Quantification and Phenotypic Characterization of Circulating Tumor Cells for Monitoring Response to a Preventive HER2/neu Vaccine-Based Immunotherapy for Breast Cancer: A Pilot Study (original) (raw)
Improved understanding of the mechanisms of tumor-specific, cell-mediated immune responses has sparked interest in the development of cancer vaccines, which have enhanced specificity and are associated with minimal toxicity compared with conventional therapy. In general, cancer vaccines target tumor-associated antigens (TAAs), which are capable of being recognized by immune effectors. One well-described TAA is HER2/neu. Because HER2/neu is overexpressed in up to 30% of breast cancers and several other adenocarcinomas, it has been investigated as a possible target for an anticancer vaccine in patients with breast cancer.1–5
Many cancer vaccination strategies have been described from whole tumor cell vaccines to naked DNA vaccines, but our group has chosen to focus on peptide vaccines, which are the purest way to stimulate and monitor a cellular immune response to TAA. Peptide vaccines use disease-specific antigenic epitopes derived from TAA for the induction of peptide-specific cytotoxic T lymphocytes (CTL) that recognize and lyse tumor cells expressing the immunogenic peptide on their surface. These vaccines have been shown to be effective in inducing clonal expansion of epitope-specific CTL, promoting inter- and intra-antigenic epitope spreading to peptide-epitope-related sequences, and in generating sustained antigen-specific antitumor immunity.6,7 Ease of administration is an additional benefit of peptide vaccines. The designated peptide combined with an immunoadjuvant can be injected intradermally without requiring a delivery system; therefore, peptide vaccines are simple, easily produced, and efficiently exported to the community.
With respect to HER2/neu, several peptides derived from the protein have been investigated for use as anticancer vaccines. The most studied of the HER2/_neu_-derived peptides is E75 (HER2/neu aa:369-375–KIFGSLAFL), which is derived from the extracellular domain of the protein.8 Promising preclinical studies of this peptide led to clinical trials using E75 in patients with metastatic disease.9–11 These trials demonstrated that E75 is safe and capable of inducing a peptide-specific systemic T-cell-mediated immune response.
Our group has extensively studied the E75 peptide as a vaccine; however, we have pursued a different strategy in our clinical trials. We have shifted focus from the treatment of late-stage disease to the prevention of disease recurrence in patients at risk of relapse. In our ongoing clinical trials, we have enrolled patients with both node-positive (NP) and high-risk node-negative (NN) breast cancer who have been rendered disease-free after surgery, chemotherapy, and, if indicated, radiation. Because the E75 peptide is HLA-A2–restricted, HLA-A2+ patients have been administered the vaccine while HLA-A2- patients have served as unvaccinated controls. Interim analysis of 171 patients enrolled in our trial has shown a recurrence rate of 5.6% in the vaccinated patients vs 14.2% for the observation group (P = .04) at a median follow-up of 20 months.12
Monitoring of immunological response is an important component of cancer vaccine trials. The immunological response to our peptide vaccine is determined in vivo by induction of peptide-specific immunity manifested by local cutaneous inoculation site reaction or delayed-type hypersensitivity (DTH) response. In vitro evidence of immunologic recognition of the vaccinated peptide is assessed by phenotypic assays (HLA-A2 immunoglobulin dimer or tetramer assays) looking for clonal expansion of peptide-specific CTL, functional studies such as the ELISPOT assay that measures cytokine production, or standard chromium release assays that measure CTL-mediated tumor cell killing.7,12,13 Unfortunately, the majority of currently used immunologic assays is performed in vitro, and these assays have significant limitations; hence, new approaches are needed to identify in vivo monitoring techniques of peptide-vaccine-induced immune responses that correlate with clinical outcomes.14,15
Substantial numbers of circulating tumor cells (CTCs) have been identified in up to 70% of patients with metastatic breast cancer.16 In these patients, the CTC burden has been shown to predict treatment efficacy, progression-free, and overall survival in patients with metastatic breast cancer prior to and at any point after initiation of systemic therapy.17–19 The relevance of CTCs identified in the peripheral circulation of patients rendered disease-free with conventional therapy but thought to be at risk for recurrence is less well studied. We hypothesize that the presence of CTCs, specifically HER2/neu + CTCs, may identify a subset of patients that could benefit from vaccination with a HER2/_neu_-derived peptide. This pilot study was therefore undertaken to demonstrate proof-of-principle that CTCs could be detected and phenotyped in breast cancer patients rendered disease-free but at high risk of recurrence. In addition, we have sought to investigate the potential utility of enumerating and phenotyping CTCs in assessing a clinically significant immune response to vaccination in the context of a preventive vaccine trial.
METHODS
Patients and Clinical Trial
We are conducting IRB-approved prospective clinical trials of the HLA-A2-restricted HER2/_neu_-derived immunogenic peptide, E75 (IND#9187), in NP and high-risk for recurrence NN patients with HER2/_neu_-expressing primary breast cancer (IHC 1–3+ and/or positive FISH > 1.2) following completion of standard of practice breast cancer therapies (i.e., surgery, chemotherapy, immunotherapy, and radiation as appropriate per standard individualized cancer care). Study participants are cancer-free clinically, immunologically intact by recall anergy testing, have good performance status (ECOG < 2), and have completed standard primary breast cancer therapy. HLA-A2 status was determined at study enrollment: HLA-A2+ patients were enrolled in the treatment group and HLA-A2- patients were followed prospectively as untreated controls. With the initiation of this CTC pilot study, all 16 patients currently active in the NP trial were enrolled in this subprotocol regardless of where they were in the vaccination series. Some patients were enrolled prior to initiation of the series (R0), during the series (R3), at completion (R6), or at their 6 month follow-up (RC6).
Peptide Vaccination
Commercially available, purified, and sterile E75 peptide (1000 μg) (NeoMPS, Inc, San Diego, CA) was administered in conjunction with an immunoadjuvant, GM-CSF (250 μg) (Immunex Corporation, Seattle, WA) intradermally in the same extremity monthly for 6 months. Blood samples were drawn from the vaccinated patients prior to each inoculation and at 1 and 6 months following completion of the 6-month vaccination series. National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 definitions of adverse events were applied to assess local injection site and systemic toxicity.
Assessment of Immunological Response
Peripheral blood mononuclear cells from the patients were used to prepare dendritic cells and to serve as a source of E75-peptide-stimulated lymphocytes for in vitro assays. Specifically, functional enzyme-linked immunospot assay (ELISPOT) and E75 peptide-loaded HLA-A2:Ig dimer assay were performed as described previously.7,13 Immunological response to the E75 peptide vaccine was assessed in vivo by local cutaneous inoculation site reaction. Briefly, a DTH reaction was assessed with 100 μg of E75 in 0.5 cc of normal saline (without GM-CSF) and 0.5 cc normal saline as a volume control. The DTH reaction was measured in two dimensions at 48–72 hours using the sensitive ballpoint-pen method and reported as the orthogonal mean and compared with control.
Isolation, Identification, Enumeration, and Phenotyping of CTCs from Blood
The CellSearch System (Veridex, LLC, Warren, NJ) consists of the CellSave Preservative Tube, CellSearch Circulating Tumor Cell Kit, MagNest cell presentation device, CellTracks AutoPrep System, and the CellTracks Analyzer II.
The CellSearch Circulating Tumor Cell Kit contains a ferrofluid-based capture reagent and immunofluorescent reagents. The ferrofluid reagent consists of nanoparticles with a magnetic core surrounded by a polymeric layer coated with antibodies targeting the EpCAM antigen (anti-Ep-CAM ferrofluid) to immunomagnetically enrich and capture CTCs. After immunomagnetic capture and enrichment, fluorescent reagents are added for identification, enumeration, and HER2/neu phenotyping of CTCs. The fluorescent reagents include: anti-CK-Phycoerythrin (PE) specific for the intracellular protein cytokeratin 8, 18, and 19 (characteristic of epithelial cells), 4′, 6- diamidino-2-phenylindole (DAPI) dye to fluorescently label the cell nucleus, anti-CD45-Allophycocyanin (APC) specific for leukocytes, and buffers for cell washing and resuspension.20 An additional reagent, CellSearch HER-2/neu Tumor Phenotyping Reagent was added to assess HER2/neu positivity.
Blood was collected from all 16 NP patients active in the E75 trial at the initiation of this pilot trial to enumerate total and HER2/neu + CTC/20 mL of blood prior to, during, or after vaccination using the CellSearch System (Veridex). All patients were enrolled during the defined pilot study period regardless of where they were in the vaccination series. All evaluations were performed without knowledge of patient vaccination time course or clinical status. Peripheral venous whole blood was collected aseptically, prevaccination, and postvaccination, and drawn into two evacuated 10 mL blood draw CellSave Preservative Tubes containing an anticoagulant, EDTA, and a proprietary cell-stabilizing reagent. Samples were maintained at room temperature and processed within 72 hours of blood collection. All blood tubes were labeled with unique identifier study numbers, without patient identifying data or clinical information.
Twenty mL of blood was transferred from the CellSave Preservative Tube into a labeled tube provided with the CellSearch Kit and mixed with dilution buffer, centrifuged at 800×G for 10 minutes, and then processed on the CellTracks AutoPrep System within 1 hour of sample preparation. The CellTracks AutoPrep System is a fully automated sample preparation device for CTC capture and staining.
Following centrifugation, plasma and buffer layers were aspirated automatically and ferrofluids added. After incubation and magnetic separation, the plasma and unlabeled cells were aspirated, and the target cells resuspended in buffer. The immunomagnetically labeled cells were added to permeabilization buffer along with staining reagents to fluorescence label the cells and incubated. Magnetic separation was repeated, and excess staining reagents aspirated.
The reagent/sample mixture was dispensed by the CellTracks AutoPrep System into a cartridge that is inserted into a MagNest cell presentation device, comprising a single cartridge containing two magnets. The magnetic field of the MagNest device attracted the magnetically labeled epithelial cells to the surface of the cartridge. The CellTracks Analyzer II (a semiautomated four-color fluorescence microscope) automatically scanned the entire surface of the cartridge, acquired images, and displayed collocated events of CK-PE and DAPI fluorescence. Images were presented in a gallery format for final classification. An event was classified as a tumor cell when its morphological features were consistent with that of a tumor cell demonstrating DAPI+ nuclear and intracellular CK+ staining (positive cytokeratin 8, 18, 19-Phycoerythrin staining), positive staining for EpCAM+, but lack of expression of CD45 (negative CD45-Allophycocyanin staining), hence, a phenotype characterized by EpCAM+, CK+, DAPI+, CD45-. HER2/_neu_-expressing tumor cells were defined by characteristic morphological features of an epithelial tumor cell with HER2/neu staining and EpCAM+, CK+, DAPI+, CD45-phenotype. Results were reported as the number of CTC/20 mL of whole blood. All CTC enumeration and phenotyping was conducted by researchers blinded to the knowledge of clinical data.
Statistical Analysis
Demographic and categorical parameters were compared using an independent sample t test (not assuming equal variance), Mann-Whitney, or the χ2 test with Yates’ correction as appropriate. Mean levels of CTCs, DTH reactions, and in vitro immunological response assays prevaccination and postvaccination were compared by paired two-tailed t test. A _P_-value <.05 was considered statistically significant in all comparisons.
RESULTS
Analysis of CTCs and HER2/neu + CTCs in Disease-Free Patients with Breast Cancer
Sixteen consecutive disease-free patients with NP breast cancer treated with standard multimodality therapy were selected to enumerate total and HER2/neu + CTCs per 20 mL of blood during vaccination. Clinical and pathological characteristics of this patient population are shown in Table 1.
TABLE 1. Demographics and pathological tumor characteristics in 16 breast cancer patients at high risk of recurrence who were rendered disease-free with standard multimodality therapy and who then underwent vaccination with the HER2/neu-derived peptide E75
Total CTCs (1 or more) were detected in 14 (87.5%) of these patients (mean: 3.4 ± 0.2 CTC/20 mL). Thirteen of the 16 patients (81.3%) had at least one HER2/neu + CTC (mean: 2.1 ± 0.1 CTC/20 mL). Importantly, multiple (two or more) CTCs were identified in 10 of the 16 patients (62.5%) (Fig. 1a). Eight (50%) patients had multiple HER2/neu + CTCs detected (Fig. 1b).
FIG. 1.
(a) Detectable (at least one) and multiple (≥2) CTC/20 mL of peripheral blood in 16 disease-free NP patients with breast cancer. (b) Detectable (at least one) and multiple (≥2) HER2/neu + CTC/20 mL of peripheral blood in 16 disease-free NP patients with breast cancer.
Correlation of CTC Number with Established Clinical Prognostic Factors in Breast Cancer
To correlate the number of CTCs with known clinical prognostic factors, we analyzed the nine patients that had peripheral blood drawn for CTC enumeration prior or early in the vaccination series. Patients were empirically divided into two groups for analysis: those having 0–1 CTC/20 mL (CTC-Low, n = 4) and those with ≥2 CTC/20 mL (CTC-High, n = 5) (Table 2).
TABLE 2. Prognostic factors in patients with Low- versus High-CTC per 20 mL of blood prior to completion of the vaccination series
By definition, the CTC-Low patients had significantly lower number of total CTCs (median: 1 CTC/20 mL, range: 0–1) than the CTC-High group (median: 7 CTC/20 mL, range: 2–14, P = .02). The CTC-Low group also had fewer HER2/neu + CTCs (median: 0.5 CTC/20 mL, range: 0–1) than the CTC-High group (median: 5 CTC/20 mL, range: 2–9, P = .02).
Correlations with established clinical prognostic factors revealed significantly larger primary breast cancers in the CTC-Low group compared with the CTC-High group (T2–T4 tumors: 100% for CTC-Low, 20% for CTC-High, P = .048). There was no significant difference in the mean time from previous treatment to measurement of CTCs between groups (CTC-Low: 17 ± 8 months vs CTC-High: 10 ± 8 months, P = .57). Interestingly, a trend was also demonstrated for declining number of HER2/neu + CTC/20 mL as primary tumor HER2/neu expression (as determined by IHC) increased (Fig. 2).
FIG. 2.
Number of HER2/neu + CTC/20 mL based on HER2/neu IHC expression category (1–3+); bars indicate median number of HER2/neu + CTCs in each group.
Levels of CTCs and HER2/neu + CTCs in Unmatched Patients Before and After HER2/neu Vaccination
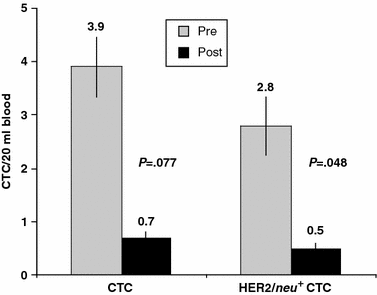
As an initial indicator of the potential impact of HER2/neu peptide vaccination on the number of CTCs, a group of patients (n = 9) was assessed prior to the vaccination series and compared with a separate group of patients (n = 7) who had previously completed the vaccination series. A reduction in total CTCs was detected when the two groups were assessed (mean total CTC/20 mL: 3.9 ± 1.5 vs 0.7 ± 0.4, P = .077). A statistically significant reduction was noted for HER2/neu + CTCs in the same unmatched group of patients prevaccination and postvaccination (mean HER2/neu + CTC/20 mL: 2.8 ± 1.0 vs 0.5 ± 0.2, P = .048) (Fig. 3).
FIG. 3.
Total and HER2/neu + CTCs in unmatched group of 16 disease-free patients with breast cancer prevaccination and 6ix or more months postvaccination with E75 peptide.
Hence, not only were enumerated CTCs reduced over the course of vaccination, but perhaps more importantly, a significant reduction in HER2/_neu_-expressing CTCs was observed over the same period in patients vaccinated with HER2/neu protein-derived immunogenic peptide.
Levels of CTCs and HER2/neu + CTCs Serially Measured During the HER2/neu Vaccination Series
A subset of nine patients had blood drawn to enumerate CTCs at multiple time points over the course of the vaccination series. CTCs were detected in eight of nine (88.9%) disease-free patients prior to vaccination (mean: 4.8 ± 0.5 CTC/20 mL) following completion of standard breast cancer therapy. One (11.1%) patient had no CTCs detected, but seven patients (77.8%) had two or more identified. All eight (88.9%) patients with identified CTCs demonstrated HER2/neu + CTCs (mean: 2.7 ± 0.3 CTC/20 mL).
For this subset of nine patients, all but two patients demonstrated a decrease in total CTCs over the course of the vaccination series, while the remaining two patients showed stable numbers of 0 and 1 CTC, respectively (Fig. 4).
FIG. 4.
Total CTCs in the subset of nine patients that had blood drawn over the course of the vaccination series. R0, prior to series initiation; R3, during series; R6, completion of series; RC6, 6-month follow-up after completion.
When the same nine-patient subset is compared for CTCs at early versus late time points in a matched or paired analysis, a significant reduction in both total (mean total CTC/20 mL: 4.8 ± 1.5 vs 0.3 ± 0.2, P = .009) and HER2/neu + CTC (mean HER2/neu + CTC/20 mL: 3.0 ± 0.9 vs 0.4 ± 0.2, P = .013) (Fig. 5) is seen.
FIG. 5.
Total and HER2/neu + CTCs in a group of nine disease-free patients with breast cancer that had blood drawn for CTCs over the course of the vaccine series. Matched or paired analysis of early versus late time points.
Temporal Correlation in Reduction of CTC Numbers with In Vitro and In Vivo Immune Response to the HER2/neu Vaccine
To demonstrate an immunologic response to vaccination, all patients had a DTH reaction assessed. The nine patients demonstrating a significant reduction in total and HER2/neu + CTCs also displayed a significant increase in DTH in response to vaccination. At the same time that the CTCs were being followed, these patients developed DTH reactions that averaged 22.2 ± 3.8 mm compared with 2.8 ± 2.0 mm in controls (P = .0001; Fig. 6A). The ELISPOT assat was used to investigate the number of E75 peptide-specific IFN-γ-producing peripheral blood mononuclear cells (PBMC). The functional ELISPOT assay performed on fresh, ex vivo, unstimulated PBMC demonstrated an increase in E75-specific IFN-γ-producing cells after vaccination in 6 of 14 patients (Fig. 6B). The phenotypic HLA-A2:Ig dimer assay was used to assess vaccine-specific immunologic response. Four of the six patients with dimer assay data and enumerated CTCs early and late in the vaccination series demonstrated clonal expansion of E75-specific CD8+ T cells (median 0.36–0.65%) and CTCs (median 4.5–0 per 20 mL) (Fig. 6C).
FIG. 6.
Immune response monitoring among patients with CTCs measured. Panel (a) shows all vaccinated patients’ postvaccine DTH response to E75 (22.2 ± 3.8 mm) compared with saline control (2.8 ± 2.0 mm; P = .0001), demonstrating increased in vivo response to the vaccine. Panel (b) shows peptide-specific IFN-γ-secreting cells at baseline and maximum postvaccine response as measured by functional ELISPOT assay. Panel (c) shows clonal expansion of E75-specific CD8+ T cells in individual patients early and late in the vaccination series. E75 peptide-specific CD8+ T cells are shown by E75 peptide–loaded HLA-A2:Ig dimer staining and flow cytometric analysis; % of all CD8+ T cells that are E75-specific are shown per patient. The panel shows responses to vaccine with E75-specific T-cells on left side and the same patients’ quantified CTCs on right side measured at the same time points.
DISCUSSION
The current pilot study was undertaken to assess the potential for expanded utility of an assay to enumerate CTCs in disease-free NP breast cancer patients who have completed conventional multi-modality cancer therapy. This exploratory study also determined the relationship between E75 peptide vaccination and total and HER2/neu + CTC number. The findings of the present study indicate that CTCs are detectable using the CellSearch System for the isolation, enumeration, and phenotypic characterization of CTCs in patients without clinical evidence of breast cancer but who are at high risk of recurrence. A significant reduction in not only total CTCs but also in HER2/neu + CTCs was demonstrated over the course of vaccination in patients immunized with the E75-peptide vaccine. These preliminary data suggest that enumeration of CTCs may serve as a surrogate marker for monitoring immunologic response in studies of vaccines targeting breast cancer. More importantly, this assay system may be the first clinically relevant monitoring tool for vaccine trials.
One difficulty encountered by investigators administering vaccine trials is how best to monitor clinically relevant biological activity of these peptide vaccines. Presently, immune assays performed with peripheral blood cells are used to assess the specific type and magnitude of T-cell responses over multiple time points in cancer vaccine trials. The majority of currently employed immunologic assays have significant limitations. For example, the requirement for in vitro T-cell expansion to assess T-cell responses in cytotoxity/proliferation assays limits the sensitivity of this method of assessing peptide-specific immunological response. Second-generation ex vivo functional T-cell assays (dimer/multimer, ELISPOT, cytokine flow cytometry) too are limited by lack of sensitivity given potentially interfering background cytokines and inconsistent assay conditions.14 Third-generation ex vivo multivariable cytometry assays provide phenotypic and functional aspects of detected antigen-specific T-cell subset populations; however, this form of sophisticated monitoring for immune response remains to be correlated definitively with clinical endpoints.
Hence, the various immune assays presently in use lack standardization, are constrained by considerable variability, and demonstrate limited sensitivity. A recent Cancer Vaccine Clinical Trial Working Group consensus conference addressed shortcomings of present-day immune assays and recommended criteria, though imperfect, for assessing immune response (e.g., two or more assays at three time points).15 No single immune assay or combination of assays currently in use establishes efficacious peripheral blood immune end points that correlate with clinical outcomes. Clearly, new approaches are needed to reliably identify in vivo correlates of peptide vaccine-induced immune responses.15
The quantity and phenotype of CTCs may prove to be surrogate markers for clinical efficacy in patients with minimal residual disease administered a peptide vaccine. CTCs are present in nearly 40% of patients with NN breast cancer, and peripheral blood tumor cell persistence has been shown from 7 to 22 years after treatment in low-risk breast cancer patients with no clinical evidence of disease.16,21 Antitumor immunity is one important component of complex mechanisms maintaining tumor dormancy. A CTC level ≥ 5 CTC/7.5 mL peripheral blood prior to beginning treatment and at first follow-up visit has been proven to be an independent adverse prognostic factor in patients with metastatic breast cancer.17 Moreover, after initiation of systemic chemotherapy for metastatic breast cancer, ≥5 CTC/7.5 mL peripheral blood at time of restaging are not only independently associated with adverse progression-free and overall survival, but are also predictive of reduced efficacy of systemic therapy.18 Importantly, the finding that elevated levels of CTCs ≥5/7.5 mL of blood at any time during systemic treatment of metastatic breast cancer is a reliable predictor of disease progression and mortality has established enumeration of CTCs as a standard of practice for patients with advanced breast cancer.19
Although multiple reports have demonstrated the prognostic and predictive value of CTCs in advanced breast cancer, their role in clinically disease-free patients at risk for recurrence is undetermined.17–19 Although preliminary, two important findings in this study warrant further investigation. The inverse correlation between tumor size and circulating tumor cell number suggests that CTCs may represent a reflection of aggressive tumor biology rather than primary tumor burden. The surprising number of HER2/neu + CTC/20 mL in patients with IHC-determined 1–2+ HER2/_neu_-expressing primary tumors suggests that not only patients with tumors overexpressing HER2/neu (3+), but also those with any degree of HER2/neu expression (1–3+) may be suitable candidates for a preventive peptide vaccine. Our data suggest a potential role for monitoring CTCs in clinically disease-free patients administered a peptide vaccine. Given the failings of current immune response monitoring techniques, the possibility that CTCs may also be used either for monitoring response to vaccination or predicting clinically efficacious immune response is intriguing. Moreover, the ability to phenotype tumor cells in peripheral blood may have important implications in terms of patient selection for breast cancer immunotherapy based on predicted peptide-vaccine-induced antitumor immunity.
The current pilot study provides proof-of-principle that CTCs can be enumerated and HER2/neu phenotyping can be performed in NP patients clinically free of disease following standard therapies including surgery, chemotherapy, immunotherapy, and radiation. The finding that total and HER2/neu + CTCs are reduced significantly in disease-free patients over the course of a vaccination series suggests that peripheral blood CTCs may be a useful way to monitor immune response to peptide vaccination. Though beyond the scope of the present study, it is also possible that CTCs may be useful in identifying patients that may benefit from preventive vaccination; CTCs may thereby improve patient selection for ongoing clinical trials. Furthermore, CTCs may have a role in predicting disease recurrence in vaccinated patients. Ongoing Phase II trials will provide the opportunity to critically evaluate these potential roles for CTCs monitoring.
References
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244:707–12
Article PubMed CAS Google Scholar - Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci USA 1995; 92:432–6
Article PubMed CAS Google Scholar - Disis ML, Schiffman K, Guthrie K, et al. Effect of dose on immune response in patients vaccinated with an HER-2/neu intracellular domain protein-based vaccine. J Clin Oncol 2004; 22:1916–25
Article PubMed CAS Google Scholar - Murray JL, Przepiorka D, Ioannides CG. Clinical trials of HER-2/neu-specific vaccines. Semin Oncol 2000; 27 Suppl 11:71–5
PubMed CAS Google Scholar - Correa I, Plunkett T. Update on HER-2 as a target for cancer therapy: HER2/neu peptides as tumour vaccines for T cell recognition. Breast Cancer Res 2001; 3:399–403
Article PubMed CAS Google Scholar - Mittendorf EA, Gurney JM, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Vaccination with a HER2/neu peptide induces intra- and inter-antigenic epitope spreading in patients with early stage breast cancer. Surgery 2006; 139:407–18
Article PubMed Google Scholar - Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol 2005; 23:7536–45
Article PubMed CAS Google Scholar - Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med 1995; 181:2109–17
Article PubMed CAS Google Scholar - Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res 1998; 58:4902–8
PubMed CAS Google Scholar - Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin Cancer Res 2002; 8:1014–8
PubMed CAS Google Scholar - Murray JL, Gillogly ME, Przepiorka ME, et al. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res 2002; 8:3407–18
PubMed CAS Google Scholar - Peoples GE, Khoo S, Dehqanzada ZA, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for prevention of recurrence in high-risk breast cancer patients. Breast Cancer Res Treat 2006; 100 (Suppl 1):S6(Abstract 4)
Google Scholar - Woll MM, Fisher CM, Ryan GB, et al. Direct measurement of peptide-specific CD8+ T cells using HLA-A2:Ig dimer for monitoring the in vivo immune response to a HER2/neu vaccine in breast and prostate cancer patients. J Clin Immunol 2004; 24:449–61
Article PubMed CAS Google Scholar - Keilholz U, Martus P, Scheibenbogen C. Immune monitoring of T-cell responses in cancer vaccine development. Clin Cancer Res 2006; 12:2346s–52s
Article PubMed CAS Google Scholar - Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother 2007; 30:1–15
Article PubMed Google Scholar - Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch System. Clin Cancer Res 2007; 13:920–8
Article PubMed CAS Google Scholar - Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351:781–91
Article PubMed CAS Google Scholar - Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005; 23:1420–30
Article PubMed Google Scholar - Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006; 12:4218–24
Article PubMed CAS Google Scholar - Kahn HJ, Presta A, Yang LY, et al. Enumeration of circulating tumor cells in the blood of breast cancer patients after filtration enrichment: correlation with disease stage. Breast Cancer Res Treat 2004; 86:237–47
Article PubMed Google Scholar - Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 2004; 10:8152–62
Article PubMed Google Scholar