Cocaine cue–induced dopamine release in the human prefrontal cortex (original) (raw)
Research Paper
, Aryandokht Fotros, Paul Gravel, Kevin F. Casey, Kevin Larcher, Jeroen A.J. Verhaeghe, Sylvia M.L. Cox, Andrew J. Reader, Alain Dagher, Chawki Benkelfat and Marco Leyton
J Psychiatry Neurosci September 01, 2016 41 (5) 322-330; DOI: https://doi.org/10.1503/jpn.150207

Abstract
Background: Accumulating evidence indicates that drug-related cues can induce dopamine (DA) release in the striatum of substance abusers. Whether these same cues provoke DA release in the human prefrontal cortex remains unknown.
Methods: We used high-resolution positron emission tomography with [18F]fallypride to measure cortical and striatal DA D2/3 receptor availability in the presence versus absence of drug-related cues in volunteers with current cocaine dependence.
Results: Twelve individuals participated in our study. Among participants reporting a craving response (9 of 12), exposure to the cocaine cues significantly decreased [18F]fallypride binding potential (BPND) values in the medial orbitofrontal cortex and striatum. In all 12 participants, individual differences in the magnitude of craving correlated with BPND changes in the medial orbitofrontal cortex, dorsolateral prefrontal cortex, anterior cingulate, and striatum. Consistent with the presence of autoreceptors on mesostriatal but not mesocortical DA cell bodies, midbrain BPND values were significantly correlated with changes in BPND within the striatum but not the cortex. The lower the midbrain D2 receptor levels, the greater the striatal change in BPND and self-reported craving.
Limitations: Limitations of this study include its modest sample size, with only 2 female participants. Newer tracers might have greater sensitivity to cortical DA release.
Conclusion: In people with cocaine use disorders, the presentation of drug-related cues induces DA release within cortical and striatal regions. Both effects are associated with craving, but only the latter is regulated by midbrain autoreceptors. Together, the results suggest that cortical and subcortical DA responses might both influence drug-focused incentive motivational states, but with separate regulatory mechanisms.
Introduction
The prefrontal cortex (PFC) integrates and interprets sensory information, processing value, directing attention, and planning actions based on experience.1,2 In cocaine-dependent individuals, these regions and processes are engaged by drug-related cues, increasing the likelihood of craving and drug pursuit.3–5 For each of these effects, dopamine (DA) is a plausible contributing transmitter. Mesocortical DA transmission plays a critical role in cognitive and executive functions,6 and, in laboratory animals, DA infusions into the PFC reinstate cocaine-seeking behaviours7,8 while DA depletion or receptor antagonists reduce the drug’s reinforcing efficacy.9 Based on these observations, it has been proposed that PFC abnormalities seen in cocaine-dependent individuals10 are related at least in part to experience-dependent changes in DA transmission.11,12
Dopamine release in the human PFC and striatum can be measured following an amphetamine challenge with positron emission tomography (PET) and the 18F-labelled tracer fallypride.13,14 More recently, the radioligand’s PFC sensitivity to nonpharmacological manipulations of the DA system has also been demonstrated using psychological stressors.15–17 Here, we used [18F]fallypride and high-resolution PET to assess the ability of drug-related cues to elicit DA release in the PFC of volunteers meeting criteria for current cocaine dependence. Given the role of DA in conditioned approach and incentive learning,18 we hypothesized that exposure to familiar, drug-related stimuli would enhance DA signalling in prefrontal and, in particular, orbitofrontal regions, contributing to the generation of motivational states that lead to drug seeking.5,19 Based on earlier reports identifying an association between PFC activation and self-reported craving,3,20,21 a positive correlation between PFC DA release and craving was predicted.
To test 2 additional hypotheses, we also quantified [18F]fallypride binding in the substantia nigra (SN) and ventral tegmental area (VTA). First, since midbrain D2 autoreceptors provide inhibitory feedback to mesostriatal DA cells but are not present on mesocortical cell bodies,22,23 we predicted that individual differences in midbrain [18F]fallypride binding values would correlate negatively with drug cue–induced striatal DA release but not cortical DA release; this would also serve as a further test of the physiologic validity of the measures. Second, as vulnerability to addiction-related behaviours has been linked to increased DA activity in the striatum24,25 and impaired D2 autoreceptor function,26–29 we predicted an influence of the SN/VTA on the expression of the cue-induced craving response. An integrated mechanism was then modelled for testing.
Methods
Participants
Our study participants have been described previously.30 They were non–treatment-seeking cocaine users who met DSM-IV criteria for current cocaine dependence and were recruited from the community through local advertisements. Following a brief telephone screening interview, each potential candidate was invited to an in-depth face-to-face evaluation using the Structured Clinical Interview for DSM-IV. Participants were free of current Axis-I psychiatric disorders other than substance use disorders, had never experienced head trauma with loss of consciousness, and were physically healthy, as determined by a medical examination, electrocardiogram, and standard laboratory tests. Women were excluded if they had a seropositive pregnancy test. Reflecting a growing interest in the covariates of symptoms rather than diagnoses, the present study focused on variability among those with cocaine use disorders rather than contrasting the group with cocaine-naive individuals. The study was carried out in accordance with the Declaration of Helsinki and approved by the Research Ethics Board of the Montreal Neurological Institute (MNI). All participants provided written, informed consent.
Procedures
Each participant underwent 1 MRI and 2 PET sessions carried out on separate days. Participants arrived at the MNI at approximately 10 am on each PET scan day. Collection of baseline subjective and physiologic measurements and drug screen tests lasted approximately 1 hour. Participants were asked to abstain from psychotropic drugs for at least 24 hours before the PET sessions, and on the morning of each test day, urine drug screens were administered (Triage Drugs of Abuse Panel, Biosite Diagnostics). Female participants all tested negative on a urine pregnancy test before each PET session (Assure FastRead hCG Cassette, Conception Technologies) and were scanned during the follicular phase of their menstrual cycles.
Two hours before scanning began on the neutral cue session, participants developed an autobiographical script with the investigator in which they recalled a relaxing, uneventful day that they could narrate in detail. The development and rehearsal of this script lasted for 30 min. They were then presented with paper clips, pencils, and erasers, asked to doodle or write a few sentences and erase them, and manipulate the paper clips. This object manipulation lasted about 15 min. Participants were then shown a 10-min video clip of people in everyday situations. Additional non–drug-themed videos were watched while lying on the PET bed.
Procedures were similar on the cocaine cue test session. As seen in previous studies, sustained craving states suitable for use during long PET scans can be evoked by a multimodal combination of personalized cues, the manipulation of drug paraphernalia, and viewing realistic depictions of drug use.12,30,38 Thus, participants developed an autobiographical script in which they described in detail a subjectively positive drug experience. Intranasal cocaine powder users were presented with a mirror, razor blade, straw, and bag of white powder (lactose). Crack cocaine users were provided with a crack pipe, spoon and rock-shaped crystal (salt). Participants were told that the substance was genuinely cocaine or crack. They were asked to use the razor to divide the powder into lines several times and to hold the straw, or to touch and smell the crystal and put it in the pipe or spoon. This object manipulation lasted for 15 min. For the following 10 min, participants watched a cocaine-themed video. Additional cocaine-themed videos were watched while lying on the PET bed through video glasses connected to a laptop. The videos showed images of people buying, using, and becoming intoxicated by cocaine (powder or crack depending on the participant’s preferred form of the drug), as well as images of the drug itself and drug paraphernalia.
Psychophysiological measurements (heart rate, blood pressure) were obtained continuously throughout the PET sessions using an automated sphygmomanometer. Following the cocaine cue session, participants were seen by a psychiatrist and debriefed before being released.
Positron emission tomography imaging
The PET scans were obtained using a Siemens high-resolution research tomograph (HRRT) with a spatial resolution range between 2.3 and 3.4 mm full-width at half-maximum. Each PET session consisted of a bolus injection of [18F]fallypride and 2 dynamic image acquisition scans (90 min and 60 min), separated by a 30-min break when participants could leave the scanner (Appendix 1, available at jpn.ca). This approach, designed to reduce participant discomfort during lengthy scans, has been widely used, and excellent test–retest reliability has been demonstrated.31 The potential influence of the break on the final binding estimates is deemed minimal as 1) the 90-min scan before the break is considered sufficient for an optimal cortical signal,32 2) scan durations longer than 120 min have no impact on cortical binding potential (BPND) values,33 and 3) our time-activity curves do not show marked postbreak deviations (Appendix 1, Fig. S3). Gantry lasers were used for repositioning. A 6-min 137Cs transmission scan for attenuation correction was performed before every emission scan session. The 33 frames of dynamic PET images were reconstructed in time frames of progressively longer duration, from 10 s to 600 s, including the break (30 min) at the 27th frame (Appendix 1).
Quantification of [18F]fallypride BPND
[18F]fallypride BPND values express the association between the estimated concentration of available DA D2/D3 receptors (Bavail), the dissociation constant of the radiotracer from D2/D3 receptors (Kd), and the free fraction of nonspecifically bound tracer in the brain (FND)[BPND = FND × (Bavail ÷ Kd)]34 and were calculated using the simplified reference tissue model.35 We use a nonlinear regression to fit a region of interest (ROI) time activity curve that is obtained by averaging over the voxels. The noise level is lower than in voxel-wise data that require methods such as the basis function or linear regression with spatial constraint.36 The reference region was cerebellar grey matter based on the evidence that specific binding in this region is negligible with respect to other brain areas.37,38 Regions of interest were defined on each individual’s MRI scan in stereotaxic space, and BPND values were derived for intergroup comparisons using Turku PET centre tools (/turkupetcentre.net; Appendix 1).
Behavioural measures
Drug craving and subjective mood states were assessed using 17 Likert-like visual analogue scale (VAS) items (happy, rush, high, euphoria, excited, anxious, energetic, mind racing, alert, bored, interested, urge for cocaine, desire cocaine, crave cocaine, want cigarette, want alcohol, and want other drug). The VAS was administered at baseline, 30 min before the start of the scan and then every 30 min after the start of the scan. We administered the Cocaine Selective Severity Assessment Scale as a measure of early cocaine withdrawal symptoms and signs at the beginning of each scan day.
To define the magnitude of the craving response to cues, similarly to Wong and colleagues39 we calculated a mean score of the 6 postinjection responses to the VAS items “want,” “crave,” “desire” and “urge for cocaine” on each test session, and then divided it by the baseline pretest response on the same day, to account for any craving upon presentation to the facility. The ratio between scores on the cocaine and neutral cue days was then computed for each VAS item and averaged to obtain a final composite score that represented an _n_-fold increase in craving upon exposure to drug versus neutral cues. We used this score to classify participants who experienced greater cocaine craving on the drug cue session versus those who did not (“noncravers,” craving score ≤ 1-fold increase).
Statistical analysis
Repeated-measures 2-way analyses of variance (ANOVAs) of differences in BPND between test sessions (neutral cues, cocaine cues) were calculated with ROI and session as within-subjects factors, 1 including the cortical ROIs (mPFC, medial and lateral orbitofrontal cortex [OFC], dorsolateral PFC [DLPFC] and anterior cingulate cortex [ACC]), and 1 with striatal ROIs (ventral striatum [VST], associative striatum [AST], and sensorimotor striatum [SMST]). We applied Greenhouse–Geisser corrections as appropriate. These were followed by Bonferroni-corrected post hoc comparisons to define the source of significant differences. Magnitude of BPND reduction was calculated as %ΔBPND = (BPND_cue − BPND_neutral) ÷ BPND_neutral × 100.
To test the hypothesis that midbrain DA receptors influence cue-induced craving through the striatum, we performed mediation analysis,40 using the SPSS macro Indirect.sbs developed by A.F. Hayes. By convention paths a and b, respectively, represent the effect of the causal variable X on a mediator M, and the effect of M on the outcome variable Y partialling out the effect of X; the “indirect effect” of X on Y is the product of a and b (a × b). Path c is the “total effect” of X on Y and is equal to the sum of a × b and _path c_’, that instead represents the direct effect of X on Y, controlling for M.
The significance of the indirect path a × b is a necessary requirement to infer a mediation effect. We performed the bootstrapping procedure (with 5000 resamples), implemented by Preacher and Hayes as a test for significance. We then checked for reversed causal effect by inverting the predictor and outcome variables.
Associations between continuous variables were analyzed with the Pearson correlation coefficient once normality was established through a Shapiro–Wilk test and visual inspection of histograms, Q-Q plots, and P-P plots.
Results
Twelve volunteers participated in this study. Sample demographic and drug use characteristics are presented in Table 1. All participants were active cocaine users, currently using at least once a week and for an average of 16 years (range 3–25 yr, mean 7.5 ± 4.5 g of cocaine/wk). All participants had current or a history of other illicit substance use (see the study by Fotros and colleagues30 and Appendix 1, Table S1) but reported cocaine as their drug of choice.
Table 1
Demographic and cocaine use characteristics of study participants (n = 12)
Behavioural and psychophysiological measure analyses
As previously reported,30 exposure to the drug cues compared with the neutral cues markedly increased VAS-measured craving scores (want, urge, desire, crave cocaine: _t_11 = 3.5, p = 0.005) and various other subjective states (rush, anxious, excited, mind-racing, interested, and euphoria: all _t_11 > 2, p < 0.04). Based on the composite craving score, 9 out of 12 participants (75%) were identified as cocaine cue–induced cravers (mean 2.66 ± 0.29 fold increase relative to neutral day craving). Three participants did not show a craving response (mean 0.74 ± 0.18 fold change; cravers v. non-cravers: _t_10 = 3.66, _p_ = 0.004). The craving response was not associated with age or with any of the cocaine use characteristics listed in Table 1 (all _r_ ≤ 0.43, _p_ ≥ 0.16). Exposure to the cues did not lead to significant changes in heart rate (_t_11 = −2.03, _p_ = 0.07) or blood pressure (_t_11 < 1.2, _p_ > 0.25).
PET [18F]fallypride BPND analyses
Within the cortex of the cravers, a statistically significant effect of session (_F_1,8 = 6.88, p = 0.030; partial η2 = 0.463) indicated decreased BPND values following exposure to drug cues. The session × ROI interaction was not significant (_F_4,32 = 2.06, p = 0.11), though post hoc tests indicated that the cocaine cue–induced reductions peaked in the mOFC (~20%, post hoc test p = 0.010, significance survived following Bonferroni correction; Cohen’s d = 1.07) and ACC (11%, p = 0.06; Cohen’s d = 0.78; Table 2). An effect of session was also seen in the striatum, as previously reported30 (_F_1,8 = 5.46, p = 0.048; partial η2 = 0.406), with no session × ROI interaction (_F_2,16 = 1.32, p = 0.29).
Table 2
[18F]fallypride binding potential values for the PFC ROI and the midbrain
Binding reductions were not associated with age in any of the regions examined. Years of cocaine use were inversely correlated with magnitude of binding reduction in the ACC only (r = −0.57, p = 0.05). Adding this variable as a covariate in the main analysis did not alter the effect of session (_F_1,7 = 5.61, p = 0.05).
Correlation and mediation analyses
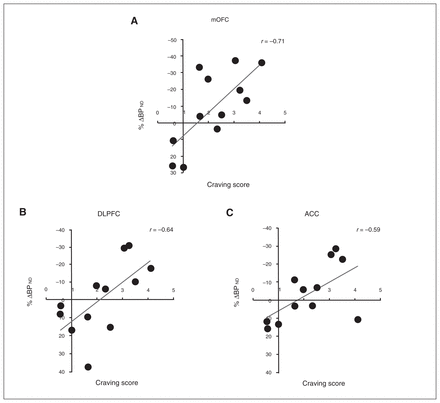
In the full sample of 12 participants, individual differences in the cocaine cue–induced craving score correlated robustly with BPND reductions in the mOFC (r = 0.71, p = 0.009), DLPFC (r = 0.64, p = 0.026), ACC (r = 0.58, p = 0.046), VST (r = 0.75, p = 0.005), AST (r = 0.75, p = 0.005), and SMST (r = 0.80, p = 0.002); the greater the craving response, the greater the change in BPND values (Fig. 1). In general, PFC and striatal responses tended to covary (Table 3). Subjective state measures other than craving score did not correlate with DA release (data not shown).
Fig. 1
Correlations between [18F]fallypride %Δ binding potential (BPND) in (A) the medial orbitofrontal cortex (mOFC; r = 0.71), (B) the dorsolateral prefrontal cortex (DLPFC; r = 0.64), and (C) the anterior cingulate cortex (ACC; r = 0.59) and craving score in the whole sample (n = 12). The craving score is to be interpreted as _n_-fold increase of baseline (neutral cue session) craving.
Table 3
Frontostriatal and mesostriatal correlational analysis*
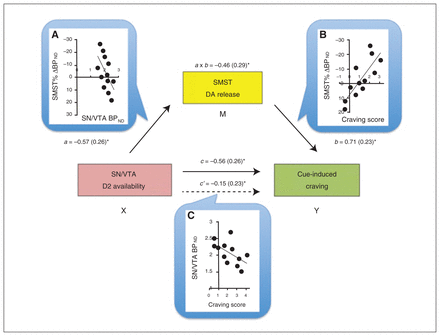
Baseline BPND values in the SN/VTA negatively correlated with the craving scores (r = −0.57, p = 0.06) and Δ BPND values within the striatum (Table 3), but, as expected, not in the PFC (Table 4). To help interpret these associations further, we conducted mediation analyses to test the prediction that midbrain receptor availability influences cue-induced craving by modulating DA release in the striatum. In a first path model, VST responses were not a significant mediator of the VTA–craving association. The BPND changes in the SMST, in comparison, were a significant mediator, explaining the SN/VTA BPND–craving score association (a × b indirect effect: B = −1.48, confidence interval = −3.81 to −0.29; Fig. 2).
Fig. 2
Mediation model for the association between baseline availability of D2 receptors in the substantia nigra/ventral tegmental area (SN/VTA), dopamine (DA) release in the sensorimotor striatum (SMST) and cue-induced craving score. Dopamine release in the SMST was a full mediator of the SN/VTA–craving association. Next to each arrow are the path B coefficients (standardized) with standard errors. Note that a negative coefficient sign indicates a negative effect in the direction of the path. (A) The scatterplot displays the negative association between baseline binding potential (BPND) in the midbrain (SN/VTA) and DA release in the SMST. Path a reflects the effect of the predictor SN/VTA (X) on the mediator (M) SMST. (B) Direct correlation between DA release in the SMST and craving score: the higher the DA release, the higher the craving experienced. Path b is the effect of the mediator SMST on the outcome measure (Y) craving score. (C) The scatterplot shows the negative correlation between baseline SN/VTA BPND and craving score. Path c and path _c_′ are, respectively, the “total effect” and the “direct effect” of X on Y. Of note, the direct effect of the midbrain on craving is no longer significant when controlling for the mediator, suggestive of a mediation effect in this model. a × b reflects the indirect effect through the mediator, assessed through the bootstrapping procedure. *p < 0.05.
Table 4
Mesocortical correlational analysis*
Discussion
To our knowledge, the present report provides the first evidence of drug cue–induced DA release in human frontal cortex. The statistically most robust effect was found in the mOFC with a threshold response in the ACC. Individual differences in the magnitude of responses in the mOFC, DLPFC and ACC all correlated positively with self-reported craving.
Previous PET studies in cocaine abusers have demonstrated effects of drug cues on cortical cerebral blood flow and glucose metabolism preferentially within the ACC,4,20 DLPFC and mOFC.3 Similarly, fMRI studies have identified drug cue–induced activations in the ACC and left DLPFC of crack cocaine users,21 effects that are larger than those elicited by erotic images.41
The above effects of cocaine- and other reward-paired cues on cortical activation might reflect, in part, increased DA release. In laboratory animals, PFC DA release occurs in response to reward-paired stimuli,42,43 emotional arousal,44 stressful events,45 and cognitive tasks that require attention46 and working memory.47 Within the ventromedial PFC (vmPFC), DA infusions promote goal-directed behaviours.48 The mOFC influences behaviour related to outcome expectancy49 and attribution of value5,50 by integrating inputs from other PFC, limbic (amygdala) and sensory areas. The ACC, in comparison, influences selective attention and decision processing, especially for reward-related events.51 The vmPFC also appears to play an important role in the transition to outcome-insensitive habit behaviours.52 In part, this may reflect vmPFC (mOFC, ACC) projections to the VST where much of the motivational information is translated into action.
Previous PET studies have identified [18F]fallypride displacement within the PFC following exposure to laboratory stressors. These effects occurred in the vmPFC16 and dmPFC,17 with no significant effect in the DLPFC. Although in our study there is some regional overlap with the effects reported by Lataster and colleagues,16 this may reflect their roles in attentional salience. Indeed, unlike what was seen in the studies of stress, the cocaine cues did not induce autonomic responses (heart rate and blood pressure).
A higher baseline availability of D2 receptors in the SN/VTA was associated with lower craving and lower DA release in the striatum. D2 autoreceptors, located on mesostriatal projections, are likely involved in these effects.53,54 The inverse association with craving is interesting in light of previous reports that lower midbrain D2 receptor levels are associated with personality traits and behaviours closely linked to vulnerability to addictions.25,28,55,56 Individual differences in midbrain DA autoreceptors in cocaine-dependent individuals might pre-exist drug use, be perturbed by the drug use, or result from a combination of these effects.
The generation of craving states is thought to reflect the confluence of multiple processes, including the precognitive impetus to approach, cognitive appraisal, and action planning. In our analysis, a significant mediator in the modulation of these craving responses to familiar cues was SMST DA release (Fig. 2). Although DA release in both the VST and SMST were associated with receptor levels in the midbrain, only the sensorimotor portion fit the path model. This is in line with observations that, following repeated drug use, striatal–midbrain loops come to engage more dorsal aspects of the striatum,30,39,57 contributing to the development, selection and execution of habit-like compulsive approach to highly familiar cues.58 Since DA release in the mOFC/ACC also correlated positively with SMST DA release and craving factor scores, the results are at least consistent with propositions that motivational states are generated within subcortical sites, such as the striatum, and represented in the cortex from where top–down regulatory control can be exerted.1,2,59 The associations observed here might reflect a learned response whereby DA-related alterations in meso–fronto–striatal connectivity direct the individual toward a maladaptive pursuit.60,61 The heterogeneous craving response might reflect the varied individual emotional engagement with the cues and their assigned incentive salience.59
Limitations
The primary objective of the present study was to test whether exposure to drug cues would evoke DA release in prefrontal cortical regions in cocaine-dependent volunteers and to compare this to now well-established effects in the striatum. Though the small sample size underscores the need for caution, the observed effects were predicted a priori. The analysis also afforded the opportunity to conduct a large number of exploratory analyses. These results, though intriguing, require an additional level of caution. Thus, our findings should be interpreted in light of the following considerations. First, as noted, our sample size was limited and the number of female participants was low. This reflects, in part, the strict entry criteria, which yielded a relatively homogeneous sample who met the criteria for cocaine dependence, had no co-occurring Axis-I disorders and were tested while not intoxicated. Second, session order was kept fixed (neutral scan session first, cue scan session second) across participants to avoid a drug cues–PET environment pairing that could then affect the neutral cue session. Supporting our procedural choice, the test–retest variability of [18F]fallypride is approximately 10% and does not reflect a change in a unique direction.31 In comparison, our strongest effect in the mOFC was double this magnitude; indeed, if an effect is induced in the same direction consistently, meaningful differences can be detected even when they are smaller than the test–retest variability. Third, we were not able to follow-up most participants to assess whether the reported effects predicted drug use after the scan. Future studies will be needed to clarify this possibility. Fourth, the suitability of labelled fallypride to detect a DA signal in the cortex has been questioned, specifically in response to an amphetamine challenge.62,63 A low signal-to-noise ratio in extrastriatal regions, combined with high intersubject variability, have likely prevented reaching statistically significant drug-induced binding decreases, though with percent displacement above normal test–retest variations14,31 and previous successful reports.13,14 In the present study, the sensitivity of the method may have been increased by using a higher-resolution PET scanner (HRRT), fallypride labelled with 18F instead of 11C, the lower intersubject variability, the nonpharmacological challenge used, and the behaviour-based division of subgroups. [11C]FLB457 is another commonly used high-affinity radiotracer that by virtue of a favourable pharmacokinetic profile may be more sensitive than [18F]fallypride to subtle changes in extrastriatal binding.38 However, the shorter half-life of [11C]FLB457 limits its use for striatal quantifications; in comparison, with [18F]fallypride stable measures can be achieved with scans of 2 hours or longer.32 Indeed, the use of [18F]fallypride to measure DA release in the striatum is well-supported,28,30,64 and the present study confirms that informative data can be collected from cortical, striatal and midbrain regions during the same scan. Fifth, the use of the cerebellum as reference region in D2/D3 PET imaging has been a subject of discussion.65 Albeit very low compared with striatal regions,37,66 the presence of D2/D3 receptor density in the cerebellum could influence BPND estimations in lower-density cortical regions. With [18F]fallypride, however, the nondetectable specific binding37 and the absence of a mass effect on cerebellar volume of distribution67 support its selection as reference region. Finally, in light of the small sample size, the mediation analysis presented should be interpreted with caution as preliminary statistical support for the proposed model.
Conclusion
The present results suggest the following. First, in cocaine-dependent individuals, drug cues can induce DA release within the limbic cortex. Second, cocaine cue–induced DA release within the striatum is related to individual differences in midbrain DA autoreceptor availability. Third, individual differences in the magnitude of the DA responses correlate positively with increases in craving, potentially reflecting the transfer of information within cortico–striatal–thalamic–cortical circuitry. In people with severe cocaine use disorders, drug-focused incentive motivational states might reflect DA release in both striatal and cortical regions, each with separable regulatory mechanisms.
Acknowledgements
This work was supported by an operating grant to M. Leyton from the Canadian Institutes of Health Research (MOP-36429). The authors thank the personnel at the McConnell Brain Imaging Centre for technical assistance.
Footnotes
Competing interests: M. Leyton declares having received operating funds through his institution from GlaxoSmithKline in 2006–2007 to conduct a study on naltrexone. No other competing interests declared.
Contributors: A. Fotros, S. Cox, A Dagher, C. Benkelfat and M. Leyton designed the study. A. Fotros, J. Verhaeghe, S. Cox, A. Reader and M. Leyton acquired the data, which M. Milella, P. Gravel, K. Casey, K. Larcher and M. Leyton analyzed. M. Milella, K. Larcher and M. Leyton wrote the article, which all authors reviewed and approved for publication.
Received June 1, 2015.
Revision received September 9, 2015.
Accepted October 19, 2015.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵