Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity (original) (raw)
Research Paper
, Alexander C. McFarlane, Robyn L. Bluhm, Kathryn A. Moores, C. Richard Clark, Marnie E. Shaw, Peter C. Williamson, Maria Densmore and Ruth A. Lanius
J Psychiatry Neurosci July 01, 2010 35 (4) 258-266; DOI: https://doi.org/10.1503/jpn.090175

Abstract
Background: Working memory processing and resting-state connectivity in the default mode network are altered in patients with post-traumatic stress disorder (PTSD). Because the ability to effortlessly switch between concentration on a task and an idling state during rest is implicated in both these alterations, we undertook a functional magnetic resonance imaging study with a block design to analyze task-induced modulations in connectivity.
Methods: We performed a working memory task and psychophysiologic interaction analyses with the posterior cingulate cortex and the medial prefrontal cortex as seed regions during fixation in 12 patients with severe, chronic PTSD and 12 healthy controls.
Results: During the working memory task, the control group showed significantly stronger connectivity with areas implicated in the salience and executive networks, including the right inferior frontal gyrus and the right inferior parietal lobule. The PTSD group showed stronger connectivity with areas implicated in the default mode network, namely enhanced connectivity between the posterior cingulate cortex and the right superior frontal gyrus and between the medial prefrontal cortex and the left parahip-pocampal gyrus.
Limitations: Because we were studying alterations in patients with severe, chronic PTSD, we could not exclude patients taking medication. The small sample size may have limited the power of our analyses. To avoid multiple testing in a small sample, we only used 2 seed regions for our analyses.
Conclusion: The different patterns of connectivity imply significant group differences with task-induced switches (i.e., engaging and disengaging the default mode network and the central-executive network).
Introduction
Posttraumatic stress disorder (PTSD) is characterized by disturbances in concentration and memory1 that have been linked to underlying alterations in working memory performance compared with both healthy controls with no exposure to trauma2 and healthy controls with trauma exposure.3–5 In a recent article, data were presented that indicate abnormal recruitment of network regions involved in working memory updating during a simple working memory maintenance task in patients with PTSD.6 Subtraction analyses of these data supported the notion that attending to simple working memory tasks, like those requiring only maintenance, demand a greater effort in PTSD patients than in healthy controls; this possibly explains the concentration problems described in the DSM-IV diagnostic criteria for PTSD. Studies also have connected PTSD symptomatology with diminished connectivity of the default mode network during rest.7,8 Because the ability to effortlessly switch between concentration on a task and an idling state during rest may be implicated in both these alterations, we undertook a functional magnetic resonance imaging (fMRI) study with a block design and a comparatively short fixation condition to study the underlying functional connectivity of areas in the default mode network during a low-demand fixation condition and a complex task.
Whereas a previous neuroimaging study showed evidence of attenuated connectivity during the resting state among default mode network regions in PTSD patients during a relatively long resting-state condition,7 modulations in connectivity due to task-induced switching between default mode networks and central-executive and salience networks have yet to be studied. To examine the effects of working memory load on connectivity in these networks, we used psychophysiologic interaction analyses to examine connectivity with seed regions in the medial prefrontal cortex (mPFC) and the posterior cingulate cortex (PCC) in patients with severe, chronic PTSD and matched, healthy controls.
Recent neuroimaging studies have lead to the hypothesis that rest is characterized by an organized baseline level of activity that is attenuated during goal-oriented mental activity. It has been hypothesized that the brain maintains this “default mode” in the absence of cognitive demands,9–11 possibly to facilitate a state of readiness to respond to environmental changes.12 Other authors have linked default mode network activity to self-referential processing because key regions like the posterior cingulate PCC and mPFC have been shown to subserve introspective mental imagery, self-reflection and self-awareness.13–16 A recent meta-analysis17 identified various areas as components of the default mode network, such as the PCC, anterior cingulate cortex (ACC), middle temporal gyrus and mPFC. The stability of the default mode network across the lifespan18–20 as well as across different states (light sedation21), wakefulness and early stages of sleep22 has been shown, and the functional connectivity was matched by a computational model with high fidelity.23
Tasks that activate the executive network have been consistently shown to evoke decreased activation (deactivation) in the default mode network. McKiernan and colleagues24 showed that task-related deactivation increased with task difficulty. Two previous studies25,26 examined the connectivity of the default mode network during very demanding cognitive tasks and found significantly diminished functional connectivity within the default mode network under high working memory load.
Different groups have recently discussed the notion that the default mode network might comprise different subsystems.16,27 Uddin and colleagues28 revealed considerable differences by analyzing the anticorrelations of seed regions in the mPFC and PCC, suggesting that the activity of distinct nodes of the default mode network may differentially modulate activity in task-positive networks. They suggest that future research should therefore distinguish between these network components and analyze their connectivity separately.
Alterations in default mode network connectivity have emerged as possible markers for psychiatric disorders such as schizophrenia,29 social phobia,30 depression,31 bipolar disorder32 and autism.33 In PTSD, altered functional connectivity in default mode network regions has been shown using emotion-relevant paradigms such as facial affect perception and trauma script–driven imagery.34–36 A recent study carried out by our group analyzed functional connectivity of seed regions in the PCC and the mPFC separately during rest in PTSD patients and healthy controls.7 Direct comparison between groups showed significantly reduced connectivity among default mode network areas in the PTSD group. The PTSD group still showed some, although diminished, connectivity between the PCC seed region and the right superior frontal gyrus (Brodmann area [BA] 9) and left thalamus; however, connectivity of the mPFC seed region was strictly limited to adjunct areas in the mPFC. A prospective study in a group of acutely traumatized patients8 showed that resting-state connectivity of the PCC with the right amygdala predicts future PTSD symptoms, suggesting that the integrity of the default mode network is compromised in PTSD and that the extent of these deficits reflects clinical measures of PTSD. To extend our knowledge about the specificity of these alterations, experimental paradigms that manipulate the activity of the default mode network are needed.
In this study, we investigated the relation between the task-negative default mode network and task-positive networks involved in switching to working memory updating. It has been suggested that there are 2 differentiable task-positive networks: a “salience network” that includes the dorsal ACC and the orbitofrontal-insular cortices, and an “executive-control network” that connects the dorsolateral frontal cortex with the parietal cortex.37,38 Converging evidence for the executive system stems from studies describing the same network structures across a variety of executive-type tasks.39–43 The salience network, which encompasses the frontoinsular circuit and the anterior cingulate, is uniquely positioned to initiate the control of signals that activate the central-executive network.38,44–47 It has been linked to monitoring task performance48 and the modulation of arousal during cognitively demanding tasks.49
These networks mediate higher-order control and likely facilitate the disengagement of systems that are not task-relevant, including the default mode network.46,50 Recent studies have therefore emphasized the salience and executive networks as being critically involved in switching from an idling state into a task-oriented state.38,45,51,52 Therefore, we hypothesized that the relative engagement of these networks is central to the differences in the ability of PTSD patients to engage and disengage from tasks.
We sought to determine whether connectivity with the default mode network nodes differs significantly within the 2 groups between working memory task and the control condition and whether connectivity alterations between groups emerge when comparing the working memory task with the control condition in PTSD patients and healthy controls.
Based on recent publications indicating that the default mode network might exhibit more internal differentiation than generally assumed,28 it seemed plausible that the connectivity patterns for the 2 seed regions would also differ between the PTSD group and the control group. We therefore hypothesized that during the working memory task, both seed regions would show greater connectivity with areas of networks involved in attending to the task (such as the salience network and the executive network) in healthy controls than in PTSD patients.
Methods
Participants
We included 12 patients with PTSD and 12 controls who had not experienced trauma. We matched controls to patients based on years of education, occupational status and estimated verbal intelligence quotient based on the National Adult Reading Test.53 Participants were all right-handed and gave written informed consent to participate. We excluded those with head injury or loss of consciousness (> 1 h), major illness, hospitalization or general anesthetic (≤ 2 yr prior), epilepsy, neurologic, learning or developmental disorders, or current psychopathology diagnosed by use of the Diagnostic Interview Schedule–Screening Interview (DISSI54).
Psychiatrists at the Queen Elizabeth Hospital (Adelaide, South Australia) diagnosed PTSD according to DSM-IV criteria.55 We assessed comorbidity using the Composite International Diagnostic Interview (CIDI, DSM-IV Version 2.1; www.whocidi.org/instruments_papi.php), and we excluded patients with current panic disorder, lifetime psychosis or alcohol abuse or dependence within the preceding year.
Ethics approval was obtained for this study from the Social and Behavioural Research Ethics Committee at Flinders University and the Research Ethics Committee at the Royal Adelaide Hospital.
Psychological measures
Trained clinical interviewers measured PTSD symptom severity (Table 1) using the Clinician-Administered PTSD Scale (CAPS56) and the Impact of Event Scale (IES22). All participants completed the General Health Questionnaire (GHQ57), the Beck Depression Inventory (BDI58) and the State-Trait Anxiety Inventory (STAI59). For information about neuropsychological measures, please refer to a previous article on this data set.6
Table 1
Descriptive statistics for psychological adjustment measures for study participants
Activation and control tasks
We investigated the integrity of the default mode network during working memory processing to substantiate the link between default mode network alterations and PTSD pathology. We chose a 1-back working memory updating task because the neurofunctional substrate of working memory is not limited to prefrontal cortex activation but requires the concerted interplay of widespread interacting networks including the parietal cortex, subcortical regions and cerebellar areas. We used a visuoverbal target detection task that required participants to attend to a block of serially presented words on a computer monitor and to detect infrequent targets by making an appropriate finger response. The task manipulated working memory updating processes by using a flexible target identity. The working memory task required participants to attend to a serially presented set of words on a computer monitor and to detect infrequent targets by making an appropriate finger response. The target was defined as any consecutively repeated word, which required participants to continually update the target identity in working memory with each new word presented throughout the block.
The control condition was a simple fixation task, requiring attention either to the response instruction or to a line of 5 asterisks in the centre of the screen. We chose this control task to resemble the activation task as closely as possible; it therefore differed considerably from previous resting state analyses because it was relatively short in duration and thus necessitated fast switches between the control condition and the activation task. It also prompted the participants to keep their eyes open and fixated on the stimulus, which has been shown to result in stronger default mode network activations than the closed-eyes condition.60
Study design
To ensure frequent switching between an idling state and task-induced activation, we used a block design, presenting the activation task (8 volumes) twice interspersed with the fixation task (4 volumes) within each of 16 imaging runs. Each task was preceded by an instruction block (4 volumes duration), amounting to a total acquisition of 512 volumes per participant. The order of the working memory tasks was counterbalanced between runs and across participants. Full details of this working memory paradigm are provided in the study by Moores and colleagues.6 There were 2 variations of this task in each run concerning the elicited button press response; however, because we were interested in the effects of cognitive effort on default network connectivity, rather than specific effects associated with a particular variation of the task, we combined the response variations to model a single “task” condition for this study. The control condition consisted of periods of viewing either 5 asterisks in the centre of the screen or a notice of which variation of the task would be performed next.
The stimulus sequences consisted of 16 words for each block (4 words repeated 4 times) selected without replacement from a master list. The probability of any word including targets was 25%. The master list comprised 338 concrete nouns obtained from the MRC Psycholinguistic Database (version 2.0061) that met the following criteria: (a) 4–7 letters, (b) 2–3 syllables, (c) written frequency between 20 and 50 times62 and (d) no irregular plurals. Because we were investigating trauma-neutral information processing, we excluded words with emotive impact (128 words). The PTSD patients reviewed the reduced master list to exclude any words with personal emotive impact. Lowercase words were presented in colour (red, blue, green, yellow) at the centre of a black screen using Gentask software (Neurosoft Inc.). Word colour was not relevant for this study. Stimuli were generated in Arial font (150 point) and presented with horizontal (4.54°) and vertical (1.04°) visual angles. The fixation stimulus consisted of a row of 5 asterisks presented in the centre of a black screen, reflecting the average word length. Stimulus duration (including asterisks) was constant at 300 ms and stimulus onset asynchrony varied pseudorandomly around 4 seconds (standard deviation [SD] 0.2 s). Stimuli were rear projected using an EPSON EMP-3300 (Seiko Epson Corp.) onto a Daylite Insta-Theatre screen and viewed via a mirror located on the head coil.
Magnetic resonance image data acquisition
We collected MRI data on a Siemens VISION (Magnotom 4000) 1.5-T MRI scanner with a circularly polarized head coil. Two high-resolution _T_1-weighted sagittal structural MRI volumes were obtained (magnetization-prepared rapid acquisition with gradient echo sequence, repetition time [TR] 9.7 ms, echo time [TE] 4 ms, inversion time [TI] 200 ms, delay time 0 ms, flip angle 12°, field of view [FOV] 256 mm × 256 mm, matrix 256 × 256, 180 slices, 1 mm isovoxels, scan time 8 min 20 s) for each participant. We used a specialized gradient echo, echoplanar imaging trapezoidal mosaic sequence developed in the Functional Imaging Laboratory (Wellcome Department of Imaging Neuroscience, University College London, UK) in collaboration with Siemens. We acquired axial fMRI volumes every 3.494 seconds over the whole brain (80 acquisitions per run, total time 4 min 39 s) in 34 slices (TR 0.76 ms, TE 50 ms, TD1 [echo time] 20 ms, TD2 [measurement delay time] 188.2 ms, flip angle 90°, matrix 64 × 64, FOV 320 mm × 320 mm, pixel size 5 mm × 5 mm, slice thickness 4 mm with a 1-mm interslice gap yielding 5 mm 3 isovoxels). No stimuli were presented during the acquisition of the first 3 volumes of each run while steady-state magnetization was achieved.
Preprocessing of fMRI data
We performed image preprocessing steps and statistical analysis using Statistical Parametric Mapping (SPM2, Well-come Department of Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm). We processed single participant data using standardized preprocessing steps (motion detection, realignment, spatial normalization, Gaussian smoothing at 10 mm full-width at half-maximum isotropic Gaussian filter) and a general linear model. Global scaling removed differences common to the whole brain within and between sessions in global signal intensity.63 Recently, the use of this preprocessing step in studies examining connectivity has been a subject of discussion. Murphy and colleagues64 and Weissenbacher and colleagues65 argue that it could be the sole cause of anti-correlated resting state networks in functional connectivity analyses. We decided to use global scaling because we were not analyzing anticorrelations in this paradigm and because data presented by Fox and colleagues66 and Weissenbacher and coworkers65 indicate that global scaling enhances the detection of system-specific correlations and doubles connection specificity. Weissenbacher and colleagues65 compared different preprocessing approaches in human and simulated data sets and recommend applying global scaling to maximize the specificity of positive resting-state correlations. We used high-pass filtering with a cut-off at 128 seconds to minimize the impact of serial autocorrelations in the fMRI time series that can result from scanner drift.
Psychophysiological interaction analysis
We conducted novel analyses on a sample of patients who had been scanned for a previous study.6 In the current study, we used psychophysiological interaction analyses to examine alterations in the connectivity between each of 2 seed regions (the PCC and mPFC, both nodes of the default mode network) and the rest of the brain in PTSD patients and matched healthy controls. Psychophysiological interaction analyses are designed to measure context-sensitive changes in effective connectivity between one or more brain regions67 by comparing connectivity in one context (in the current study, a working memory updating task) with connectivity during another context (in this case, a fixation condition). We used seed regions in the mPFC and PCC because both these nodes of the default mode network act independently across different cognitive tasks, might subserve different subsystems within the default mode network and have both been associated with alterations in PTSD.8
We performed connectivity analyses using the psycho-physiological interaction analysis methods implemented in SPM2. For each participant, an average time course was extracted from the 2 seed regions of interest, defined as a 10-mm sphere around coordinates derived from a previous study of the default mode network.68 The PCC analysis was centred at Montreal Neurological Institute (MNI) coordinates (x, y, z) −6, −50, 36 and the mPFC analysis at 0, 50, 0. We conducted each psychophysiological interaction analysis individually for each participant and 2 seed regions. The resulting contrast images derived from these analyses were then entered into 2-sample t tests comparing PTSD patients with healthy controls. Group comparisons of the psychophysiological interaction analyses were thresholded at a cluster size of k > 5 and p = 0.001 (uncorrected).
To detect differences in default mode network connectivity unrelated to the working memory task, we also analyzed the connectivity between the default network areas during the control condition alone using volume of interest–based correlations that were thresholded at p = 0.001 (false discovery rate [FDR]–corrected).
We also examined connectivity during each of the rest and task blocks separately using the method described by Fair and colleagues42 to isolate the images from each condition. We then correlated activity in these images with activity in the seed region to determine whether the seed region was positively or negatively correlated, during each of rest and task, with areas showing significant changes in connectivity in the psychophysiological interaction analysis.
Throughout this article, brain areas are identified as part of the different networks on the basis of coordinate comparisons with areas previously implicated with the default mode network by Spreng and colleagues17 and Uddin and colleagues28 and with the salience and executive network by Seeley and coworders38 and Sridharan and coworkers.46 We considered any region to be part of the corresponding network if the published coordinate was within 2 cm of the peak identified by our analysis (or part of a larger cluster encompassing it) as well as within the same neuroanatomical structure. We converted MNI coordinates to Talairach space as necessary using BioImage Suite (www.bioimagesuite.org/Mni2Tal/index.html).
Results
Participants
We collected data from 12 patients with PTSD (7 men, 5 women; mean age 44.83, SD 9.32 yr) and 12 controls who had not experienced trauma (7 men, 5 women; mean age 40.41, SD 10.93 yr). The controls were matched to patients based on years of education, occupational status and estimated verbal intelligence quotient (PTSD: mean 110.83, SD 4.74; controls: mean 113.75, SD 5.24; _t_22 = −1.429, p = 0.167).
The mean duration of PTSD in the patients was 6.8 years, and 4 PTSD patients showed delayed onset of symptoms (≥ 6 mo). Precipitating traumas included assault (4), witnessing human injury or death (7) and motor vehicle (2) and other accidents (1). Current comorbidities included major depressive disorder (3), agoraphobia (1), nicotine dependence (3) and somatoform disorders (3). Five patients were taking psychoactive medication, typically selective serotonin reuptake inhibitors. One patient was taking fluoxetine, 2 were taking sertraline and 2 were taking citalopram, with one of these patients also prescribed diazepam and quetiapine. No control participants were taking medication, and participants in both groups denied recreational drug use during an initial screening interview and a diagnostic interview.
The PTSD sample had an average IES total score of 59.91 and CAPS total severity score of 74.20, indicating severe PTSD (Table 1). Compared to those in the control group, the PTSD patients had higher depression scores (BDI _t_21 = 4.83, p < 0.001), state (STAI _t_21 = 4.03, p = 0.001) and trait (STAI _t_21 = 4.42, p = 0.001) anxiety and generalized psychological distress (GHQ _t_21 = 6.10, p < 0.001).
Within-group analyses
The results of our within-group functional connectivity analyses for the 2 groups are presented in Table 2. In the healthy control group, no brain areas showed greater connectivity with the mPFC seed region during the working memory task than during the control condition (p > 0.001). The PCC seed region showed significantly greater connectivity with a number of adjunct areas as well as with the inferior frontal gyrus (BA 9). Also, a number of areas implicated in the salience and executive networks showed enhanced connectivity with the PCC, including the left superior temporal gyrus (BA 22), right inferior frontal gyrus (BA 47) and right inferior parietal lobule (BA 39).
Table 2
Within-group results* for the working memory task versus the control condition
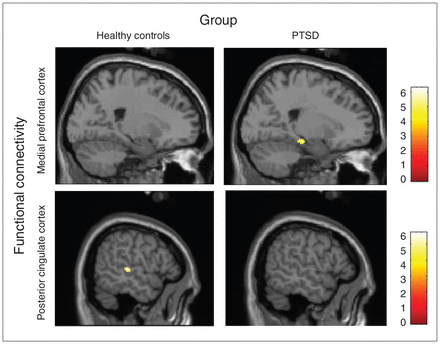
In the PTSD group, the bilateral parahippocampal gyri (BA 28, 30), left middle occipital gyrus (BA 19) and left medial frontal gyrus (BA 6) showed significantly stronger connectivity with the mPFC seed region during the working memory task compared with the control condition. No areas were significantly connected with the PCC seed region (Fig. 1).
Fig. 1
Functional connectivity with the medial prefrontal cortex and the posterior cingulate cortex seed regions (thresholded at p = 0.001, uncorrected). The patients with posttraumatic stress disorder (PTSD) showed significant connectivity with the parahippocampal gyrus during the working memory updating task. The control group showed significant connectivity with the superior temporal gyrus during the working memory updating task.
Between-group analyses
During the working memory task, compared with the control condition, the healthy control group showed significantly stronger connectivity than the PTSD group (p < 0.001) with areas implicated in the salience and executive networks (Table 3, Table 4). The mPFC seed region was significantly more connected with the orbital gyrus (BA 11) and the pre-supplementary motor area (superior frontal gyrus, BA 6). The PCC seed region was functionally connected with areas in the left middle frontal gyrus (BA 8, 9), the left inferior frontal gyrus (BA 9) and the left middle frontal gyrus (BA 10). It also showed significantly greater connectivity with adjunct areas as well as with the superior temporal gyrus (BA 22).
Table 3
Between-group results* for areas showing significant connectivity with the posterior cingulate for the working memory task versus the control condition
Table 4
Between-group results* for areas showing altered connectivity with the medial prefrontal cortex for the working memory task versus the control condition
In contrast, the PTSD group showed stronger connectivity with areas previously implicated in the default mode network than did the healthy control group during the working memory task versus the control condition (Table 3, Table 4), namely enhanced connectivity between the PCC seed region and the right superior frontal gyrus (BA 10) and between the mPFC seed region and the left parahippocampal gyrus (BA 35). The PTSD group also exhibited significantly greater connectivity between the PCC and the left fusiform gyrus (BA 19, 37) and the right medial frontal gyrus (BA 10). The mPFC seed region had a significantly stronger connection with the left fusiform gyrus (BA 20) and the left hippocampus.
Correlations during the control condition
To determine if these group differences originated from underlying differences during the control condition and therefore might exist independently of the engagement and disengagement necessary during the trial, we analyzed brain areas significantly correlated with the 2 seed regions during the control condition. Both groups showed a number of default mode network areas significantly correlated with the seed regions (p < 0.001, FDR-corrected). In the control group, these included the anterior (BA 32) and posterior (BA 29) cingulate, the medial frontal gyrus (BA 10) and the superior temporal gyrus (BA 22). In the PTSD patients, significant correlations with the precuneus (BA 7, 31), medial frontal gyrus (BA 10) and superior temporal gyrus (BA 38) were observed. Whereas there were no statistically significant differences between the groups, it is noteworthy that the PTSD group also exhibited significant correlations with a number of brain areas involved in switching between the default mode network and task-positive networks (i.e., the salience and executive networks) that were absent in the control group. These areas included the right inferior frontal gyrus (BA 45, 47), the precentral gyrus (BA 9) and the inferior parietal lobule (BA 40).
Discussion
We focused on 2 nodes of the default mode network, the PCC and the mPFC, and we investigated alterations in connectivity patterns associated with a working memory task and a control condition in patients with severe, chronic PTSD and healthy controls. Inspection of the within-group results for the 2 seed regions revealed striking differences in the underlying functional connectivity during working memory processing. Whereas the healthy control group showed greater connectivity between the PCC and frontal (right inferior frontal gyrus), temporal (superior temporal gyrus) and parietal (right inferior parietal lobule) regions implicated in switching between these states, the PTSD group did not show enhanced connectivity with any regions during updating. Notably, the opposite pattern occurred for the mPFC seed region. Here the healthy controls showed no enhanced functional connectivity during the working memory task, whereas the PTSD patients had greater connectivity between the mPFC seed region and the bilateral parahippocampal gyri, left middle occipital gyrus and left medial frontal gyrus. Because the parahippocampal gyri are considered part of the default mode network,17,27,28 these data indicate enhanced connectivity in the anterior part of the default mode network during the working memory task in the PTSD group. This failure to suppress default mode activity during tasks has been linked to decreased activity in task-related regions,69 attentional lapses and decrements in performance.63,70
Additional areas that showed significantly greater connectivity with the mPFC during the task in the PTSD group included the left middle occipital gyrus (BA 19) and left medial frontal gyrus (BA 6). This is possibly linked to their role in processing working memory tasks71 and facilitating the response finger movements.72,73 The connectivity between the PCC and the inferior frontal gyrus exhibited by the control group is in line with previous studies linking it to the execution of working memory tasks.74
Taken together, these within-group data indicate that the PTSD group has enhanced connectivity within the default mode network during tasks compared with rest and that they have strikingly different connectivity patterns with regard to the 2 seed regions.
The general trend of these results was supported by the between-group statistics. Whereas the PTSD group showed greater connectivity between the seed regions and default mode network areas during the working memory task (i.e., superior frontal gyrus, parahippocampal gyrus), the healthy control group successfully suppressed the default mode network during the task, as indicated by significantly stronger connectivity between areas known to be part of the salience and executive networks and the 2 seed regions. The mPFC seed region was significantly more connected with the orbital gyrus, which is considered to be part of the executive network, and the supplementary motor area (superior frontal gyrus), which is implicated in the salience network. The PCC seed region was functionally connected with areas in the left middle frontal gyrus and the left inferior frontal gyrus, which is implicated in the executive network,38 and the left middle frontal gyrus, which is implicated in the salience network.38 In addition, the superior temporal gyrus showed enhanced connectivity in the control group, most likely owing to the verbal nature of the working memory task used in this study.75
The PTSD group also showed significantly stronger connectivity with the left fusiform gyrus. Different locations within the fusiform gyrus have been identified as default mode network areas;17,27 however, the cluster identified by our analysis does not encompass the published peaks. The fusiform gyrus has also repeatedly been shown to exhibit abnormal activity in PTSD and other anxiety disorders related to its role in emotion processing,76–79 so proper interpretation of this finding will necessitate further research.
Taken together, these results not only support the hypothesis that there is greater heterogeneity in the default mode network than generally assumed, but also that PTSD patients show a striking pattern of connectivity alterations compared with controls. These alterations can best be described as an imbalance concerning the 2 seed regions, with enhanced connectivity with the anterior part of the default mode network and diminished connectivity with the posterior part.
We found no significant group differences when comparing the brain areas significantly correlated with the 2 seed regions during the control condition. Therefore, the group differences during the task cannot be attributed to underlying differences independent of the working memory task. Nevertheless, it is noteworthy that the PTSD group exhibited significant correlations with a number of brain areas that are part of the salience and executive networks, which were absent in the healthy control sample (right inferior frontal gyrus, pre-central gyrus and inferior parietal lobule). In addition to their stronger connectivity within the default mode network during task, the PTSD patients also showed indications of sustained engagement of higher-order control regions during the control condition.
These different patterns of connectivity and the recruitment of substantially different neural networks between the medial prefrontal cortex and posterior cingulate between the controls and PTSD patients imply significant differences in their orientation during a working memory task and significant difficulties with the task-induced switches (i.e., engaging and disengaging the default mode network and the central-executive network).
Limitations
The limitations of our results predominantly relate to the PTSD sample studied. To investigate the long-lasting symptoms that accompany a significant reduction of the general level of functioning, we studied alterations in severe, chronic PTSD, which did not allow us to exclude patients taking medications. In addition, the small sample size might have limited the power of our analyses. To avoid multiple testing in a small sample, we only used 2 seed regions for our analyses. Future studies should add a resting state scan without any visual input to allow for comparison of default mode network connectivity during the short control condition and a longer resting state.
Conclusion
This study is the first to explore the underlying network connectivity in the context of alteration in working memory performance in PTSD, linking the previously separate research areas of functional connectivity during rest and working memory alterations. These results call for future studies that not only carefully explore differences between seed regions of interest but also focus on task-induced differences when studying functional connectivity alterations in PTSD.
Acknowledgements
This study was supported by the Australia Research Council (grant no. A00105227) and the National Health and Medical Research Council of Australia (grant no. 981270.
Footnotes
Competing interests: None declared for Drs. Daniels, Shaw, Williamson and Lanius and Ms. Densmore. Dr. McFarlane and his institute have received National Health and Medical Research Council (NHMRC) program and project grants. Dr. Bluhm has received payment for writing and reviewing the a manuscript from The University of Western Ontario as part of her postdoctoral fellowship. Drs. Moores’s and Clark’s institutions have received NHMRC grants.
Contributors: Drs. Daniels, McFarlane, Bluhm, Moores and Lanius designed the study. Drs. McFarlane, Moores and Clark and Ms. Densmore acquired the data, which all authors analyzed. Drs. Daniels, McFarlane, Bluhm and Lanius wrote the article. All authors reviewed the article and approved its publication.
Received December 2, 2009.
Revision received February 11, 2010.
Accepted February 11, 2010.