Protective roles of ginseng against bacterial infection (original) (raw)
INTRODUCTION
Because Asia has a specific topography and soil that differs from that of other continents, it provides unique environmental conditions that support the growth of several medicinal plants, which have been used as agricultural products, food, dietary supplements, health supplements, and medicines [1]. Botanical medication has been used to treat various diseases in Asia for thousands of years. Among these, ginseng (Panax ginseng Meyer, family Araliaceae) is one of the most widely known and used oriental medicinal plants [2]. Ginseng is a shade plant that prefers a cool and dry climate, like that of Korea [1]. The genus “_Panax_” was named by the Russian botanist Carl Anton Meyer, from the Greek “_pan_” meaning “all” and “_axos_” meaning “cure” [1]. The main species of ginseng are P. ginseng C.A. Meyer (Korea ginseng), Panax quinquefolius L. (American ginseng), Panax notoginseng Burkill (Tienchi ginseng), and Panax japonicus C.A. Meyer (Japanese ginseng) [3]. By 2009, Korea was the second-highest global producer of ginseng after China [1]. Ginseng is globally distributed throughout 35 countries in various forms following processing via drying, steaming, and heating [2].
–
Several studies have recently reported the beneficial effects of ginseng on diseases such as cancer; immune disorders; diabetes; and liver, neuronal, cardiovascular, and infectious diseases [4][5][6][7][8][9][10][11]. Although extracts of ginseng root, leaves, and stems exhibit various pharmacological effects, most pharmacologically active compounds are thought to be present in the root, which has been the focus of previous studies. A significant change in the element accumulation occurs during the life cycle of ginseng. P. ginseng C.A. Meyer cultivated in Korea is harvested following long cultivation (4-6 years), which allows for the increased composition of secondary metabolites [12]. It is consumed after traditional processing methods, including air drying (white ginseng; after 4–6 years’ cultivation), steaming and heating (red ginseng; after 6 years’ cultivation). Red ginseng is steamed at 98°C–100°C for 2-3 h and then dried at <15% humidity. Because the streaming process enhances its biological activity, red ginseng is more widely used as an herbal medicine than white ginseng [13][14][15]. Ginseng contains various bioactive components including tetracyclic triterpenoids (ginsenosides), polyacetylenes, polyphenolic compounds, and acidic polysaccharides of which ginsenoside is highly pharmacologically active.
–
Although most microorganisms do not induce diseases, some harmful pathogens cause infections in their hosts. When a host is vulnerable to a pathogen, it cannot respond adequately to protect against the infectious disease. Infections are triggered by pathogenic microorganisms, such as bacteria, viruses, parasites, or fungi. The mechanisms of infectious disease development are complex because they depend on interactions between the host, the pathogen, and the environment [16].
–
Antibiotics are medicines used to prevent and treat bacterial infections. Antibiotic resistance occurs when bacteria change in response to the use of these medicines. Bacteria, not humans or animals, become antibiotic-resistant. These bacteria may infect humans and animals, and the infections they cause are harder to treat than those caused by non-resistant bacteria. Antibiotic resistance leads to higher medical costs, prolonged hospital stays, and increased mortality. Thus, there is an urgent need to re-think the prescription and use of antibiotics. Even if new medicines are developed, without behavior change, antibiotic resistance will remain a major threat. Behavior changes must also include actions to reduce the spread of infections through vaccination, hand washing, practicing safer sex, and good food hygiene [17][18][19]. Thus, there is an urgent need to develop novel alternative remedies [20]. The pharmacological effects of natural products, especially the antimicrobial activities of plants, are considered to offer attractive novel treatment strategies. Plants interact with various microorganisms and produce small-molecule (<500 Da) antimicrobial compounds that limit the harmful effects of pathogenic microorganisms. Thus, many hundreds of plants have been widely used as traditional medicines [21]. Additionally, the combination of natural products and antibiotics exerts a synergistic effect against infectious diseases, resulting in an enhanced antibacterial effect on drug-resistant bacteria and reducing the dosage of existing antibiotics, which alleviates their toxicity [22][23].
–
Currently, food-related immune system enhancement has attracted attention because of the global emergence of infectious disease epidemics [24]. Infections can cause different phenomena depending on the immune system status of the host. Healthy individuals can defend their bodies against a pathogenic invader and remain asymptomatic, but immunocompromized people could acquire a severe infectious disease from the same pathogen. The size of the immunocompromized population is rising because of increasing longevity, changing nutritive conditions of modern people, and the development of long-term cures for various diseases [25]. With this increase in infectious disease, ginseng could provide an effective antibacterial treatment. Ginseng has been investigated for its effect on various aspects of disease treatment, especially its role in protection against microbial attack. This review focuses on the effect of ginseng against bacterial infection.
THE MAJOR COMPONENT OF GINSENG
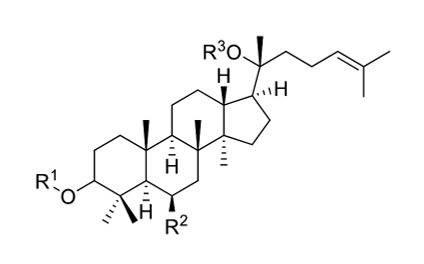
Ginseng comprises saponin and non-saponin constituents. Saponins are glycosides attached to either a saccharide or non-saccharide component (sapogenin and aglycone) (Fig. 1). Ginsenosides refer specifically to ginseng saponins, named to distinguish them from the saponins of other plants. Ginsenosides are specific secondary metabolites of Panax sp. and comprise the major pharmacological component of the ginseng plant. Over 30 ginsenosides have been isolated and identified in raw or processed ginseng. Ginsenosides are classified as dammarane or oleanane type, depending on their aglycone skeleton. Dammarane-type ginsenosides, the dominant ginsenoside, are protopanaxadiols (PPDs), protopanaxatriols (PPTs), or ocotillols. PPD-group saponins comprise ginsenosides Ra1, Ra2, Ra3, Rb1, Rb2, Rb3, and Rd; quinquenosides R1 and Rs1–Rs3; and malonyl ginsenosides Rb1, Rb2, Rc, and Rd. PPT-group saponins include ginsenosides Re, Rf, Rg1, Rg2, Rh1, and F1 and notoginsenosides R1 and R2. The ocotillol-group ginsenosides, identified in Panax species such as P. quinquefolius, P. japonicus, and Panax vietnamensis, comprise majonoside R2 and pseudoginsenoside F11. Ginsenoside Ro has only been identified among the oleanane-group saponins and is a minor component of P. ginseng [12].
–
Korean ginseng contains dammarane-type ginsenosides and a unique saponin found in the Panax genus that is nontoxic and displays antibacterial activity against non-hemolytic bacteria. 32 ginsenosides have been isolated from Korea Red Ginseng (KRG), whereas 22, 13, and 14 ginsenoside species have been isolated from white ginseng, P. quinquefolius, and P. notoginseng, respectively [26]. The compound panaxan contains 21 species (R0, R1, R2, Ra, Rb1, Rb2, Rc5, Rd5, Re, Rf5, RgI, Rg2, Rg3, RgS5 RhI, Rh2, Rh4, RsI, Rs2, Rs35 or Rs4), and its principal sugar components include glucose, arabinose, galactose, xylose, and rhamnose [27]. Several studies have revealed that ginseng components other than ginsenosides also possess pharmaceutical properties. For example, the polysaccharide ginsan, isolated from the ginseng root, can induce physiological effects such as cytokine modulation and lymphoid cell stimulation [28].
ANTIBACTERIAL ACTIVITY OF GINSENG
Microbial infections cause several distinct diseases, requiring different antibiotic treatments, but inappropriate antibiotic usage triggers antibiotic resistance and leads to various toxic side effects in the host [29]. The emergence of multiple-drug-resistant bacteria can render existing antibiotics useless. To address this threat, alternative approaches, such as the use of natural products, have been attempted. This involves targeting bacterial pathways that indirectly kill pathogens and protect the host from bacterial invasions. Such properties have been identified for ginseng [30].
–
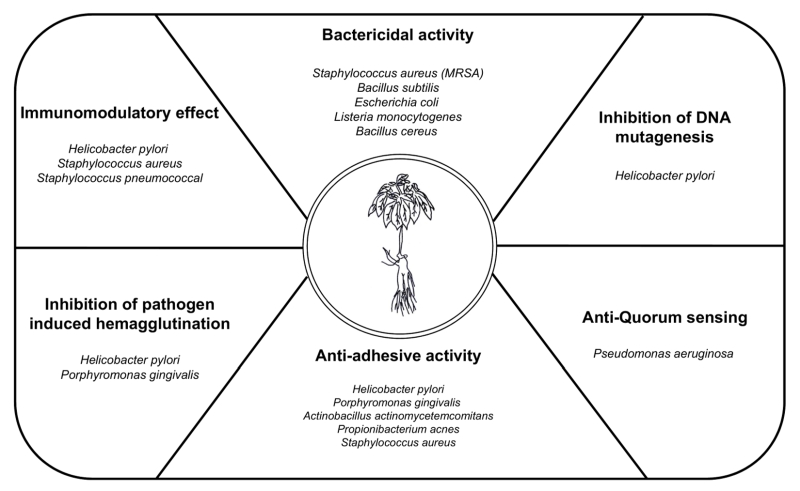
Ginseng indices bactericidal activity, inhibition of DNA mutagenesis, anti-quorum sensing, anti-adhesive activity, inhibition of pathogen-induced hemagglutination, immune-modulatory functions and demonstrates a protective role against pathogen-induced inflammation. The next sections describe the antibacterial effect of ginseng on several representative pathogens (Table 1 and Fig. 2).
–
Helicobacter pylori
Helicobacter pylori (H. pylori) is a highly motile, Gram-negative, microaerophilic bacterium that can infect the stomach, impacting human health [31]. H. pylori infects 50% of the world’s population. Most infections are asymptomatic, but some people demonstrate an improper response to the pathogenesis of H. pylori, developing peptic ulcers, gastric cancer, or malignant lymphoma [32]. H. pylori colonizes the epithelial surfaces of the stomach mucosa in individuals with active chronic gastritis.
–
The adhesion of pathogenic bacteria to host cells is crucial to initiate host infection, allowing entry into cells beyond the host barrier and subsequent pathogen multiplication [33]. Recent research has shown that ginseng extract inhibits cell adhesion, thereby blocking the initiation of pathogenic infections. Pectin-type polysaccharide PG-F2 isolated from P. ginseng possesses a marked anti-adhesion activity against many microbes [34]. Furthermore, ginseng protects against pathogen-induced DNA damage and regulates cell death, as observed in H. pylori infection.
–
As H. pylori induces gastric inflammation, ulceration, and DNA damage, it has been defined as a class I carcinogen by the WHO. H. pylori is the most extensively studied and well-known pathogen which is affected by ginseng via various pathways. Acidic polysaccharides from the roots of P. ginseng C.A. Meyer (Araliaceae) and the leaves of Artemisia capillaris (Asteraceae) exert anti-adhesive effects on H. pylori infecting human gastric cells and erythrocytes [35][36][37]. In recent studies, fermented ginseng extracts, containing the lactic acid bacterium Lactobacillus plantarum MG 208, exhibited powerful anti-H. pylori activity, including anti-bacterial, anti-adhesion, and urease inhibition effects. This extract contained a larger concentration of Rd and R1 ginsenosides compared with other fermented ginseng extracts, which explains its stronger antibacterial activities [38].
–
Other studies have demonstrated that red ginseng extract (RGE) exerts a protective effect against cytotoxicity and DNA mutagenesis induced by H. pylori and can reduce proinflammatory activity in gastric mucosal cells. An RGE pretreatment of <100 µg/mL induces protective effects in gastric epithelium cells. RGE also inhibits DNA damage and apoptosis induced by H. pylori by inhibiting ERK1/2 signaling. This reduces caspase-3 activation and subsequent programmed cell death, consequently diminishing proinflammatory cytokine IL-8 or 5-lipoxygenase mRNA expression [39][40][41][42]. A study by Bae et al. (2001) revealed the inhibition of H. pylori growth by polyacetylenes and PPT isolated from RGEs. Moreover, panaxytriol was shown as partially effective in inhibiting H. pylori growth (MIC50 = 50 µg/mL). Ginsenoside Rh1 and PPT can cause minor inhibitory effects on H+/K+ ATPases, which are involved in the final step of stomach acid secretion [43]. In an animal model using Mongolian gerbils, RGE showed inhibitory effects on H. _pylori_-induced inflammation. One week after infection with H. pylori via intra-gastric inoculation, the control diet gerbil group was compared with gerbils given a diet containing 200 mg RGE for 6 weeks. Although the RGE diet supplementation did not affect the viable H. pylori count in the stomach, RGE inhibited induction of inflammatory mediators (KC, IL-1β, iNOS, MOP activity, and LPO level) in the gastric mucosa of the gerbils [44].
–
White ginseng extract (WGE) has also demonstrated anti-H. pylori activity, cytotoxicity, and anti-inflammatory activity in vitro. The antibacterial activity of WGE against H. pylori was measured using a disk diffusion assay, and it was concluded that the growth inhibition was dependent on the WGE dosage. Additionally, WGE exerts a cytotoxic effect on various human cancer cell lines such as A-549 (human lung carcinoma), HEC-1-B (human endometrial adenocarcinoma), and HeLa (human uterine adenocarcinoma), but not normal cells. Also, an analysis of anti-inflammatory activity using RAW 264.7 cells showed a reduction of nitric oxide (NO) production by WGE treatment [45].
–
Pseudomonas aeruginosa
Pseudomonas aeruginosa (P. aeruginosa) is a common environmental Gram-negative, rod-shaped bacterium that is an opportunistic bacillus. P. aeruginosa can colonize under various conditions by utilizing many environmental compounds as energy sources [46]. Infections are common in individuals with cystic fibrosis, thermal injury, chronic wounds, chronic obstructive lung diseases, and urinary tract infections, and in immunocompromized patients with acquired immune deficiency syndrome (AIDS) and AIDS-related complex [39][47][48][49]. Because P. aeruginosa can form biofilms, treatment with antibiotics or via the host immune system is challenging. Thus, P. aeruginosa has acquired resistance to many antibiotics [50], and therapeutics directly targeting biofilms are required to eliminate P. aeruginosa infection. In a 2011 study, Hong et al. showed that although aqueous ginseng extract did not directly affect the growth of P. aeruginosa, it reduced biofilm formation in vitro [51].
–
Some pathogenic bacteria use quorum sensing (QS), a cell-to-cell communication mechanism, during the infectious process. QS responds to changes in cell-population density and regulates gene expression systems, and is crucial for establishing an infection. Through QS, bacteria produce and release signaling molecules called autoinducers that affect bacterial behavior based on cell density. Bacteria present in biofilms, surface-attached groups of microbial cells enveloped in an extracellular matrix, communicate with others in the biofilm by synthesizing and reacting to these chemical signals [52]. Bacterial biofilms can cause chronic infections by limiting the effectiveness of antibiotics. Thus, biofilm reduction is vital in infectious disease treatment. Susceptibility tests with in vitro biofilm models have demonstrated that antibiotics are only effective against bacterial biofilms at concentrations hundreds or even a thousand times the minimum inhibitory concentration (MIC) measured in suspension culture [53][54]. Ginseng has demonstrated anti-QS activity by suppressing the efficacy of virulence factor production which is related to QS control [53] and inhibition of biofilm formation [39]. Recent research has revealed that QS in P. aeruginosa is required for biofilm formation [52]. P. aeruginosa pathogenesis is related to QS through the formation of various extracellular virulence factors and biofilms. Therefore, QS could be a novel target for the treatment of P. aeruginosa infections. A 6-year-old dried form of ginseng reduced the levels of QS signaling molecules such as N-butanoyl-L-homoserine lactone and N-3-(oxododecanoyl)-L-homoserine lactone. These signaling molecules are critical components that induce the production of virulence factors [53]. A motility test has demonstrated that ginseng activated swimming and twitching motilities but reduced swarming motility, which is essential for biofilm development [51]. The effect of ginseng treatment on P. aeruginosa pneumonia in an animal model promoted a Th1-like response, which might activate the phagocytes and NK (natural killer) cells, leading to improved bacterial clearance in the lungs which results in a reduced severity of lung pathology and an easier control of the bacterial infection [55][56].
–
Staphylococcus aureus
Staphylococcus aureus (S. aureus) is a Gram-positive commensal bacterium and major pathogen that can trigger severe clinical infections. It is widely distributed globally and is strongly resistant to the natural environment. S. aureus colonizes one-third of the human population and commonly exists on the skin and nasal surfaces of healthy people. This bacterium can colonize nares, skin, and the respiratory tract and invade the skin, tissue, and the bloodstream. When S. aureus infects the skin, it causes abscesses, sinusitis, and food poisoning. Following bloodstream invasion, S. aureus replicates and disseminates throughout the body, triggering severe infections such as sepsis and endocarditis [57]. S. aureus is able to build biofilms and is a major antibiotic-resistant pathogen. Therefore, the treatment of S. aureus infections is critical. Although the development of new antibiotics is progressing, S. aureus acquires effective resistant mechanisms to antibiotics at a rapid rate. Antibiotic-resistant S. aureus includes two types, namely, methicillin and vancomycin resistant strains. Methicillin-resistant S. aureus (MRSA) is resistant to methicillin and other beta-lactam antibiotics, including cephalosporin, ampicillin, and nafcillin, and to almost all antibiotics, which makes treatment of S. _aureus_-infected patients complicated [58][59]. Ginsenoside extracted from KRG, with an MIC50 of 100 μg/mL, has shown antibacterial activity against Gram-positive and Gram-negative bacteria including MRSA and exhibits a similar killing effect as propionic acid, which is a well-known bactericidal agent against MRSA. To identify the antibacterial activity of ginsenoside, a bacterial membrane mimic liposome containing fluorescent marker was used. Treatment with ginsenosides induced the acceleration of fluorescent dye leakage, indicating that ginsenoside disturbs bacterial membranes, thereby causing an antibacterial effect. Combination therapies of antibiotics with ginsenoside have been employed to expand the usage of antibiotics and to prevent the development of resistant strains. The combined effect of ginsenosides and the commercial antibiotics kanamycin and cefotaxime against MRSA has been investigated, and it was concluded that these combinations exerted a synergistic effect against MRSA [60].
–
Ginsan, a polysaccharide isolated from P. ginseng, has induced increased NO production and potent phagocytic activity by macrophages. Ginsan stimulation of the macrophages has enhanced anti-septicemic activity and increased the production of proinflammatory cytokines. Additionally, ginsan treatment has increased proinflammatory cytokine production in the murine fibroblast cell line L929 [61]. Furthermore, Ginsan has demonstrated anti-septicemic activity in mouse models. Ginsan has enhanced survival rates and reduced bacterial burden in the blood during S. _aureus_-infected sepsis in mice. Moreover, a combination of ginsan and vancomycin induced higher protective effects than the respective single treatments, as measured by mice survival rates [61][62][63]. These results suggest that ginsan possesses a potent anti-septicemic activity by stimulating macrophages and acting as an immunomodulator against sepsis caused by S. aureus infections in vitro and in vivo.
–
The underlying mechanisms of ginsan include its anti-septic activity, affecting the toll-like receptor (TLR) pathway. Ginsan treatment has been shown to reduce proinflammatory and anti-inflammatory cytokine production in S. aureus_-infected mice, and ginsan treatment of peritoneal macrophages stimulated by S. aureus has suppressed the expression of TLR2, TLR4, TLR9, and the adaptor protein myeloid differentiation primary response 88. Ginsan has also inhibited mitogen-activated protein kinase signaling and NF-κB activation induced by S. aureus [62][63][64]._
–
The processing of ginseng using heat transforms its components and has been shown to enhance its antibacterial activity against S. aureus. The potent antimicrobial compound Rg3, an absent ginsenoside in non-heated ginseng, is produced by heating ginseng at 100°C for 16 h and exhibits a higher antimicrobial activity via a reduction in the cell membrane potential [65].
–
Porphyromonas gingivalis
Porphyromonas gingivalis (P. gingivalis) is a Gram-negative, rod-shaped, non-motile, anaerobic, and pathogenic bacterium. It causes periodontal diseases and colonizes the periodontal pocket, gastrointestinal tract, respiratory tract, and colon. This pathogen induces aggressive inflammation which destroys the gingiva supporting the teeth and eventually leads to tooth loss. P. gingivalis rapidly adheres to and enters host cells to induce proinflammatory cytokines such as IL-1β and IL-6 [66]. PG-HMW and PG-F2, acidic polysaccharides isolated from the roots of P. ginseng, have been shown to inhibit the attachment of P. gingivalis to human oral adenocarcinoma cells such as KB cells [34]. Furthermore, PG-F2 has been shown to inhibit P. _gingivalis_-mediated hemagglutination. These results suggest that PG-F2 could be developed as the base of a dietary component or as a novel anti-adhesive drug for protection against periodontal diseases [67][68]. Additionally, steaming of the American ginseng leaf has been shown to induce conversion from polar ginsenosides to less polar ginsenosides. These heat-transformed saponins easily disturbed cell integrity and exhibited higher antibacterial activity than unprocessed saponins against P. gingivalis [69].
–
Listeria monocytogenes
Listeria monocytogenes (L. monocytogenes) is a facultative pathogenic bacterium that induces listeriosis. It is a small rod-shaped, Gram-positive, facultatively anaerobic bacterium and the most recognized globally virulent intracellular food-borne pathogen. Approximately 20%–30% of food-borne listeriosis is fatal. It is assumed that Listeria triggers 1,600 illnesses in the United States annually, of which 400–500 are fatal [70].
–
Several procedures have been utilized to extract functional components from ginseng, primarily by using different solvents, such as methanol, ethanol and water [71][72]. In a study in 2012, Choi et al. showed that a water extract of KRG has demonstrated antibacterial activity against L. monocytogenes (MIC50 = 1.0 mg/mL) but not with an ethanol extract [71]. Furthermore, Lee et al. showed that ginseng extracts produced from ginseng byproducts, such as stems and leaves, using subcritical water extraction (SWE) have exhibited anti-L. monocytogenes activity. SWE at a high temperature enhanced the extraction yield of the phenolic portion in ginseng stems and leaves and also resulted in higher antibacterial activity. Treatment using a 0.2% of SWE ginseng extract has induced morphological cell damage and the loss of structural integrity of bacterial cell walls. From the results obtained by measuring the leakage of cellular materials through the cytoplasmic membrane during treatment with SWE ginseng extract, it is expected that the antibacterial activity demonstrated against L. monocytogenes is induced by disrupting membrane integrity [72].
–
Bacillus cereus
Bacillus cereus (B. cereus) is a Gram-positive, spore-forming, facultative anaerobe bacterium. It is arranged in chain patterns and is motile because of a flagellum. This pathogen forms heat-resistant spores and can exist in and poison food. B. cereus is environmentally widespread and has been isolated from soil and plants. It also flourishes in insects and the intestinal tracts of mammals. The bacterium produces many virulence factors, including toxins such as emetic toxin and enterotoxins [73]. These toxins can cause two types of illness: one type characterized by diarrhea and the other, called emetic toxin, by nausea and vomiting. These bacteria are present in foods and can multiply quickly at room temperature. The pathogenicity of B. cereus, whether intestinal or non-intestinal, is associated with the production of a tissue-destructive/reactive exoenzyme. Additionally, food poisoning by intestinal infection triggers a systemic and local infection in immunologically compromised and immunocompetent individuals [74]. Treatment with ginseng has been shown to have an antibacterial effect against B. cereus [71]. A study has compared the antibacterial activities among extracts of fine ginseng roots with various solvents. The results revealed that the hexane fraction demonstrated the highest antibacterial activity compared with that of the other fractions [75]. Furthermore, the dried stems and leaves of ginseng extract produced by SWE at 190°C have demonstrated antibacterial activity against B. cereus by causing bacterial cell membrane damage and inhibition of cell growth, as observed using transmission electron microscopy [72]. A recent study has shown that heating ginseng enhanced its antimicrobial activity against B. cereus. Ginseng was extracted using methanol and ethanol and processed at various time points. The antimicrobial activity of the heat-treated ginseng extracts was measured using a disk diffusion method. The results indicated that the ginseng extract heated to 100°C demonstrated the highest antimicrobial activity against B. cereus. Changes to the ginsenoside composition and contents via the heating process enhanced the bacterial growth inhibition effect [65].
–
Staphylococcus pneumoniae
Streptococcus pneumoniae (S. pneumoniae) is a Gram-positive bacterium which is the most common cause of pneumonia, and its infection results in 50% of sepsis cases. Ginsan has demonstrated antiseptic effects against sepsis induced by S. pneumoniae; a KRG water extract was shown to reduce the severity of pneumococcal sepsis. Upon KRG treatment, mice infected with S. pneumoniae D39 experienced a smaller reduction in body weight and an enhanced survival rate. Additionally, bacterial colonization was reduced, and lung inflammation decreased following treatment with KRG. Moreover, S. _pneumonia-_mediated TLR/NF-κB activation was inhibited by KRG treatment in vitro in the same manner as for S. aureus. KRG increased PI3K-AKT signaling, thereby enhancing cell survival in S. _pneumonia_-infected RAW 264.7 cells [76][77].
| TABLE 1. Summary of the ginseng effects on bacterial infection. |
|---|
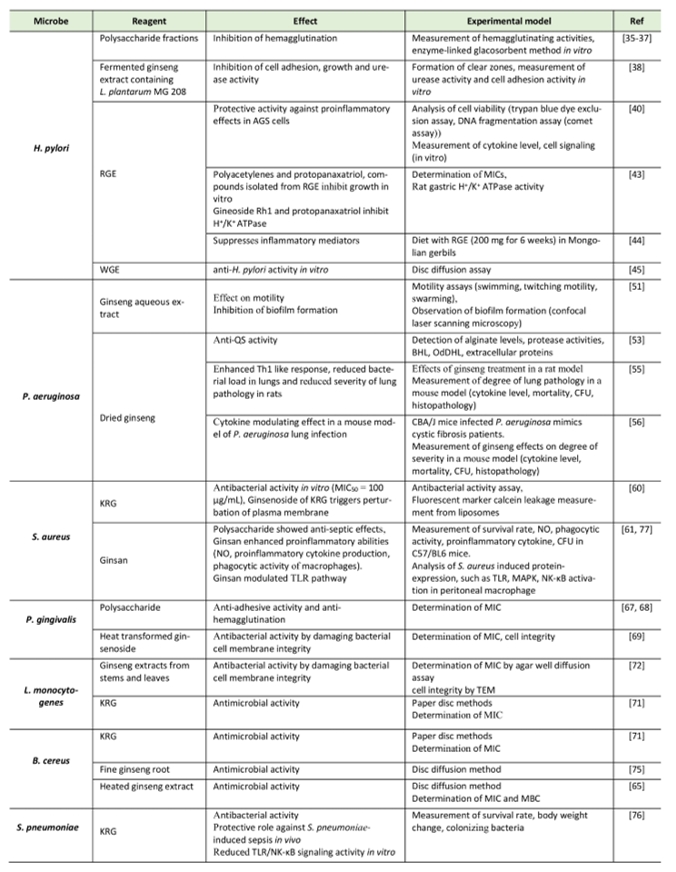 |
| Abbreviations: KRG: Korean red ginseng, MBC: minimum bactericidal concentration, MIC: minimum inhibitory concentration, RGE: red ginseng extract, TEM: transmission electron microscopy. [35][36][37][38][40][44][45][51][55][56][60][61][65][67][68][69][71][72][75][76][77] |
CONCLUSIONS AND FUTURE PERSPECTIVES
Several studies have suggested that using ginseng to cure infectious diseases could protect the host against pathogen infection. Ginseng has effects that not only directly kill bacteria but also work against the regulation of bacterial adhesion, inflammation, cytotoxicity, and hemagglutination (Table. 1 and Fig. 2). Although the importance of infectious diseases caused by viruses was recently highlighted, bacterial infections remain the most serious problem. Emerging infectious diseases and antibiotic resistance present an immense global predicament, which is limited by the availability of effective antibacterial agents and vaccines. Additionally, an indiscreet use of antibiotics to solve these infections triggers severe side effects in patients. Because of these problems, natural products like ginseng have been highlighted as treatments for bacterial infection with a verified relatively low toxicity. However, because the causal relationship between specific active components and the bioactivities of ginseng is unclear, additional research is required to understand the use of ginseng as an antimicrobial agent. On the other hand, ginseng research could be applied to the food industry to prevent food poisoning because several food pathogens are affected by the antibacterial activity of ginseng. Additionally, the effect of ginseng byproducts outside the root have largely been ignored, but a recent study has revealed that various portions of ginseng demonstrate biologic effects. Years of cultivation of ginseng are critical because of the accumulation of biologically active ginseng components over time. The processing of ginseng, such as heating, drying, and boiling, transforms these components, enhancing the antibacterial effect of ginseng as shown in some studies. As we have explained, the optimal use of ginseng will require the development of additional studies. Using ginseng as a natural antibiotic could be a powerful way to deal with bacterial infections.
References
- O. Lee, G. Sathiyaraj, Y. Kim, J. In, W. Kwon, J. Kim, and D. Yang, "Defense Genes Induced by Pathogens and Abiotic Stresses in Panax ginseng C.A. Meyer", Journal of Ginseng Research, vol. 35, pp. 1-11, 2011. http://dx.doi.org/10.5142/jgr.2011.35.1.001
- T.K. Yun, "Brief Introduction of Panax ginseng C.A. Meyer", Journal of Korean Medical Science, vol. 16, pp. S3, 2001. http://dx.doi.org/10.3346/jkms.2001.16.S.S3
- I. Baeg, and S. So, "The world ginseng market and the ginseng (Korea)", Journal of Ginseng Research, vol. 37, pp. 1-7, 2013. http://dx.doi.org/10.5142/jgr.2013.37.1
- S. Helms, "Cancer prevention and therapeutics: Panax ginseng.", Alternative medicine review : a journal of clinical therapeutic, 2004. http://www.ncbi.nlm.nih.gov/pubmed/15387718
- K. Radad, G. Gille, L. Liu, and W. Rausch, "Use of ginseng in medicine with emphasis on neurodegenerative disorders.", Journal of pharmacological sciences, 2006. http://www.ncbi.nlm.nih.gov/pubmed/16518078
- I. Benzie, and S. Wachtel-Galor, " Herbal medicine : biomolecular and clinical aspects.", Taylor & Francis Group, Chapter 1:1-11., 2011.
- D. Yoo, M. Kim, M. Park, J. Song, F. Quan, K. Park, Y. Cho, and S. Kang, "Protective Effect of Korean Red Ginseng Extract on the Infections by H1N1 and H3N2 Influenza Viruses in Mice", Journal of Medicinal Food, vol. 15, pp. 855-862, 2012. http://dx.doi.org/10.1089/jmf.2012.0017
- K. Im, J. Kim, and H. Min, "Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection", Journal of Ginseng Research, vol. 40, pp. 309-314, 2016. http://dx.doi.org/10.1016/j.jgr.2015.09.002
- J.S. LEE, E. KO, H.S. HWANG, Y. LEE, Y. KWON, M. KIM, and S. KANG, "Antiviral activity of ginseng extract against respiratory syncytial virus infection", International Journal of Molecular Medicine, vol. 34, pp. 183-190, 2014. http://dx.doi.org/10.3892/ijmm.2014.1750
- S. Kim, Y. Lee, and J. Cho, "Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death.", Biological & pharmaceutical bulletin, 2014. http://www.ncbi.nlm.nih.gov/pubmed/24882407
- V. Vuksan, J. Sievenpipper, E. Jovanovski, and A.L. Jenkins, "Current Clinical Evidence for Korean Red Ginseng in Management of Diabetes and Vascular Disease: A Toronto's Ginseng Clinical Testing Program", Journal of Ginseng Research, vol. 34, pp. 264-273, 2010. http://dx.doi.org/10.5142/jgr.2010.34.4.264
- Y. Kim, D. Zhang, and D. Yang, "Biosynthesis and biotechnological production of ginsenosides", Biotechnology Advances, vol. 33, pp. 717-735, 2015. http://dx.doi.org/10.1016/j.biotechadv.2015.03.001
- W.Y. Kim, J.M. Kim, S.B. Han, S.K. Lee, N.D. Kim, M.K. Park, C.K. Kim, and J.H. Park, "Steaming of ginseng at high temperature enhances biological activity.", Journal of natural products, 2000. http://www.ncbi.nlm.nih.gov/pubmed/11141123
- L. Wang, X. Yang, X. Yu, Y. Yao, and G. Ren, "Evaluation of Antibacterial and Anti-inflammatory Activities of Less Polar Ginsenosides Produced from Polar Ginsenosides by Heat-transformation", Journal of Agricultural and Food Chemistry, vol. 61, pp. 12274-12282, 2013. http://dx.doi.org/10.1021/jf404461q
- S. Lee, H. Jo, B. Im, S. Kim, W. Whang, and S. Ko, "Changes in the Contents of Prosapogenin in the Red Ginseng (Panax ginseng) Depending on Steaming Batches", Journal of Ginseng Research, vol. 36, pp. 102-106, 2012. http://dx.doi.org/10.5142/jgr.2012.36.1.102
- U. Office, " Emerging Infectious Diseases: Review of State and Federal Disease Surveillance Efforts.", GAO-04-877, pp 1-64., 2004.
- M.S. Morehead, and C. Scarbrough, "Emergence of Global Antibiotic Resistance", Primary Care: Clinics in Office Practice, vol. 45, pp. 467-484, 2018. http://dx.doi.org/10.1016/j.pop.2018.05.006
- A. Petchiappan, and D. Chatterji, "Antibiotic Resistance: Current Perspectives", ACS Omega, vol. 2, pp. 7400-7409, 2017. http://dx.doi.org/10.1021/acsomega.7b01368
- K.U. Jansen, C. Knirsch, and A.S. Anderson, "The role of vaccines in preventing bacterial antimicrobial resistance", Nature Medicine, vol. 24, pp. 10-19, 2018. http://dx.doi.org/10.1038/nm.4465
- J. Davies, and D. Davies, "Origins and Evolution of Antibiotic Resistance", Microbiology and Molecular Biology Reviews, vol. 74, pp. 417-433, 2010. http://dx.doi.org/10.1128/MMBR.00016-10
- K.W. Martin, and E. Ernst, "Herbal medicines for treatment of bacterial infections: a review of controlled clinical trials.", The Journal of antimicrobial chemotherapy, 2003. http://www.ncbi.nlm.nih.gov/pubmed/12562687
- S. Buhner, " Herbal antibiotics : natural alternatives for treating drug-resistant bacteria.", Second edition. Storey Publishing, LLC., 2012.
- S. Hemaiswarya, A.K. Kruthiventi, and M. Doble, "Synergism between natural products and antibiotics against infectious diseases", Phytomedicine, vol. 15, pp. 639-652, 2008. http://dx.doi.org/10.1016/j.phymed.2008.06.008
- P.C. Calder, and S. Kew, "The immune system: a target for functional foods?", The British journal of nutrition, 2002. http://www.ncbi.nlm.nih.gov/pubmed/12495459
- C. Dye, "After 2015: infectious diseases in a new era of health and development", Philosophical Transactions of the Royal Society B: Biological Sciences, vol. 369, pp. 20130426, 2014. http://dx.doi.org/10.1098/rstb.2013.0426
- S. Park, H. Kim, J. Kim, J. Lee, S. Kim, H. Shin, and T. Yi, "Immunostimulatory Effect of Fermented Red Ginseng in the Mouse Model", Preventive Nutrition and Food Science, vol. 19, pp. 10-18, 2014. http://dx.doi.org/10.3746/pnf.2014.19.1.010
- C. Konno, K. Sugiyama, M. Kano, M. Takahashi, and H. Hikino, "Isolation and Hypoglycaemic Activity of Panaxans A, B, C, D and E, Glycans of_Panax ginseng_Roots1", Planta Medica, vol. 50, pp. 434-436, 1984. http://dx.doi.org/10.1055/s-2007-969757
- K. Kim, Y. Lee, I. Jung, S. Park, H. Chung, I. Lee, and Y. Yun, "Acidic Polysaccharide from_Panax ginseng_, Ginsan, Induces Th1 Cell and Macrophage Cytokines and Generates LAK Cells in Synergy with rIL-2", Planta Medica, vol. 64, pp. 110-115, 1998. http://dx.doi.org/10.1055/s-2006-957385
- J.M.A. Blair, M.A. Webber, A.J. Baylay, D.O. Ogbolu, and L.J.V. Piddock, "Molecular mechanisms of antibiotic resistance", Nature Reviews Microbiology, vol. 13, pp. 42-51, 2014. http://dx.doi.org/10.1038/nrmicro3380
- J. Pizarro-Cerdá, and P. Cossart, "Bacterial Adhesion and Entry into Host Cells", Cell, vol. 124, pp. 715-727, 2006. http://dx.doi.org/10.1016/j.cell.2006.02.012
- Y. Cho, Y. Jung, H. Sung, and C. Joo, "Frequent Genetic Defects in the HIV-1 5'LTR/gag Gene in Hemophiliacs Treated with Korean Red Ginseng: Decreased Detection of Genetic Defects by Highly Active Antiretroviral Therapy", Journal of Ginseng Research, vol. 35, pp. 413-420, 2011. http://dx.doi.org/10.5142/jgr.2011.35.4.413
- M. Kawakubo, Y. Ito, Y. Okimura, M. Kobayashi, K. Sakura, S. Kasama, M.N. Fukuda, M. Fukuda, T. Katsuyama, and J. Nakayama, "Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection.", Science (New York, N.Y.), 2004. http://www.ncbi.nlm.nih.gov/pubmed/15310903
- D. Ribet, and P. Cossart, "How bacterial pathogens colonize their hosts and invade deeper tissues", Microbes and Infection, vol. 17, pp. 173-183, 2015. http://dx.doi.org/10.1016/j.micinf.2015.01.004
- J. LEE, J.S. SHIM, M. CHUNG, S. LIM, and K.H. KIM, "Inhibition of Pathogen Adhesion to Host Cells by Polysaccharides from_Panax ginseng_", Bioscience, Biotechnology, and Biochemistry, vol. 73, pp. 209-212, 2009. http://dx.doi.org/10.1271/bbb.80555
- N.I. Belogortseva, J.Y. Yoon, and K.H. Kim, "Inhibition of Helicobacter pylori hemagglutination by polysaccharide fractions from roots of Panax ginseng.", Planta medica, 2000. http://www.ncbi.nlm.nih.gov/pubmed/10821045
- J.S. Woo, B.H. Ha, T.G. Kim, Y.H. Lim, and K.H. Kim, "Array", Biotechnology Letters, vol. 23, pp. 507-511, 2001. http://dx.doi.org/10.1023/A:1010360412969
- J. Lee, E.K. Park, C. Uhm, M. Chung, and K.H. Kim, "Inhibition of Helicobacter pylori adhesion to human gastric adenocarcinoma epithelial cells by acidic polysaccharides from Artemisia capillaris and Panax ginseng.", Planta medica, 2004. http://www.ncbi.nlm.nih.gov/pubmed/15254854
- J. Yang, S.Y. Choi, S. Park, N. Paek, and S.S. Kim, "Anti-Helicobacter Pylori effect of fermented ginseng extracts with Lactobacillus plantarum MG 208", Journal of the Korean Society for Applied Biological Chemistry, vol. 55, pp. 53-56, 2012. http://dx.doi.org/10.1007/s13765-012-0009-0
- H. Wu, N. Høiby, L. Yang, M. Givskov, and Z. Song, "Effects of Radix Ginseng on microbial infections: a narrative review", Journal of Traditional Chinese Medicine, vol. 34, pp. 227-233, 2014. http://dx.doi.org/10.1016/s0254-6272(14)60083-2
- S. Park, M. Yeo, J. Jin, K. Lee, J. Jung, R. Choue, S.W. Cho, and K. Hahm, "Rescue of Helicobacter pylori–Induced Cytotoxicity by Red Ginseng", Digestive Diseases and Sciences, vol. 50, pp. 1218-1227, 2005. http://dx.doi.org/10.1007/s10620-005-2763-x
- S. Park, M. Yeo, J. Jin, K. Lee, S.S. Kim, S.Y. Choi, and K. Hahm, "Inhibitory Activities and Attenuated Expressions of 5-LOX with Red Ginseng in Helicobacter pylori-Infected Gastric Epithelial Cells", Digestive Diseases and Sciences, vol. 52, pp. 973-982, 2007. http://dx.doi.org/10.1007/s10620-006-9440-6
- S. LEE, Y.W. SHIN, and K. HAHM, "Phytoceuticals: Mighty but ignored weapons against Helicobacter pylori infection", Journal of Digestive Diseases, vol. 9, pp. 129-139, 2008. http://dx.doi.org/10.1111/j.1751-2980.2008.00334.x
- E.A. Bae, M.J. Han, N.I. Baek, and D.H. Kim, "In vitro anti-Helicobacter pylori activity of panaxytriol isolated from ginseng.", Archives of pharmacal research, 2001. http://www.ncbi.nlm.nih.gov/pubmed/11534760
- M. Bae, S. Jang, J.W. Lim, J. Kang, E.J. Bak, J. Cha, and H. Kim, "Protective effect of Korean Red Ginseng extract against Helicobacter pylori-induced gastric inflammation in Mongolian gerbils", Journal of Ginseng Research, vol. 38, pp. 8-15, 2014. http://dx.doi.org/10.1016/j.jgr.2013.11.005
- H. Jee, K. Chang, S. Moon, S. Park, and H. Paik, " Anti-Helicobacter pylori, Cytotoxic, and Anti-inflammatory Activities of White Ginseng Extract.", Food Sci Biotechnol 17(5):1106-1109., 2008.
- P.A. Williams, and M.J. Worsey, "Ubiquity of plasmids in coding for toluene and xylene metabolism in soil bacteria: evidence for the existence of new TOL plasmids.", Journal of bacteriology, 1976. http://www.ncbi.nlm.nih.gov/pubmed/1254555
- F. Franzetti, M. Cernuschi, R. Esposito, and M. Moroni, "Pseudomonas infections in patients with AIDS and AIDS-related complex.", Journal of internal medicine, 1992. http://www.ncbi.nlm.nih.gov/pubmed/1588272
- J.B. Lyczak, C.L. Cannon, and G.B. Pier, "Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist.", Microbes and infection, 2000. http://www.ncbi.nlm.nih.gov/pubmed/10967285
- S.H. Hyun, E.S. Kim, S.M. Lee, J.S. Kyung, S.M. Lee, J.W. Lee, M.R. Kim, J.T. Hong, and Y.S. Kim, "Comparative Study on Immuno-Enhancing Effects of Red Ginseng Fractions", Journal of the Korean Society of Food Science and Nutrition, vol. 43, pp. 1665-1673, 2014. http://dx.doi.org/10.3746/jkfn.2014.43.11.1665
- P.S. Stewart, and J.W. Costerton, "Antibiotic resistance of bacteria in biofilms.", Lancet (London, England), 2001. http://www.ncbi.nlm.nih.gov/pubmed/11463434
- H. Wu, B. Lee, L. Yang, H. Wang, M. Givskov, S. Molin, N. Høiby, and Z. Song, "Effects of ginseng on_Pseudomonas aeruginosa_motility and biofilm formation", FEMS Immunology & Medical Microbiology, vol. 62, pp. 49-56, 2011. http://dx.doi.org/10.1111/j.1574-695X.2011.00787.x
- M.B. Miller, and B.L. Bassler, "Quorum Sensing in Bacteria", Annual Review of Microbiology, vol. 55, pp. 165-199, 2001. http://dx.doi.org/10.1146/annurev.micro.55.1.165
- Z. Song, K. Kong, H. Wu, N. Maricic, B. Ramalingam, H. Priestap, L. Schneper, J. Quirke, N. Høiby, and K. Mathee, "Panax ginseng has anti-infective activity against opportunistic pathogen Pseudomonas aeruginosa by inhibiting quorum sensing, a bacterial communication process critical for establishing infection", Phytomedicine, vol. 17, pp. 1040-1046, 2010. http://dx.doi.org/10.1016/j.phymed.2010.03.015
- H. Ceri, M.E. Olson, C. Stremick, R.R. Read, D. Morck, and A. Buret, "The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms.", Journal of clinical microbiology, 1999. http://www.ncbi.nlm.nih.gov/pubmed/10325322
- Z. Song, H.K. Johansen, V. Faber, C. Moser, A. Kharazmi, J. Rygaard, and N. Høiby, "Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats.", Antimicrobial agents and chemotherapy, 1997. http://www.ncbi.nlm.nih.gov/pubmed/9145852
- Z. Song, C. Moser, H. Wu, V. Faber, A. Kharazmi, and N. Høiby, "Cytokine modulating effect of ginseng treatment in a mouse model of Pseudomonas aeruginosa lung infection", Journal of Cystic Fibrosis, vol. 2, pp. 112-119, 2003. http://dx.doi.org/10.1016/S1569-1993(03)00065-1
- L. Thomer, O. Schneewind, and D. Missiakas, "Pathogenesis of Staphylococcus aureus Bloodstream Infections", Annual Review of Pathology: Mechanisms of Disease, vol. 11, pp. 343-364, 2016. http://dx.doi.org/10.1146/annurev-pathol-012615-044351
- . WHO, "Antimicrobial resistance: global report on surveillance 2014.", World Health Organization, pp 1-257., 2014.
- F.D. Lowy, "Antimicrobial resistance: the example of Staphylococcus aureus", Journal of Clinical Investigation, vol. 111, pp. 1265-1273, 2003. http://dx.doi.org/10.1172/JCI18535
- W.S. Sung, and D.G. Lee, "The Combination Effect of Korean Red Ginseng Saponins with Kanamycin and Cefotaxime against Methicillin-Resistant Staphylococcus aureus", Biological and Pharmaceutical Bulletin, vol. 31, pp. 1614-1617, 2008. http://dx.doi.org/10.1248/bpb.31.1614
- D. Lim, K. Bae, I. Jung, C. Kim, Y. Yun, and J. Song, "Anti-Septicaemic Effect of Polysaccharide from Panax ginseng by Macrophage Activation", Journal of Infection, vol. 45, pp. 32-38, 2002. http://dx.doi.org/10.1053/jinf.2002.1007
- J. Ahn, J. Song, Y. Yun, G. Jeong, and I. Choi, "Protection of_Staphylococcus aureus_-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan", FEMS Immunology & Medical Microbiology, vol. 46, pp. 187-197, 2006. http://dx.doi.org/10.1111/j.1574-695X.2005.00021.x
- J.H. Kim, Y. Yi, M. Kim, and J.Y. Cho, "Role of ginsenosides, the main active components of Panax ginseng , in inflammatory responses and diseases", Journal of Ginseng Research, vol. 41, pp. 435-443, 2017. http://dx.doi.org/10.1016/j.jgr.2016.08.004
- C. Yang, S. Ko, B. Cho, D. Shin, J. Yuk, S. Li, J. Kim, R.M. Evans, J. Jung, D. Song, and E. Jo, "The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin‐induced lethal shock", Journal of Cellular and Molecular Medicine, vol. 12, pp. 1739-1753, 2008. http://dx.doi.org/10.1111/j.1582-4934.2007.00181.x
- S. Na, J. Kim, Y.K. Rhee, and S. Oh, "Enhancing the antimicrobial activity of ginseng against Bacillus cereus and Staphylococcus aureus by heat treatment", Food Science and Biotechnology, vol. 27, pp. 203-210, 2017. http://dx.doi.org/10.1007/s10068-017-0209-9
- J. Mysak, S. Podzimek, P. Sommerova, Y. Lyuya-Mi, J. Bartova, T. Janatova, J. Prochazkova, and J. Duskova, "Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview", Journal of Immunology Research, vol. 2014, pp. 1-8, 2014. http://dx.doi.org/10.1155/2014/476068
- J. Lee, J.S. Lee, M. Chung, and K.H. Kim, "_In Vitro_Anti-Adhesive Activity of an Acidic Polysaccharide from_Panax ginseng_on_Porphyromonas gingivalis_Binding to Erythrocytes", Planta Medica, vol. 70, pp. 566-569, 2004. http://dx.doi.org/10.1055/s-2004-827160
- J. Lee, J.S. Shim, J.S. Lee, M. Kim, M. Chung, and K.H. Kim, "Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria", Carbohydrate Research, vol. 341, pp. 1154-1163, 2006. http://dx.doi.org/10.1016/j.carres.2006.03.032
- P. Xue, Y. Yao, X. Yang, J. Feng, and G. Ren, "Improved antimicrobial effect of ginseng extract by heat transformation", Journal of Ginseng Research, vol. 41, pp. 180-187, 2017. http://dx.doi.org/10.1016/j.jgr.2016.03.002
- V. Ramaswamy, V.M. Cresence, J.S. Rejitha, M.U. Lekshmi, K.S. Dharsana, S.P. Prasad, and H.M. Vijila, "Listeria--review of epidemiology and pathogenesis.", Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi, 2007. http://www.ncbi.nlm.nih.gov/pubmed/17332901
- Y. Choi, S. Kim, J. Huh, Y. Han, and M. Lee, "Antibacterial and Antioxidative Activity of Roasted Coffee and Red Ginseng Mixture Extracts", Journal of the Korean Society of Food Science and Nutrition, vol. 41, pp. 320-326, 2012. http://dx.doi.org/10.3746/jkfn.2012.41.3.320
- K.A. Lee, W.J. Kim, H.J. Kim, K. Kim, and H. Paik, "Antibacterial activity of Ginseng (Panax ginseng C. A. Meyer) stems–leaves extract produced by subcritical water extraction", International Journal of Food Science & Technology, vol. 48, pp. 947-953, 2012. http://dx.doi.org/10.1111/ijfs.12046
- L.P. Stenfors Arnesen, A. Fagerlund, and P.E. Granum, "From soil to gut:_Bacillus cereus_and its food poisoning toxins", FEMS Microbiology Reviews, vol. 32, pp. 579-606, 2008. http://dx.doi.org/10.1111/j.1574-6976.2008.00112.x
- E.J. Bottone, "Bacillus cereus , a Volatile Human Pathogen", Clinical Microbiology Reviews, vol. 23, pp. 382-398, 2010. http://dx.doi.org/10.1128/CMR.00073-09
- K. Lim, H. Kang, S. Kang, and B. Lee, " Antioxidant and antimicrobial activities of various solvent fractions of fine ginseng root.", Food Sci Biotechnol 18(2):513-518., 2009.
- C.T. Nguyen, T.T. Luong, S.Y. Lee, G.L. Kim, H. Kwon, H. Lee, C. Park, and D. Rhee, "Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation", Phytomedicine, vol. 22, pp. 1055-1061, 2015. http://dx.doi.org/10.1016/j.phymed.2015.07.005
- J. Ahn, I. Choi, J. Shim, E. Yun, Y. Yun, G. Jeong, and J. Song, "The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll‐like receptor‐mediated inflammatory signals", European Journal of Immunology, vol. 36, pp. 37-45, 2005. http://dx.doi.org/10.1002/eji.200535138
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ko-rea government (MSIP) (No. 2016R1D1A1A02937312). This work was supported by the research fund of Hanyang Univer-sity (HY-2015-N).