Frontiers | Chemokine Receptor-Specific Antibodies in Cancer Immunotherapy: Achievements and Challenges (original) (raw)
Introduction
Cancer treatment is expanding from non-specific cytotoxic chemotherapies to targeted therapies. These are focused on fighting cancer cells, modifying the tumor microenvironment, or enhancing anti-tumor immunity (1–3). Malignant and stromal cells secrete a variety of proteins, including matrix components, proteolytic enzymes, growth factors, pro-inflammatory cytokines, and chemokines (2, 4). Among them, tumor-associated chemokines play a central role in cancer biology, favoring leukocyte infiltration, promoting tumor growth, angiogenesis, and immune evasion (5–10).
Many tumor cells over-express functional chemokine receptors, undetectable on their normal counterparts. These receptors respond to chemokine signals by promoting cell survival, proliferation, adhesion, or migration, but also direct metastasis formation on tissues or organs where the corresponding ligands are secreted (9). Since the most common cause of death in cancer patients are metastases (11, 12), chemokine receptors expressed on the surface of cancer cells are considered suitable targets for the generation of new anti-tumor drugs (8, 13–17). As a consequence, a great effort has been made in the investigation of new drugs targeting chemokine receptors for cancer treatment. The initial focus was on the development of small molecules able to inhibit chemokine receptor signaling, although with limited success (18, 19). Recent achievements in the use of monoclonal antibodies (mAbs) targeting a variety of molecules for the treatment of leukemia, breast cancer, colon cancer, and melanoma (20–23), have contributed to move the efforts onto targeting chemokines and their receptors toward generating specific antibodies for therapeutic purposes.
The clinical use of an anti-chemokine receptor mAb, mogamulizumab, specific for the C–C chemokine receptor type 4 (CCR4) has been granted in Japan for the immunotherapy of patients with relapsed or refractory CCR4+ adult T-cell leukemia (ATL) (24). This antibody is currently in phase II and III clinical trials in Europe and the USA for the treatment of patients with ATL, cutaneous T-cell lymphoma (CTCL), or peripheral T-cell lymphoma (PTCL) (24, 25). In addition, antibodies specific for the CCR2 and CXCR4 receptors are also being evaluated in clinical trials, while antibodies against many other chemokine receptors have shown effectiveness in different xenograft models of cancer (26–38).
Chemokines and Their Receptors in Cancer
Chemokines are a family of small chemotactic cytokines, with 44 members in humans, which generate soluble or immobilized gradients that direct the movement of cells (39, 40). Chemokines, by controlling leukocyte trafficking and recruitment, play a central role in homeostasis and the maintenance of innate and acquired immunity (41). They are essential in mammalian development and organogenesis, and like other cytokines stimulate cell growth, differentiation, and activation (16). Chemokines are subdivided in four major groups, namely CX3C, CXC, CC, and C, based on the number and spacing of conserved cysteine residues on their N-terminus (39, 42). These proteins are functionally known as “inflammatory” or “homeostatic,” based on whether they are released upon inflammatory stimuli or constitutively secreted by cells located in lymphoid organs, respectively (43).
The biological effects of chemokines are exerted through their interaction(s) with specific surface receptors (chemokine receptors), structurally belonging to the seven transmembrane domain G protein-coupled receptor superfamily (GPCR). The chemokine receptor family contains 24 members in humans and can be subdivided, based on the class of chemokines they bind, into four subfamilies (CX3CR, CXCR, CCR, and XCR) all of them activating G proteins, and one subfamily (ACKR), containing 6 atypical receptors, unable to activate G proteins upon ligand binding (39, 44). It is worth to note that the chemokine/chemokine receptor system has redundancy, since some particular chemokines are able to bind to multiple receptors, and vice versa (41).
Chemokines and their receptors have been implicated in the pathogenesis of many inflammatory and infectious diseases including rheumatoid arthritis, multiple sclerosis, asthma, atherosclerosis, malaria, and AIDS (15, 16, 45, 46), but also in cancer (5, 47). Expression levels of chemokines and their receptors are often deregulated in malignant cells, due, for example, to inactivation of tumor suppressor genes, constitutive activation of oncogenes, or altered expression of transcription factors (8, 48–50).
Expression of chemokines and their receptors play a dual role in tumorigenicity. On the one hand, chemokines secreted by either the cancer-initiating cells or the normal cells surrounding them can help limiting tumor development by increasing leukocyte migration toward the site, and inducing long-term anti-tumor immunity. On the other hand, they may facilitate survival, proliferation, and metastatic potential of tumor cells (6, 10, 51–54). The initially secreted chemokines at the tumor site play a key role defining the composition of the tissue stroma and recruiting tumor infiltrating leukocytes bearing specific chemokine receptors (CXCR1, CXCR2, CCR2, CCR4, or CCR5, among others) (7). Therefore, many chemokines are pro-inflammatory for most tumors (4) and have key functions in tumor angiogenesis (55, 56). For example, CXCL12 (SDF-1), the only known ligand for CXCR4, is a potent endothelial cell chemoattractant (57, 58).
Tumor cells are able to hijack the chemokine receptor/chemokine system on their own benefit. Strikingly, they “convert” infiltrating leukocytes into immuno-tolerant allies (59–61), since they are able to (i) attract suppressor T-cells and neutrophils (62–65), (ii) hijack immature dendritic cells, avoiding their migration toward the lymph nodes and therefore antigen presentation, favoring a tolerogenic profile (66), and (iii) participate in the recruitment and induction of myeloid-derived suppressor cells (67).
The most frequently over-expressed chemokine receptor in malignant cells is CXCR4 (68). It is present in over 23 different types of human cancer, including those with the highest incidence, such as lung, brain, prostate, breast, pancreas, ovarian, colorectal, leukemia, and melanomas (63). CXCR4 expression on malignant cells correlates with cell survival, tumor growth, angiogenesis, higher metastatic potential, and resistance to therapeutic agents (35, 69–76). Its ligand, CXCL12, is secreted in large amounts by bone marrow, lymph node, liver, and lung cells.
CXCR4 and CXCL12 can be used to exemplify the complexity of the chemokine/chemokine receptor networks in cancer. Unlike other chemokine receptors that have several ligands, it was originally thought that CXCL12 was the unique ligand for CXCR4 and that it was unable to bind any other receptor. In 2005, it was reported that CXCL12 was also able to bind ACKR3 (formerly CXCR7) with 10 times higher affinity (77–80). ACKR3 has two ligands CXCL12 and CXCL11. This receptor, in addition to important roles in embryonic development and cardiovascular functions (79, 81, 82), also participates in breast and lung tumorigenesis and metastasis (78). The complexity of this network is even higher since the ACKR3 ligand CXCL11 is also shared by CXCR3, a chemokine receptor with two isoforms (A and B), over-expressed in many tumors, and able to bind other ligands (CXCL9 or CXCL10) (83). Altered CXCR3 isoform expression regulates cancer cell migration and invasion (84). Indeed, CXCL11 promotes proliferative signals through binding to CXCR3-A or ACKR3, whereas binding to CXCR3-B results in growth inhibitory functions (85). The complexity of several ligands being able to bind different receptors or different isoforms of a given receptor, having different outcomes (proliferation vs. growth inhibition), should be taken into account on any pharmacological intervention.
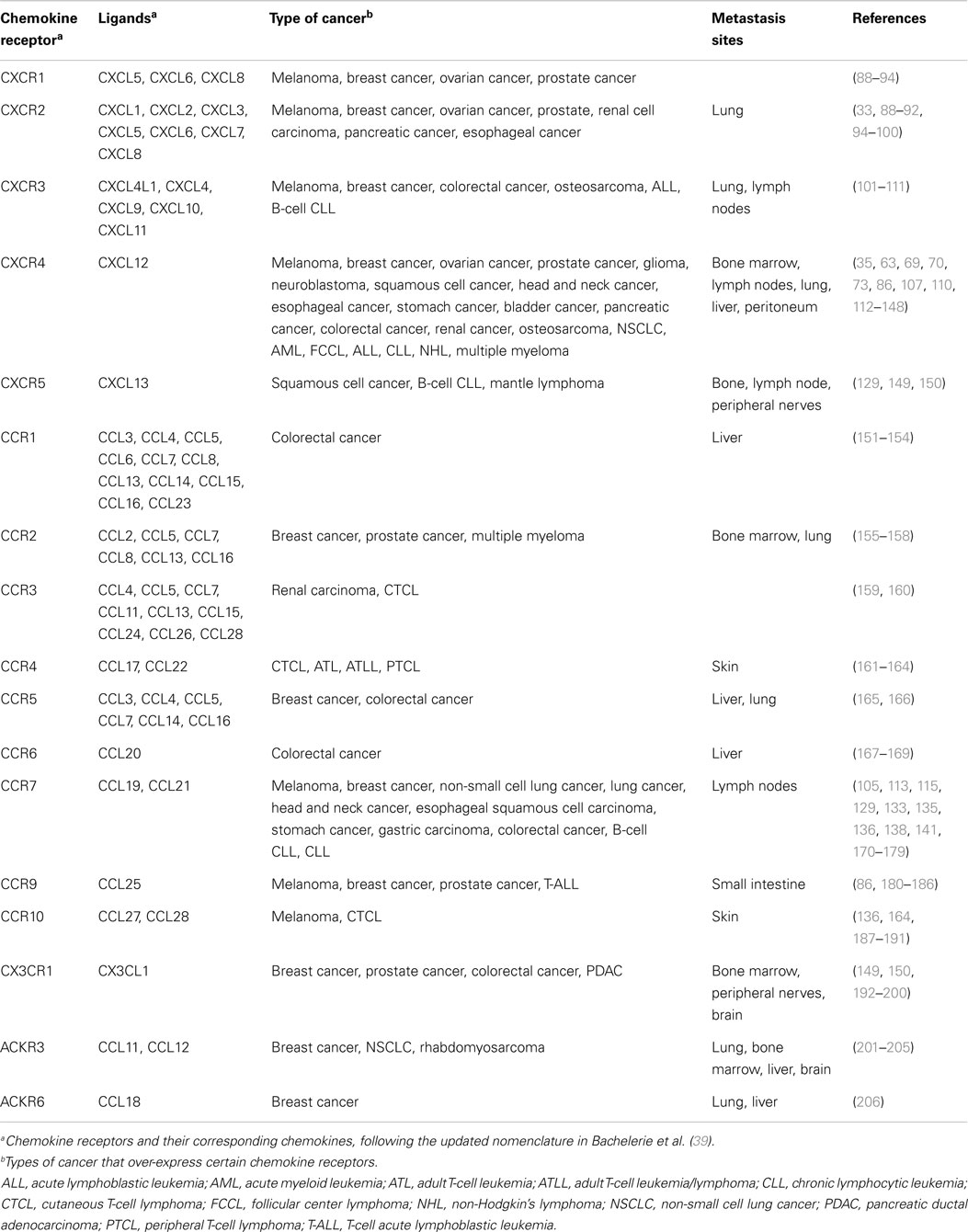
There is broad evidence indicating that the expression of a determined chemokine receptor by a tumor preferentially directs its metastasis to the organs in which the corresponding chemokine ligand is secreted (10, 86), some of them are detailed in Table 1. Extensive revision of the expression and actions of chemokine receptors in cancer exceeds the focus of this review and can be obtained from recent reviews in Ref. (86, 87).
Table 1. Human chemokine receptors and related metastases.
Antibody-Based Drugs for Cancer Therapy
Monoclonal antibodies are relatively large molecules with clear advantages for their use as therapeutic drugs. These are related to their long half-life in blood, their ability to establish specific and high affinity interactions with other molecules or with immune system cells, together with their relatively low toxicity (207–210). Drugs based on mAbs are, however, more difficult and expensive to develop and produce, and less convenient to administer than small molecule drugs. Indeed, they are able to bridge the target antigen, or cells bearing the antigen, with the innate or acquired cellular immune response (211). These characteristics, together with the development of antibody humanization techniques, phage display systems, advanced high-throughput screening methods, and transgenic mice that produce human antibodies, led to many pharmaceutical companies to invest on therapeutic mAbs. This, together with the clinical success of therapeutic antibodies during the last decade, has led to an exponential increase on the number of mAbs for cancer treatment. For instance, in oncology, chimeric and humanized mAb that entered clinical studies had approval success rates four times greater than new chemical entities, including small molecule agents (211–213). The development of therapeutic antibodies is growing fast, and includes many best-selling drugs for the treatment of cancer (rituximab, bevacizumab, trastuzumab) or immunological diseases (adalimumab, infliximab) (214).
Therapeutic antibodies for cancer treatment can be classified, on the basis of the targets they are directed to, into: (i) surface-expressed molecules on the tumor cell; (ii) cytokines, growth factors, surface receptors, or other molecules required for tumor and/or stroma proliferation or survival; and (iii) immune cell surface molecules that regulate tumor cell recognition and elimination. Conversely, on the basis of their mechanisms of action, they would be classified as mAbs that kill tumor cells through: (i) direct effects (host independent) (i.e., inhibiting receptor-ligand binding, and/or activating intracellular signaling); (ii) indirect effects (host dependent) modulating the immune response [i.e., antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), antibody-dependent phagocytosis (ADP), etc.]; (iii) being used as molecular carriers, specifically delivering cytotoxic agents, toxins, or radio-isotopes, to target malignant cells (209); and (iv) targeting regulatory molecules on host immune system cells. Therapeutic mAbs often exert their anti-tumoral functions simultaneously using several of these mechanisms of action (29).
During the last years, a broad effort has been centered on targeting regulatory molecules from the host immune system that act as “immune checkpoints” with mAbs. Examples are ipilimumab, a mAb directed against the receptor cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), or pembrolizumab (MK-3475), a mAb against the programed cell death protein-1 (PD-1), used for the treatment of metastatic melanomas (215). These mAbs inhibit the negative regulatory signals triggered by CTLA-4 or PD-1, enhancing T-cell responses against the tumor (215–218). Other mAbs, recognizing antigens on the antigen presenting cells, such as an agonist mAb against the stimulatory protein CD40 have also been postulated to harbor therapeutic effects against tumors (218, 219).
Antibodies have been generated against chemokines and their receptors. Since chemokine receptors have seven domains embedded in the cell membrane, their solubilization for obtaining the required amounts in the native conformation and the correct orientation for their use as immunogens is extremely difficult (220). These characteristics, together with their multiple post-translational modifications, low cell surface expression levels and lack of stability of their native conformations, make it particularly challenging to generate antibodies against them. Synthetic peptides had been used as immunogens, although this approach usually generates mAbs with low affinity and poor antagonistic effects (221, 222). The development of sophisticated strategies that preserve the protein native conformation during purification, along with advances in the synthesis of peptides with pre-designed structures, genetic immunization techniques, production of chemokine receptor-containing liposomes or lipoparticles, or over-expression of receptors in viral particles, enabled the generation of antibodies with higher affinities and/or able to function as strong antagonists (220). The expected in vivo efficacy of mAbs anti-chemokine receptors is higher than those against chemokines, since a cell surface-restricted receptor molecule is more efficiently targeted than delocalized secreted chemokines (220, 223). In addition, chemokine receptor targeting offers the possibility not only of blocking the signaling by preventing ligand binding to its receptor but also of tagging the tumor cells with the antibody, to trigger the host immune response against them.
Anti-chemokine receptor antibodies have been evaluated for the treatment of inflammatory and infectious diseases, including anti-CCR2 for rheumatoid arthritis and atherosclerosis (224); CCR3 and CCR4 for asthma and pulmonary inflammation (225–228); CXCR4 and CCR5 for HIV infections (229, 230); and CCR7 for pulmonary fibrosis (231). However, in the following paragraphs, we will only focus on their potential as anti-cancer drugs.
Chemokine Receptors with Antibodies in Clinical Trials for Cancer Treatment
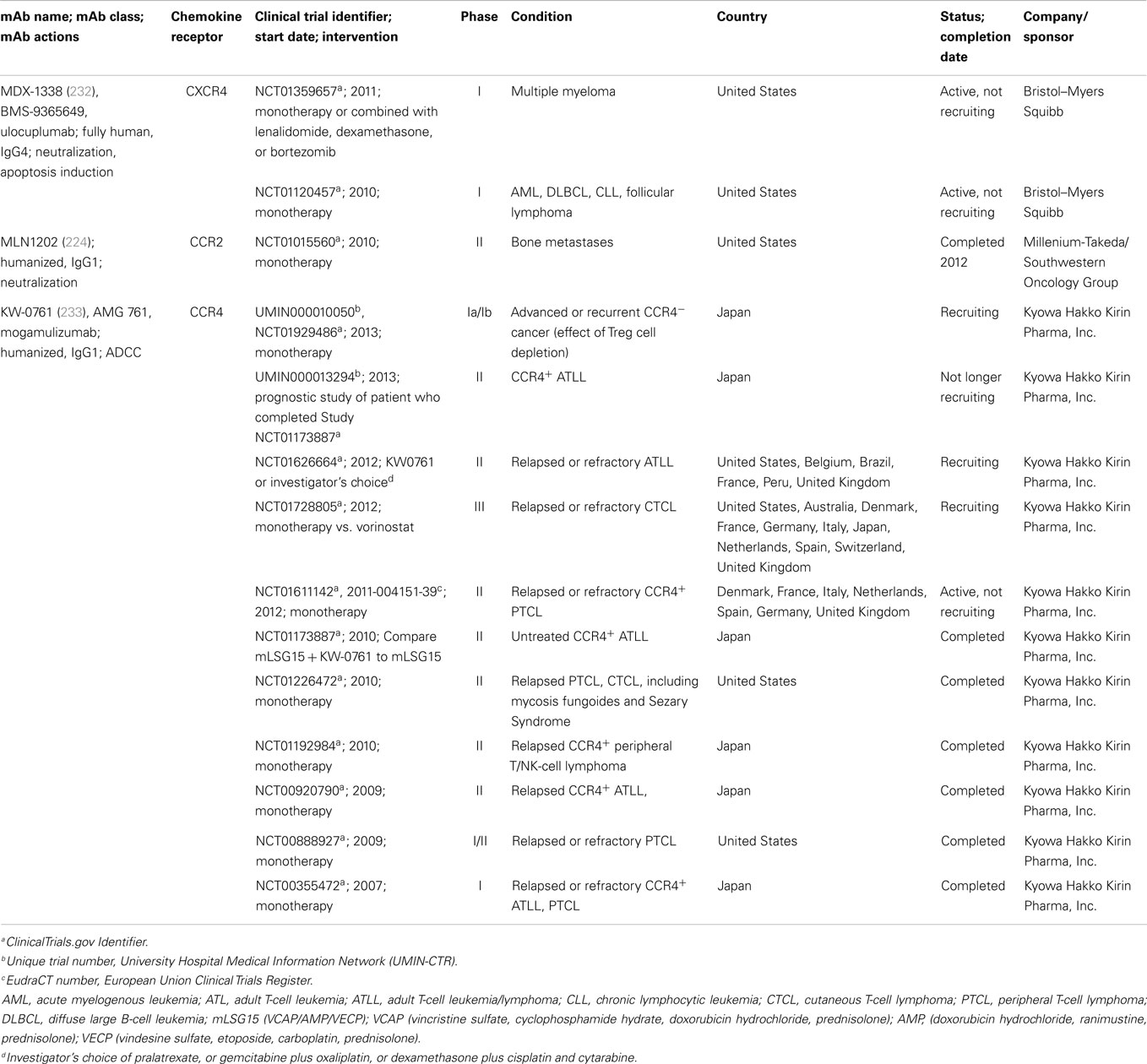
Monoclonal antibodies against CXCR4, CCR2, and CCR4 have entered clinical trials for cancer therapy. A list of trials with these antibodies is shown in Table 2, and antibodies against each of these receptors and their potential in cancer therapy are described below.
Table 2. Anti-chemokine receptors antibodies for cancer therapy in clinical trials.
CXCR4
As demonstrated by a plethora of publications, CXCR4 has a key role in fundamental aspects of cancer, including proliferation, migration, invasion, and angiogenesis (35, 69–76, 234–237), leading to a number of programs to develop therapeutic anti-CXCR4 antibodies. The most advanced candidate is MDX-1338, an anti-CXCR4 mAb also known as BMS-936564 (owned by Bristol-Myers Squibb Co.). It was raised on human Ig transgenic mice immunized with human CXCR4-transfected mouse cells (232). This antibody (IgG4) blocks CXCL12 binding to its receptor with high affinity, and inhibits CXCL12-induced migration and calcium flux. MDX-1338 shows anti-tumoral activity in xenografts of acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma. In vitro assays showed that the antibody triggers tumor cell apoptosis, allowing to propose it as one of the mechanisms of tumor growth inhibition (232).
MDX-1338 is currently undergoing two Phase I studies. The first-in-human study (ClinicalTrials.gov Identifier: NCT01120457) started in 2010 and was planned to be accomplished by the end of 2014 and to enroll up to 82 patients. This anti-CXCR4 mAb is being evaluated as a monotherapy and combined with chemotherapy to treat patients with relapsed/refractory AML, diffuse large B-cell leukemia, chronic lymphocytic leukemia (CLL), or follicular lymphoma. The aim of the trial is to determine the safety, tolerability, maximum tolerated dose, preliminary pharmacodynamics, and efficacy. A second Phase I trial (NCT01359657) started in 2011 to determine safety and tolerability of MDX-1338 as monotherapy or in combination with lenalidomide/dexamethasone or bortezomib/dexamethasone in subjects with relapsed/refractory multiple myeloma. This study is planned to enroll up to 64 patients and be finished in 2015.
Other antibody-derived molecules targeting CXCR4 are being evaluated in clinical trials. This is the case for ALX-0651 (owned by Ablynx, Belgium), a biparatopic anti-CXCR4 nanobody, directed against two different epitopes of CXCR4 (32). Nanobodies are single-domain proteins, derived from the antibody-binding fragment of camelid antibodies. Their immunoglobulins are devoid of light chains and possess only heavy-chains. Nanobodies have the advantages of their relative small size (12–15 kDa) and high solubility, which allows them to cross tissue barriers easily than mammalian immunoglobulins (with a 10-fold higher M.W.). ALX-0651 effectively mobilizes hematopoietic stem cells in a pre-clinical cynomolgus monkey model (32). A Phase I study of safety and effectiveness for this nanobody in healthy volunteers started in 2011 (NCT01374503), but no results have yet been reported.
In addition, many pre-clinical reports have demonstrated the in vivo relevance of CXCR4 as a target for cancer therapy. An early report by Müller and co-workers demonstrated a key role for chemokine receptors in metastasis, linking the expression of CXCR4 in breast carcinomas with their ability to generate regional lymph node and lung metastases (35). These data were supported by experiments in which a neutralizing anti-human CXCR4 antibody (clone 44717.111) led to a significant decrease in lung, inguinal, and axillary lymph node metastases. This work highlighted that chemokine interactions with chemokine receptors might turn to be crucial for breast cancer metastasis, showing that antibodies anti-CXCR4 may be useful to interfere with tumor progression and metastasis. Subsequently, similar results were obtained treating xenografts of a human NHL (26) and of a primary human AML (38) with another anti-human CXCR4 antibody (clone 12G5). In both models, a significant reduction on tumor progression was reported. In endometrial cancer xenografts, treatment with 12G5 mAb led to a complete inhibition of spontaneous metastases in liver and lung, and a 28-fold decrease in metastatic index in the peritoneum (30). Interestingly, on an intratibial human osteosarcoma xenograft model, 12G5 mAb reduced metastatic spread to the lung (27).
CCR2
CCR2 expression in tumor cells facilitates prostate and breast cancer metastases to the bone, where its ligand CCL2 is expressed (155, 238). Prostate cancer patients with bone metastases had higher CCL2 serum levels than patients with localized tumors (155). In vitro and in vivo experiments using CCR2 or CCL2 knocked down prostate cancer cells demonstrated that these proteins promote prostate cancer growth in bone (238). Similarly, breast cancer metastasis to bone and lung is facilitated by CCL2 interaction with the CCR2+ stromal cells of monocytic origin, including macrophages and pre-osteoclasts (238). MLN1202, a humanized, neutralizing anti-CCR2 mAb (224) (developed by Millenium Pharmaceuticals Inc., currently Takeda Pharmaceuticals Co.) went through a Phase II clinical trial for the treatment of bone metastases (NCT01015560). MLN1202 was administered to 44 patients with bone metastasis to address its effect on tumor cell proliferation, monocyte/macrophage trafficking, and osteoclast maturation. Forty-one out of 43 eligible patients completed this study, with 7% having serious adverse events. The concentration in urine of the _n_-telopeptide, a biomarker to measure bone turnover rates, decreased in 14% of the patients after 43 days of MLN 1202 treatment, suggesting a positive effect of the antibody in these patients.
CCR4
CCR4 is a chemokine receptor predominantly expressed on type 2 T helper cells (Th2), Foxp3+ regulatory T-cells (Treg), a subset of CD4+ Th17 cells, and skin-homing T-cells positive for cutaneous lymphocyte antigen (CLA) (239–243). This receptor binds two ligands, CCL17 (formerly TARC) and CCL22 (formerly MDC), and has been implicated in the pathology of inflammatory diseases and cancer, being over-expressed on many malignant ATL, CTCL, and PTCL cells (161–163).
The antibody KW-0761 (mogamulizumab), a derivative of the mouse KM2160 mAb, is a humanized defucosylated IgG1 mAb targeting CCR4 (developed by Kyowa Hakko Kirin Co.) (233). KM2160, its chimera KM2760 and the humanized version KW-0761 recognize the N-terminal region of human CCR4 (163, 233, 244). They neither block the interaction between CCR4 and their ligands nor inhibit CCR4 signaling (233). Defucosylation increases Fc-binding to the Fcγ receptors expressed on cytotoxic cells, activating them for tumor cell killing (245). In fact, KM2760 showed potent anti-tumor activity in mouse xenografts of CCR4+ cell lines derived from patients diagnosed of ATL, Hodgkin lymphoma, or CTCL (242, 246, 247). In addition, this mAb showed enhanced ADCC against primary CCR4+ ATL cells both in vitro and in vivo in an autologous setting (163, 233, 246). A phase I clinical trial of KW−0761 for patients with relapsed CCR4+ PTCL or ATL was the first one to examine the safety and efficacy of a new generation defucosylated therapeutic antibodies for cancer treatment (NCT00355472) (248).
KW-0761 was approved for therapeutic use in Japan for relapsed or refractory CCR4+ ATL in 2012, and for relapsed or refractory CCR4+ PTCL or CTCL in 2014, representing the first approved antibody drug against GPCR receptors being used for cancer therapy. In other countries, there are several clinical trials currently under way, such as a Phase II (NCT01611142) for patients with relapsed or refractory PTCL, and a Phase III for the comparison of progression-free survival, after treatment with either KW-0761 or vorinostat (a chemical inhibitor of histone deacetylases), on patients with previously treated CTCL (NCT01728805). Despite the positive effects of mogamulizumab in resistant/refractory ATL, PTCL, or CTCL, the application for its use in untreated CCR4+ ATL was withdrawn by the company, on February 2014.
A therapeutic potential for mogamulizumab has been suggested against Epstein–Barr virus (EBV)-associated T- and NK-cell lymphoproliferative diseases, which can be refractory to conventional chemotherapies (249). In particular, since this mAb induced ADCC activity against CCR4+ EBV+-T and -NK-cell lines, and inhibited the growth of EBV+ NK-cell lymphomas in xenografts (249).
It is known that Treg cells can facilitate tumor cell evasion from immune surveillance (250). Since CCR4 is expressed on Treg cells, it was conceivable that treatments targeting CCR4 might affect Treg cells. On CTCL patients, a single dose of mogamulizumab has been shown to reduce the fraction of CCR4+ malignant T-cells, with a concomitant reduction of CCR4+ Treg cells. Interestingly, the reduction of Treg cells may, in turn, improve the immune profiles of these patients (251). KM2760 has also been used, in co-treatment with NK cells, for the in vitro elimination of Treg cells (252). A patient treated with mogamulizumab suffered serious adverse reactions and the Stevens–Johnson syndrome (a milder form of toxic epidermal necrolysis), probably due to a significant reduction of its Treg cells (253). A positive interpretation of these results would suggest, however, that anti-CCR4 mAbs could be used on CCR4− tumors to deplete Treg cells from circulation and infiltrating the tumor mass. Indeed, a phase I clinical trial of mogamulizumab for CCR4− solid cancers (UMIN000010050), specifically aiming to deplete Treg cells is currently under way (253). Other adverse effects of mogamulizumab include cutaneous reactions that improve over time (254), the reactivation of hepatitis B virus (255), or diffuse panbronchiolitis (256).
There are other anti-CCR4 mAbs being screened on discovery or pre-clinical phases. Among them, mAb1567 is a humanized neutralizing anti-CCR4 antibody that exhibits potent anti-CCR4+ CTCL tumor activity in xenografts, where it displays in vitro CDC and neutrophil-mediated ADCC. mAb1567 also exerts in vitro human NK cell-mediated ADCC (29). In addition, SCID-beige mice expressing an adenovirus construct derived from mAb1567 allowed an effective in vivo treatment of CTCL (257). Furthermore, mAb2–3, an affinity-optimized variant of the humanized mAb1567, has been selected for further pre-clinical development (29). Human anti-CCR4 antibodies, generated by phage display (17G and 9E), also show in vitro efficient killing of CCR4+ tumor cells via ADCC and phagocytosis, and improved survival in xenografts (31).
Chemokine Receptors with Antibodies in Discovery or Pre-Clinical Assays for Cancer Treatment
CXCR2
CXCR2 and its ligands, the chemokines CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8, are expressed by a wide variety of human cancer types (41, 258). CXCR2 is a potent protumorigenic receptor that mediates the recruitment of immunosuppressive leukocytes into tissues, in particular of neutrophils (259). Immunopathological analyses demonstrated the expression of high levels of CXCR2 and its ligand CXCL5 in pancreatic tumors (33). Thus, it was conceivable that treatment with anti-CXCR2 antibodies would inhibit leukocyte infiltration and their pro-tumoral activities. Indeed, treatment with neutralizing anti-mouse CXCR2 antibodies of human pancreatic tumors growing as xenografts displayed reduced tumor volumes, decreased proliferation indexes, and microvessel densities (33, 260). The pancreatic tumor cell line used in that model was devoid of CXCR2 expression (261). CXCR2 can also be expressed on endothelial cells (262), where it can mediate angiogenesis (263). These data, together with a series of in vitro assays demonstrating an anti-angiogenic role for antagonist anti-CXCR2 mAbs in endothelial cells (260), suggested that inhibition of tumor growth in the xenograft model was due to angiogenesis inhibition mediated by these antibodies.
CXCR5
A different approach was used to target CXCR5. In this case, a bispecific Ab, containing paratopes recognizing CXCR5 on the one side and the CD3-T-cell co-receptor on the other, was used (36). With this tool, the authors were able to bring together the tumor cell, recognized with the anti-CXCR5 paratope, with a T lymphocyte, recognized with the anti-CD3 paratope, maintaining the ability of the antibody to bind cells of the innate immune response through the Fc region, potentiating in this way the anti-tumoral response (36). This bispecific antibody was highly efficient lysing tumor cells at low concentrations not only in vitro but also in vivo on xenograft models of B-cell lymphoma (36).
CCR7
In subcutaneously injected human mantle cell lymphoma (MCL) cells on mice, anti-CCR7 treatment caused a significant delay on tumor growth and metastasis generation. It also hindered lymphoma cell dissemination in intravenous injections (37). The data obtained were compatible with both decreased infiltration of MCL cells into different tissues and the induction of anti-MCL cell cytotoxicity in mice. Anti-CCR7 therapy might be indicated for patients carrying CCR7+ B-cell NHL or CLL (37).
CCR9
We have reported the generation and characterization of 91R, a mouse anti-human CCR9 mAb able to reduce 85% human T lymphoblastic cell tumors on mice. Tumor size reduction was concomitant with an increased apoptotic tumor cell fraction and tumor necrotic areas, as well as decreased fraction of proliferating cells and tumor vascularization. It is likely that CDC or ADCC represent the in vivo mechanisms of action of this mAb (28). These results suggest that CCR9-expressing tumors, such as acute and chronic T-cell lineage leukemia (180), prostate cancer (181), breast cancer (182), and melanomas (183), can potentially be targeted with this mAb.
ACKR3 (Formerly CXCR7)
Anti-ACKR3 nanobodies inhibited tumor growth of ACKR3+ head and neck cancer cells, reducing expression of the endothelial cell marker CD31 in the tumors growing as xenografts. These data were corroborated by in vitro analyses demonstrating that anti-ACKR3 nanobodies rather than affecting cell cycle progression, reduced the secretion of the angiogenic chemokine CXCL1 by the tumor cells, suggesting that anti-ACKR3 nanobodies could inhibit tumor vascularization. This work proposes anti-ACKR3 therapies as potential novel treatments against ACKR3+ head and neck cancer (34).
The antibodies described so far are representative of the use of anti-chemokine receptor antibodies for cancer treatment, but they represent only the tip of the iceberg, since many companies describe in their web pages, or have already presented in specialized meetings their efforts for developing new antibodies. Some of these antibodies are AT008 (anti-CCR4) and AT009 (anti-CXCR4) from Affitech; anti-CXCR5, anti-CCR2, and anti-CXCR3 from Sorrento Therapeutics Inc.; anti-CXCR4 (515H7) from Pierre Fabre (264); and anti-CXCR4 (CX-02 and CX-05) from NorthWest Biotherapeutics Inc. (265).
Conclusion and Perspectives
Chemokines and their receptors, in addition to their role on physiological responses, directing the cells toward specific sites, allowing lymphocyte maturation, survival, proliferation, and migration, they play a key role in cancer initiation, angiogenesis, tumor growth, progression, and metastasis. The over-expression by many tumors of chemokine receptors turns them and their ligands into clear targets for cancer therapy, on the initial assumption that inhibition of chemokine signaling might block tumor progression and/or metastasis.
The development of small molecules able to inhibit chemokine receptor signaling has shown limited success, with the exception of plerixafor (AMD3100), which by blocking binding of CXCL12 to CXCR4 mobilizes CXCR4+ cells, including CXCR4+ tumor cells, from the bone marrow to circulation (266, 267). Plerixafor enhances sensitivity of tumor cells to cytotoxic agents by disrupting interaction with the tumor microenvironment (268–271). Since therapeutic mAbs have provided clinical benefits to cancer patients during the last decade, therapeutic antibodies against chemokine receptors seemed a good alternative. The recent technological advances allowed the generation of highly specific, high affinity antibodies against these receptors that are currently entering the clinics.
This “theoretical” view suggesting that anti-chemokine receptor mAbs might represent an efficient way to treat cancer was fully supported by the recent approval in Japan of mogamulizumab for the treatment of ATL. It is so far the only anti-GPCR therapeutic antibody in the market and represents the proof-of-principle for the therapeutic use of mAbs targeting GPCRs, as on chemokine receptors, leading to clinical benefits on cancer patients.
Chemokine receptors are expressed, in addition to endothelial cells, on the tumor and on cells from the immune system, responsible for defending from the tumor. Therefore, any drug or antibody targeting a given chemokine receptor will act on both the tumor and immune system cells expressing it. In the case of mogamulizumab, raised against CCR4, it should be noted that there is only a fraction of T lymphocytes (within the Th2, Treg, and Th17 phenotypes) that are CCR4+, representing the few non-tumoral targets for this antibody. Since the phenotypes affected include Treg and Th17 cells, their elimination would become advantageous for treating the tumor, as the immunosuppressive responses would be reduced. Treatment with antibodies against other chemokine receptors such as CXCR4 or CCR7 might have a broad effect on the host immune response, since there is a large fraction of leukocytes expressing them. This effect is not necessarily beneficial in terms of reducing tumor size.
These data raise the question whether other anti-chemokine receptors can be safely used as targets for tumor treatment. In particular, it should be determined, for each chemokine receptor, whether there are therapeutic doses of an antibody able to effectively kill tumor cells or cells favoring tumor growth, while not affecting the normal cells from the immune system expressing that particular receptor. In other words, the aim is to find the therapeutic window where tumor cells are safely destroyed whereas the immune cells are not. This can be exemplified with data on CCR2+ tumor infiltrating cells, where if the infiltrating cells are macrophages supporting the metastatic dissemination of malignant cells, the anti-CCR2 treatment may be effective. Conversely, if the infiltrating cells are CD8+ and γδ effector T-cells that enhance immuno-surveillance by triggering Th1 responses, the treatment might turn deleterious (272, 273).
Thus, the complex network between chemokine receptors and their ligands, together with the simultaneous expression of a given receptor on cells of the immune system and on the tumor (including tumor cells, stroma cells, and/or tumor infiltrating cells) and the dichotomy of their responses clearly represent the lights and shadows of the potential of anti-chemokine receptor therapeutic antibodies for the treatment of tumors. The optimal situation would be to have a panel of therapeutic antibodies against different chemokine receptors. These antibodies could be used, depending on the tumor phenotype, in particular combinations (personalized medicine) at relatively low doses for each one of them, minimizing the probability of affecting the normal cells.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Inés Antón and M. Teresa Martín for critical reading of the manuscript and for helpful suggestions. The work in the authors’ laboratory was supported by grants from the Instituto de Salud Carlos III (PI10/00594 and PI14/00703) and the CSIC (201320E109 and 201420E109), Ministerio de Economía y Competitividad to Leonor Kremer.
References
1. Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol (2014) 24:71–81. doi:10.1016/j.semcancer.2013.08.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature (2001) 411(6835):375–9. doi:10.1038/35077241
CrossRef Full Text | Google Scholar
3. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer (2012) 12(4):237–51. doi:10.1038/nrc3237
CrossRef Full Text | Google Scholar
5. Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev (2002) 13(2):143–54. doi:10.1016/S1359-6101(01)00033-8
CrossRef Full Text | Google Scholar
8. Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev (2010) 21(1):27–39. doi:10.1016/j.cytogfr.2009.11.007
CrossRef Full Text | Google Scholar
9. Mukaida N, Baba T. Chemokines in tumor development and progression. Exp Cell Res (2012) 318(2):95–102. doi:10.1016/j.yexcr.2011.10.012
CrossRef Full Text | Google Scholar
14. Garber K. First results for agents targeting cancer-related inflammation. J Natl Cancer Inst (2009) 101(16):1110–2. doi:10.1093/jnci/djp266
CrossRef Full Text | Google Scholar
15. Garin A, Proudfoot AE. Chemokines as targets for therapy. Exp Cell Res (2011) 317(5):602–12. doi:10.1016/j.yexcr.2010.12.021
CrossRef Full Text | Google Scholar
16. Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res (2011) 317(5):575–89. doi:10.1016/j.yexcr.2011.01.005
CrossRef Full Text | Google Scholar
19. Debnath B, Xu S, Grande F, Garofalo A, Neamati N. Small molecule inhibitors of CXCR4. Theranostics (2013) 3(1):47–75. doi:10.7150/thno.5376
CrossRef Full Text | Google Scholar
21. Ascierto PA, Addeo R, Carteni G, Daniele B, De Laurentis M, Ianniello G, et al. The role of immunotherapy in solid tumors: report from the Campania society of oncology immunotherapy (SCITO) meeting, Naples 2014. J Transl Med (2014) 12(1):291. doi:10.1186/s12967-014-0291-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Jarboe J, Gupta A, Saif W. Therapeutic human monoclonal antibodies against cancer. Methods Mol Biol (2014) 1060:61–77. doi:10.1007/978-1-62703-586-6_4
CrossRef Full Text | Google Scholar
26. Bertolini F, Dell’Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, et al. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin’s lymphoma. Cancer Res (2002) 62(11):3106–12.
Pubmed Abstract | Pubmed Full Text | Google Scholar
27. Brennecke P, Arlt MJ, Campanile C, Husmann K, Gvozdenovic A, Apuzzo T, et al. CXCR4 antibody treatment suppresses metastatic spread to the lung of intratibial human osteosarcoma xenografts in mice. Clin Exp Metastasis (2014) 31(3):339–49. doi:10.1007/s10585-013-9632-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Chang DK, Sui J, Geng S, Muvaffak A, Bai M, Fuhlbrigge RC, et al. Humanization of an anti-CCR4 antibody that kills cutaneous T-cell lymphoma cells and abrogates suppression by T-regulatory cells. Mol Cancer Ther (2012) 11(11):2451–61. doi:10.1158/1535-7163.MCT-12-0278
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Hagemann UB, Gunnarsson L, Geraudie S, Scheffler U, Griep RA, Reiersen H, et al. Fully human antagonistic antibodies against CCR4 potently inhibit cell signaling and chemotaxis. PLoS One (2014) 9(7):e103776. doi:10.1371/journal.pone.0103776
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Jahnichen S, Blanchetot C, Maussang D, Gonzalez-Pajuelo M, Chow KY, Bosch L, et al. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc Natl Acad Sci U S A (2010) 107(47):20565–70. doi:10.1073/pnas.1012865107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Maussang D, Mujic-Delic A, Descamps FJ, Stortelers C, Vanlandschoot P, Stigter-van Walsum M, et al. Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo. J Biol Chem (2013) 288(41):29562–72. doi:10.1074/jbc.M113.498436
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature (2001) 410(6824):50–6. doi:10.1038/35065016
CrossRef Full Text | Google Scholar
36. Panjideh H, Muller G, Koch M, Wilde F, Scheu S, Moldenhauer G, et al. Immunotherapy of B-cell non-Hodgkin lymphoma by targeting the chemokine receptor CXCR5 in a preclinical mouse model. Int J Cancer (2014) 135(11):2623–32. doi:10.1002/ijc.28893
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Somovilla-Crespo B, Alfonso-Perez M, Cuesta-Mateos C, Carballo-de Dios C, Beltran AE, Terron F, et al. Anti-CCR7 therapy exerts a potent anti-tumor activity in a xenograft model of human mantle cell lymphoma. J Hematol Oncol (2013) 6:89. doi:10.1186/1756-8722-6-89
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res (2004) 64(8):2817–24. doi:10.1158/0008-5472.CAN-03-3693
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International union of basic and clinical pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev (2014) 66(1):1–79. doi:10.1124/pr.113.007724
CrossRef Full Text | Google Scholar
40. Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev (2000) 52(1):145–76.
Pubmed Abstract | Pubmed Full Text | Google Scholar
42. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity (2000) 12(2):121–7. doi:10.1016/S1074-7613(00)80165-X
CrossRef Full Text | Google Scholar
43. Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol (2000) 18:217–42. doi:10.1146/annurev.immunol.18.1.217
CrossRef Full Text | Google Scholar
50. Borrello MG, Alberti L, Fischer A, Degl’innocenti D, Ferrario C, Gariboldi M, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A (2005) 102(41):14825–30. doi:10.1073/pnas.0503039102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem (1998) 273(7):4282–7. doi:10.1074/jbc.273.7.4282
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis (2002) 19(3):247–58. doi:10.1023/A:1015587423262
CrossRef Full Text | Google Scholar
66. Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med (2010) 16(1):98–105. doi:10.1038/nm.2074
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
67. Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoid-like stroma and immune escape by tumors that express the chemokine CCL21. Science (2010) 328(5979):749–52. doi:10.1126/science.1185837
CrossRef Full Text | Google Scholar
69. Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res (2002) 62(24):7328–34.
Pubmed Abstract | Pubmed Full Text | Google Scholar
76. Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int (2010) 60(7):497–505. doi:10.1111/j.1440-1827.2010.02548.x
CrossRef Full Text | Google Scholar
78. Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med (2006) 203(9):2201–13. doi:10.1084/jem.20052144
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med (2003) 197(11):1537–49. doi:10.1084/jem.20021897
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
88. Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res (1998) 18(1A):77–81.
Pubmed Abstract | Pubmed Full Text | Google Scholar
89. Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res (2005) 11(11):4117–27. doi:10.1158/1078-0432.CCR-04-1518
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol (2006) 125(2):209–16. doi:10.1309/VPL5-R3JR-7F1D-6V03
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
94. Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem (2000) 275(10):6868–75. doi:10.1074/jbc.275.10.6868
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
98. Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Mol Cancer Ther (2013) 12(5):799–808. doi:10.1158/1535-7163.MCT-12-0529
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
100. Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res (2006) 66(6):3071–7. doi:10.1158/0008-5472.CAN-05-2871
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
102. Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood (2000) 95(2):627–32.
Pubmed Abstract | Pubmed Full Text | Google Scholar
105. Maekawa S, Iwasaki A, Shirakusa T, Kawakami T, Yanagisawa J, Tanaka T, et al. Association between the expression of chemokine receptors CCR7 and CXCR3, and lymph node metastatic potential in lung adenocarcinoma. Oncol Rep (2008) 19(6):1461–8.
Pubmed Abstract | Pubmed Full Text | Google Scholar
106. Monteagudo C, Martin JM, Jorda E, Llombart-Bosch A. CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factors. J Clin Pathol (2007) 60(6):596–9. doi:10.1136/jcp.2005.032144
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
110. Wu S, Gessner R, Taube T, Korte A, von Stackelberg A, Kirchner R, et al. Chemokine IL-8 and chemokine receptor CXCR3 and CXCR4 gene expression in childhood acute lymphoblastic leukemia at first relapse. J Pediatr Hematol Oncol (2006) 28(4):216–20. doi:10.1097/01.mph.0000212908.14642.a5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
114. Arya M, Patel HR, McGurk C, Tatoud R, Klocker H, Masters J, et al. The importance of the CXCL12-CXCR4 chemokine ligand-receptor interaction in prostate cancer metastasis. J Exp Ther Oncol (2004) 4(4):291–303.
Pubmed Abstract | Pubmed Full Text | Google Scholar
115. Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res (2005) 11(16):5686–93. doi:10.1158/1078-0432.CCR-05-0014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
118. Franco R, Cantile M, Scala S, Catalano E, Cerrone M, Scognamiglio G, et al. Histomorphologic parameters and CXCR4 mRNA and protein expression in sentinel node melanoma metastasis are correlated to clinical outcome. Cancer Biol Ther (2010) 9(6):423–9. doi:10.4161/cbt.9.6.10996
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
120. Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol (2006) 103(1):226–33. doi:10.1016/j.ygyno.2006.02.036
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
126. Kim J, Mori T, Chen SL, Amersi FF, Martinez SR, Kuo C, et al. Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg (2006) 244(1):113–20. doi:10.1097/01.sla.0000217690.65909.9c
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
127. Koishi K, Yoshikawa R, Tsujimura T, Hashimoto-Tamaoki T, Kojima S, Yanagi H, et al. Persistent CXCR4 expression after preoperative chemoradiotherapy predicts early recurrence and poor prognosis in esophageal cancer. World J Gastroenterol (2006) 12(47):7585–90. doi:10.3748/wjg.v12.i47.7585
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
129. Lopez-Giral S, Quintana NE, Cabrerizo M, Alfonso-Perez M, Sala-Valdes M, De Soria VG, et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol (2004) 76(2):462–71. doi:10.1189/jlb.1203652
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
130. Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res (2004) 64(22):8420–7. doi:10.1158/0008-5472.CAN-04-1343
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
132. Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol (2009) 16(9):2645–53. doi:10.1245/s10434-009-0599-x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
133. Milliken D, Scotton C, Raju S, Balkwill F, Wilson J. Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin Cancer Res (2002) 8(4):1108–14.
135. Muller A, Sonkoly E, Eulert C, Gerber PA, Kubitza R, Schirlau K, et al. Chemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapy. Int J Cancer (2006) 118(9):2147–57. doi:10.1002/ijc.21514
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
136. Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci (2004) 36(2):71–8. doi:10.1016/j.jdermsci.2004.03.002
CrossRef Full Text | Google Scholar
137. O’Hayre M, Salanga CL, Kipps TJ, Messmer D, Dorrestein PC, Handel TM. Elucidating the CXCL12/CXCR4 signaling network in chronic lymphocytic leukemia through phosphoproteomics analysis. PLoS One (2010) 5(7):e11716. doi:10.1371/journal.pone.0011716
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
138. Oda Y, Ohishi Y, Basaki Y, Kobayashi H, Hirakawa T, Wake N, et al. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: their correlation with activated Akt, LRP/MVP and P-glycoprotein expression. Cancer Sci (2007) 98(7):1020–6. doi:10.1111/j.1349-7006.2007.00492.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
139. Oda Y, Yamamoto H, Tamiya S, Matsuda S, Tanaka K, Yokoyama R, et al. CXCR4 and VEGF expression in the primary site and the metastatic site of human osteosarcoma: analysis within a group of patients, all of whom developed lung metastasis. Modern Pathol (2006) 19(5):738–45. doi:10.1038/modpathol.3800587
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
140. Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med (2003) 167(12):1676–86. doi:10.1164/rccm.200301-071OC
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
141. Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett (2007) 256(2):137–65. doi:10.1016/j.canlet.2007.05.013
CrossRef Full Text | Google Scholar
142. Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg (2004) 39(10):1506–11. doi:10.1016/j.jpedsurg.2004.06.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
143. Saur D, Seidler B, Schneider G, Algul H, Beck R, Senekowitsch-Schmidtke R, et al. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology (2005) 129(4):1237–50. doi:10.1053/j.gastro.2005.06.056
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
150. Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res (2008) 68(21):9060–9. doi:10.1158/0008-5472.CAN-08-1810
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
151. Cho YB, Lee WY, Choi SJ, Kim J, Hong HK, Kim SH, et al. CC chemokine ligand 7 expression in liver metastasis of colorectal cancer. Oncol Rep (2012) 28(2):689–94. doi:10.3892/or.2012.1815
CrossRef Full Text | Google Scholar
152. Hirai H, Fujishita T, Kurimoto K, Miyachi H, Kitano S, Inamoto S, et al. CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin Exp Metastasis (2014) 31(8):977–89. doi:10.1007/s10585-014-9684-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
153. Itatani Y, Kawada K, Fujishita T, Kakizaki F, Hirai H, Matsumoto T, et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology (2013) 145(5):1064–75e11. doi:10.1053/j.gastro.2013.07.033
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
154. Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A (2010) 107(29):13063–8. doi:10.1073/pnas.1002372107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
156. Craig MJ, Loberg RD. CCL2 (Monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Rev (2006) 25(4):611–9. doi:10.1007/s10555-006-9027-x
CrossRef Full Text | Google Scholar
158. Vande Broek I, Asosingh K, Vanderkerken K, Straetmans N, Van Camp B, Van Riet I. Chemokine receptor CCR2 is expressed by human multiple myeloma cells and mediates migration to bone marrow stromal cell-produced monocyte chemotactic proteins MCP-1, -2 and -3. Br J Cancer (2003) 88(6):855–62. doi:10.1038/sj.bjc.6600833
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
162. Ishida T, Inagaki H, Utsunomiya A, Takatsuka Y, Komatsu H, Iida S, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res (2004) 10(16):5494–500. doi:10.1158/1078-0432.CCR-04-0371
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
163. Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res (2003) 9(10 Pt 1):3625–34.
Pubmed Abstract | Pubmed Full Text | Google Scholar
164. Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, et al. Circulating clonal CLA(+) and CD4(+) T cells in Sezary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol (2005) 152(2):258–64. doi:10.1111/j.1365-2133.2004.06325.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
165. Cambien B, Richard-Fiardo P, Karimdjee BF, Martini V, Ferrua B, Pitard B, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRbeta in colorectal carcinoma. PLoS One (2011) 6(12):e28842. doi:10.1371/journal.pone.0028842
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
168. Liu J, Ke F, Xu Z, Liu Z, Zhang L, Yan S, et al. CCR6 is a prognostic marker for overall survival in patients with colorectal cancer, and its overexpression enhances metastasis in vivo. PLoS One (2014) 9(6):e101137. doi:10.1371/journal.pone.0101137
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
170. Alfonso-Perez M, Lopez-Giral S, Quintana NE, Loscertales J, Martin-Jimenez P, Munoz C. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol (2006) 79(6):1157–65. doi:10.1189/jlb.1105623
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
172. Cunningham HD, Shannon LA, Calloway PA, Fassold BC, Dunwiddie I, Vielhauer G, et al. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl Oncol (2010) 3(6):354–61. doi:10.1593/tlo.10178
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
173. Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, et al. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res (2003) 9(9):3406–12.
Pubmed Abstract | Pubmed Full Text | Google Scholar
176. Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res (2002) 62(10):2937–41.
Pubmed Abstract | Pubmed Full Text | Google Scholar
180. Qiuping Z, Qun L, Chunsong H, Xiaolian Z, Baojun H, Mingzhen Y, et al. Selectively increased expression and functions of chemokine receptor CCR9 on CD4+ T cells from patients with T-cell lineage acute lymphocytic leukemia. Cancer Res (2003) 63(19):6469–77.
Pubmed Abstract | Pubmed Full Text | Google Scholar
182. Johnson-Holiday C, Singh R, Johnson E, Singh S, Stockard CR, Grizzle WE, et al. CCL25 mediates migration, invasion and matrix metalloproteinase expression by breast cancer cells in a CCR9-dependent fashion. Int J Oncol (2011) 38(5):1279–85. doi:10.3892/ijo.2011.953
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
183. Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res (2008) 14(3):638–45. doi:10.1158/1078-0432.CCR-07-2025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
185. Mirandola L, Chiriva-Internati M, Montagna D, Locatelli F, Zecca M, Ranzani M, et al. Notch1 regulates chemotaxis and proliferation by controlling the CC-chemokine receptors 5 and 9 in T cell acute lymphoblastic leukaemia. J Pathol (2012) 226(5):713–22. doi:10.1002/path.3015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
186. van den Oord J. The CCR9-CCL25 axis mediates melanoma metastasis to the small intestine. Nat Clin Pract Oncol (2008) 5(8):440–1. doi:10.1038/ncponc1174
CrossRef Full Text | Google Scholar
192. Erreni M, Solinas G, Brescia P, Osti D, Zunino F, Colombo P, et al. Human glioblastoma tumours and neural cancer stem cells express the chemokine CX3CL1 and its receptor CX3CR1. Eur J Cancer (2010) 46(18):3383–92. doi:10.1016/j.ejca.2010.07.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
193. Ferretti E, Pistoia V, Corcione A. Role of fractalkine/CX3CL1 and its receptor in the pathogenesis of inflammatory and malignant diseases with emphasis on B cell malignancies. Mediators Inflamm (2014) 2014:480941. doi:10.1155/2014/480941
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
196. Kim M, Rooper L, Xie J, Kajdacsy-Balla AA, Barbolina MV. Fractalkine receptor CX(3)CR1 is expressed in epithelial ovarian carcinoma cells and required for motility and adhesion to peritoneal mesothelial cells. Mol Cancer Res (2012) 10(1):11–24. doi:10.1158/1541-7786.MCR-11-0256
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
197. Marchesi F, Locatelli M, Solinas G, Erreni M, Allavena P, Mantovani A. Role of CX3CR1/CX3CL1 axis in primary and secondary involvement of the nervous system by cancer. J Neuroimmunol (2010) 224(1–2):39–44. doi:10.1016/j.jneuroim.2010.05.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
198. Nevo I, Sagi-Assif O, Meshel T, Ben-Baruch A, Johrer K, Greil R, et al. The involvement of the fractalkine receptor in the transmigration of neuroblastoma cells through bone-marrow endothelial cells. Cancer Lett (2009) 273(1):127–39. doi:10.1016/j.canlet.2008.07.029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
199. Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res (2004) 64(14):4693–8. doi:10.1158/0008-5472.CAN-03-3437
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
201. Grymula K, Tarnowski M, Wysoczynski M, Drukala J, Barr FG, Ratajczak J, et al. Overlapping and distinct role of CXCR7-SDF-1/ITAC and CXCR4-SDF-1 axes in regulating metastatic behavior of human rhabdomyosarcomas. Int J Cancer (2010) 127(11):2554–68. doi:10.1002/ijc.25245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
202. Iwakiri S, Mino N, Takahashi T, Sonobe M, Nagai S, Okubo K, et al. Higher expression of chemokine receptor CXCR7 is linked to early and metastatic recurrence in pathological stage I nonsmall cell lung cancer. Cancer (2009) 115(11):2580–93. doi:10.1002/cncr.24281
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
203. Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A (2007) 104(40):15735–40. doi:10.1073/pnas.0610444104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
213. Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov (2004) 3(8):711–5. doi:10.1038/nrd1470
CrossRef Full Text | Google Scholar
217. Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett (2014) 588(2):368–76. doi:10.1016/j.febslet.2013.10.015
CrossRef Full Text | Google Scholar
218. Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res (2013) 19(5):1035–43. doi:10.1158/1078-0432.CCR-12-2064
CrossRef Full Text | Google Scholar
219. Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer (2007) 7(2):95–106. doi:10.1038/nrc2051
CrossRef Full Text | Google Scholar
220. Klarenbeek AMD, Blanchetot C, Saunders M, van der Woning S, Smit M, de Haard H, et al. Targeting chemokines and chemokine receptors with antibodies. Drug Discov Today Technol (2012) 9(4):e227–314. doi:10.1016/j.ddtec.2012.09.006
CrossRef Full Text | Google Scholar
221. Kremer L, Marquez G. Generation of monoclonal antibodies against chemokine receptors. Methods Mol Biol (2004) 239:243–60.
222. Ponath PD, Kassam N, Qin S. Monoclonal antibodies to chemokine receptors. Methods Mol Biol (2000) 138:231–42. doi:10.1385/1-59259-058-6:231
CrossRef Full Text | Google Scholar
224. Vergunst CE, Gerlag DM, Lopatinskaya L, Klareskog L, Smith MD, van den Bosch F, et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum (2008) 58(7):1931–9. doi:10.1002/art.23591
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
225. Ben S, Li X, Xu F, Xu W, Li W, Wu Z, et al. Treatment with anti-CC chemokine receptor 3 monoclonal antibody or dexamethasone inhibits the migration and differentiation of bone marrow CD34 progenitor cells in an allergic mouse model. Allergy (2008) 63(9):1164–76. doi:10.1111/j.1398-9995.2008.01747.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
226. Catley MC, Coote J, Bari M, Tomlinson KL. Monoclonal antibodies for the treatment of asthma. Pharmacol Ther (2011) 132(3):333–51. doi:10.1016/j.pharmthera.2011.09.005
CrossRef Full Text | Google Scholar
227. Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy (2009) 64(7):995–1002. doi:10.1111/j.1398-9995.2009.02095.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
229. Carnec X, Quan L, Olson WC, Hazan U, Dragic T. Anti-CXCR4 monoclonal antibodies recognizing overlapping epitopes differ significantly in their ability to inhibit entry of human immunodeficiency virus type 1. J Virol (2005) 79(3):1930–3. doi:10.1128/JVI.79.3.1930-1933.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
231. Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Flaherty KR, Martinez FJ, et al. Therapeutic targeting of CC ligand 21 or CC chemokine receptor 7 abrogates pulmonary fibrosis induced by the adoptive transfer of human pulmonary fibroblasts to immunodeficient mice. Am J Pathol (2007) 170(4):1152–64. doi:10.2353/ajpath.2007.060649
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
232. Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, et al. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res (2013) 19(2):357–66. doi:10.1158/1078-0432.CCR-12-2333
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
233. Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res (2010) 16(5):1520–31. doi:10.1158/1078-0432.CCR-09-2697
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
234. Hattermann K, Held-Feindt J, Lucius R, Muerkoster SS, Penfold ME, Schall TJ, et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res (2010) 70(8):3299–308. doi:10.1158/0008-5472.CAN-09-3642
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
236. Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Exp Cell Res (2011) 317(5):664–73. doi:10.1016/j.yexcr.2010.11.013
CrossRef Full Text | Google Scholar
237. Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res (2014) 124:31–82. doi:10.1016/B978-0-12-411638-2.00002-1
CrossRef Full Text | Google Scholar
239. Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol (2007) 8(6):639–46. doi:10.1038/ni1467
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
240. Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med (1998) 187(1):129–34. doi:10.1084/jem.187.1.129
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
244. Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res (2004) 64(6):2127–33. doi:10.1158/0008-5472.CAN-03-2068
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
245. Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem (2003) 278(5):3466–73. doi:10.1074/jbc.M210665200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
246. Ito A, Ishida T, Utsunomiya A, Sato F, Mori F, Yano H, et al. Defucosylated anti-CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi-scid, IL-2R gamma(null) mice in vivo. J Immunol (2009) 183(7):4782–91. doi:10.4049/jimmunol.0900699
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
247. Ito Y, Miyamoto T, Chong Y, Aoki T, Kato K, Akashi K, et al. Successful treatment with anti-CC chemokine receptor 4 MoAb of relapsed adult T-cell leukemia/lymphoma after umbilical cord blood transplantation. Bone Marrow Transplant (2013) 48(7):998–9. doi:10.1038/bmt.2012.268
CrossRef Full Text | Google Scholar
248. Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol (2010) 28(9):1591–8. doi:10.1200/JCO.2009.25.3575
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
249. Kanazawa T, Hiramatsu Y, Iwata S, Siddiquey M, Sato Y, Suzuki M, et al. Anti-CCR4 monoclonal antibody mogamulizumab for the treatment of EBV-associated T- and NK-cell lymphoproliferative diseases. Clin Cancer Res (2014) 20(19):5075–84. doi:10.1158/1078-0432.CCR-14-0580
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
251. Ni X, Jorgensen JL, Goswami M, Challagundla P, Decker WK, Kim YH, et al. Reduction of regulatory T cells by mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin Cancer Res (2014). doi:10.1158/1078-0432.CCR-14-0830
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
252. Kurose K, Ohue Y, Sato E, Yamauchi A, Eikawa S, Isobe M, et al. Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an anti-human CCR4 mAb (KM2760). J Thorac Oncol (2014). doi:10.1097/JTO.0000000000000364
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
254. Yonekura K, Kanzaki T, Gunshin K, Kawakami N, Takatsuka Y, Nakano N, et al. Effect of anti-CCR4 monoclonal antibody (mogamulizumab) on adult T-cell leukemia-lymphoma: cutaneous adverse reactions may predict the prognosis. J Dermatol (2014) 41(3):239–44. doi:10.1111/1346-8138.12419
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
255. Nakano N, Kusumoto S, Tanaka Y, Ishida T, Takeuchi S, Takatsuka Y, et al. Reactivation of hepatitis B virus in a patient with adult T-cell leukemia-lymphoma receiving the anti-CC chemokine receptor 4 antibody mogamulizumab. Hepatol Res (2014) 44(3):354–7. doi:10.1111/hepr.12117
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
256. Kato K, Miyamoto T, Numata A, Nakaike T, Oka H, Yurino A, et al. Diffuse panbronchiolitis after humanized anti-CCR4 monoclonal antibody therapy for relapsed adult T-cell leukemia/lymphoma. Int J Hematol (2013) 97(3):430–2. doi:10.1007/s12185-013-1278-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
258. Saintigny P, Massarelli E, Lin S, Ahn YH, Chen Y, Goswami S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res (2013) 73(2):571–82. doi:10.1158/0008-5472.CAN-12-0263
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
262. Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem (2003) 278(10):8508–15. doi:10.1074/jbc.M208231200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
264. Corvaia NBS, Wurch T, Boute N, Broussas M, Beau-Larvor C, Akla B, et al. 515H7, a novel anti-CXCR4 antibody: in vitro efficacy on CXCR4-associated signaling pathways and in vivo anti-tumor activity. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 Apr 2-6. (Vol. 71), Orlando, FL Philadelphia, PA: AACR (2011).
265. Boynton AL. CXCR4 is involved in multiple functions of cancer cells and is a compelling target for monoclonal antibody-based therapeutics. Abstract retrieved from Abstracts in ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol (2006) 24(18 Suppl):Abstr20013.
268. Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood (2009) 113(18):4341–51. doi:10.1182/blood-2008-10-186668
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
270. Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, et al. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia (2012) 14(8):709–18.
Pubmed Abstract | Pubmed Full Text | Google Scholar
273. Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, Hannani D, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res (2014) 74(2):436–45. doi:10.1158/0008-5472.CAN-13-1265
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar