proliferative defects among CD45RO+ cells of the CD4 and CD8 lineages. AIDS 7: 613–624.
Article Google Scholar - Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH 1995 Autocrine T cell suicide mediated by APO-1 (Fas/CD95). Nature 373: 438–441.
Article CAS PubMed Google Scholar - Wesselborg S, Janssen O, Kabelitz D 1993 Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol 150: 4338–4345.
CAS PubMed Google Scholar - Centers for disease control 1992 Guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus infection. MMWR 41/RR- 8: 1–17.
- Centers for Disease Control 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR 43/RR- 12: 1–10.
- Prince HE, York J, Jensen ER 1992 Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol 145: 254–262.
Article CAS PubMed Google Scholar - Carbonari M, Cibati M, Cherchi M, Sbarigia D, Pesce AM, Dell' Anna L, Modica A, Fiorilli M 1994 Detection and characterization of apoptotic peripheral blood lymphocytes in human immunodeficiency virus-infection and cancer chemotherapy by a novel flow immunocytometric method. Blood 83: 1268–1277.
Article CAS PubMed Google Scholar - Koopman G, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST, van Oers MHJ 1994 Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84: 1415–1420.
Article CAS PubMed Google Scholar - Salmon M, Pilling D, Borthwick NJ, Viner N, Janossy G, Bacon PA, Akbar AN 1994 The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol 24: 892–899.
Article CAS PubMed Google Scholar - Owen-Schaub LB, Yonehara S, Crump WL, Grimm EA 1992 DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol 140: 197–205.
Article CAS PubMed Google Scholar - Uehara T, Miyawaki T, Otha K, Tamaru Y, Yokoi T, Nakamura, Taniguchi N 1992 Apoptotic cell death of primed CD45RO+ T lymphocytes in Epstein-Barr virus-induced infectious mononucleosis. Blood 80: 452–458.
Article CAS PubMed Google Scholar - Westendrop MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH 1995 The mechanism of HIV Tat and gp120-sensitized T cell apoptosis. Nature 375: 497–500.
Article Google Scholar - Roederer M, Herzenberg LA, Herzenberg LA 1996 Changes in antigen densities on leukocyte subsets correlate with progression of HIV disease. Int Immunol 8: 1–11.
Article CAS PubMed Google Scholar
Acknowledgements
The authors thank the nurses of the“Onkologische Tagesklinik” of the Children's Hospital, University of Heidelberg, for their continuous support in patient care; our patients and their families for their willingness to participate in the study; A. Benner for help with statistical analysis; and S. Müller for secretarial assistance.
Author information
Authors and Affiliations
- University Children's Hospital and Division of Molecular Oncology, Hematology/Oncology, German Cancer Research Center, 69120, Heidelberg, Germany
Thomas Böhler, Stefanie Nedel & Klaus-Michael Debatin
Authors
- Thomas Böhler
You can also search for this author inPubMed Google Scholar - Stefanie Nedel
You can also search for this author inPubMed Google Scholar - Klaus-Michael Debatin
You can also search for this author inPubMed Google Scholar
Additional information
Supported by grants from Bundesministerium für Forschung und Technologie (BMFT), and Deutsche Leukämieforschungshilfe (DLFH), Germany.
Rights and permissions
About this article
Cite this article
Böhler, T., Nedel, S. & Debatin, KM. CD95-Induced Apoptosis Contributes to Loss of Primed/Memory but Not Resting/Naive T Cells in Children Infected with Human Immunodeficiency Virus Type 1.Pediatr Res 41, 878–885 (1997). https://doi.org/10.1203/00006450-199706000-00013
- Received: 22 August 1996
- Accepted: 14 January 1997
- Issue Date: 01 June 1997
- DOI: https://doi.org/10.1203/00006450-199706000-00013