Frontiers | Sex Differences in the Relationship of Dietary Fatty Acids to Cognitive Measures in American Children (original) (raw)
ORIGINAL RESEARCH article
Front. Evol. Neurosci., 02 November 2011
This article is part of the Research Topic NeuroNEEPS View all 6 articles
- 1 Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA
- 2 Department or Anthropology, University of California, Santa Barbara, CA, USA
Because the first neurons evolved in an environment high in the _n_−3 (omega-3) fatty acid docosahexaenoic acid (DHA), this fatty acid became a major component of neural structure and function and makes up 10% of the dry weight of the human brain. Since _n_−3 fatty acids must come from the diet, this suggests a possible positive role for dietary _n_−3 fatty acids in cognition and a possible negative role for _n_−6 fatty acids, which compete with _n_−3 for access to critical enzymes. Because human females must provide DHA for the growth of the unusually large brains of their offspring from maternal fat stored during childhood, their need for DHA is especially great. We used stepwise regression to determine whether particular dietary fatty acids and other nutrients were related to cognitive performance in over 4000 American children aged 6–16 from the Third National Health and Nutrition Examination Survey; a variety of possible biological, social, and environmental risk factors were statistically controlled. In this context the only dietary factors related to cognitive performance were _n_−3 and _n_−6 fatty acids. Dietary _n_−3 fatty acids were positively related to cognitive test scores in male and female children, while _n_−6 showed the reverse relationship, significantly so in females. In female children the positive effects of _n_−3 intake were twice as strong as in males and exceeded the negative effects of lead exposure. This suggests that increasing dietary intake of _n_−3 and decreasing _n_−6 fatty acids may have cognitive benefits in children, especially in females.
Introduction
The _n_−3 (also called omega-3) long-chain fatty acid docosahexaenoic acid (DHA, 22:6n−3), with a 22-carbon chain and six double bonds, comprises about 10% of the dry weight of the human brain (Svennerholm, 1968; Rapoport, 2003). Animal studies have shown that DHA readily crosses the blood/brain barrier (Ouellet et al., 2009) and plays a critical positive role in all aspects of neuronal growth, synaptic connections, and functioning (Cockburn, 1994; Jamieson et al., 1999; Salem et al., 2001; Chang et al., 2009). This includes roles in regulating the activity of Na + K + ATPase in the neural membrane (Bourre et al., 1989; Turner et al., 2003; Kumosani et al., 2011), neuron size (Ahmad et al., 2002), neurogenesis (Auestad and Innis, 2000; Coti Bertrand et al., 2006; Beltz et al., 2007; Novak et al., 2008; Da Costa et al., 2009; Dagai et al., 2009; He et al., 2009), neurite growth (Calderon and Kim, 2004; Sakamoto et al., 2007; Liu et al., 2008; Novak et al., 2008; Cao et al., 2009), synapse formation and function (Yoshida et al., 1997; Cansev and Wurtman, 2007; Wu et al., 2008; Cao et al., 2009; Wurtman et al., 2009), neuronal integrity and vitality (Issa et al., 2006; Mukherjee et al., 2007; Niemoller et al., 2009), gene expression in the brain (Kitajka et al., 2002), brain glucose transport (Pifferi et al., 2007), cognitive development (Heinemann and Bauer, 2006; Bongiovanni et al., 2007; Coluccia et al., 2009), and learning ability (Bourre et al., 1989; Yoshida et al., 1997; Greiner et al., 1999; Salem et al., 2001; Takeuchi et al., 2002; Shirai and Suzuki, 2004; Garcia-Calatayud et al., 2005; Lim et al., 2005; Chung et al., 2008; Holguin et al., 2008; Fedorova et al., 2009; He et al., 2009; Hooijmans et al., 2009; Jiang et al., 2009).
Because animals lack the enzyme required to make an _n_−3 double bond, at least the basal _n_−3 fatty acid, alpha-linolenic acid (ALA, 18:3n−3), must be obtained from their diets. Mammals can convert ALA to eicosapentaenoic EPA, 20:5n−3), and can subsequently convert EPA to docosapentaenoic (DPA, 22:5n−3) and then to DHA, though conversion efficiency is quite low and capacity limited, especially for EPA to DHA (Pawlosky et al., 2001). As cited above, animal studies show that a deficiency of dietary _n_−3 fatty acids leads to a decrease in neuronal size and synapse number and impaired learning ability. Many studies in human infants have shown that levels of DHA in the maternal diet or blood during pregnancy and in maternal milk or formula are positively related to cognitive and visual development in infants, as reviewed by McCann and Ames (2005); Eilander et al. (2007); Innis (2009); Ryan et al. (2010); and Schuchardt et al. (2010). Studies involving the _n_−3 content of the maternal diet may underestimate the effect of DHA, because most DHA delivered by a mother to her fetus or nursing infant derives from her fat stores rather than current intake (Sauerwald et al., 2000).
Most studies relating the level of _n_−3 fatty acids in the diet or blood to cognitive measures in older children have also found a positive relationship, including studies in Italy (Agostoni et al., 1997), Scotland (Whalley et al., 2004), Maryland (Ryan and Nelson, 2008), Finland (Aberg et al., 2009), Alabama (Neggers et al., 2009), and Wales (Kirby et al., 2010), though a Dutch study did not (De Groot et al., 2007). Two studies in which children were provided with short-term supplemental dietary _n_−3 found a positive effect on cognitive measures (Richardson and Montgomery, 2005; Dalton et al., 2009) and two did not (Osendarp et al., 2007; Kennedy et al., 2009). None of these studies have considered sex differences which, for reasons we will outline below, are to be expected.
Evolutionary Background for the Role of DHA in the Human Brain
The reliance of human and other mammalian brains on the neuronal functions of DHA appears to be the result of a very ancient evolutionary contingency. Though DHA is now a relatively scarce and limiting resource for the development of large brains in terrestrial environments, neurons first evolved in an aquatic environment where high levels of DHA were readily available. The first links in this chain of contingency apparently reach back more than 3 billion years.
Ancient cyanobacteria evolved the ability to synthesize _n_−3 ALA for incorporation into the thylakoid membrane where it plays an essential role in photosynthesis – as it does today in the chloroplasts of all green plants. Dinoflagellates and certain cyanobacteria and algae subsequently evolved a metabolic pathway to efficiently convert ALA to DHA using the enzyme delta-4-desaturase, and these phytoplankton are still the source of DHA for all aquatic animal life. Fossilized acritarchs suggest that dinoflagellates may have evolved more than 3 billion years ago (Javaux et al., 2010).
The first neurons evolved in Precambrian cnidarians feeding on dinoflagellates and other phytoplankton rich in DHA (Nichols et al., 2003; Putnam et al., 2007); hence neurons could evolve a design that was dependent on substantial supplies of DHA. When larger and more elaborate brains evolved in marine vertebrates, their neurons could continue to rely on large amounts of DHA because the phytoplankton producing this long-chain polyunsaturated fatty acid also lay at the base of their food chain. (DHA’s function is not limited to the vertebrate nervous system; it also plays important roles in muscles, blood, and mitochondria.)
Marine arthropods also have a diet rich in DHA, but when exclusively terrestrial arthropods first colonized the land, they were cut off from the DHA supplied by phytoplankton and had minimal ability to synthesize longer-chain _n_−3. Although their bodies have significant amounts of ALA obtained from plants, their nervous systems contain little or no DHA (Jerde et al., 1975; Fontaneto et al., 2011); instead they use mainly ALA (Stark et al., 1993; Shanker et al., 2006). Their inability to convert alpha-linolenic to DHA may limit the complexity of their nervous systems. Reptiles subsequently evolved this conversion ability, although their synthetic pathway differs from that of phytoplankton (lacking delta-4 desaturase), and is very much less efficient. Although allometrically small reptilian brains have some DHA, the proportion of DHA is quite low compared with mammalian brains (Mitchell et al., 2007), and their limited _n_−3 supply may have similarly constrained the growth and hence the evolution of a more complex nervous system.
The evolution of endothermy in mammals greatly increased caloric requirements, and the concomitant 10-fold increase in consumption of plants and/or insects provided much larger amounts of ALA, permitting the synthesis of larger amounts of DHA despite the inefficiency of this process in terrestrial animals. These higher levels of dietary _n_−3 allowed for a considerable expansion of the mammalian brain, which is not only allometrically much larger than a reptile brain but also contains a much higher proportion of DHA (Mitchell et al., 2007). However, mammals still have a lower proportion than fish (Stoknes et al., 2004; USDA, 2011). Higher DHA levels in mammalian mitochondrial membranes also facilitated endothermic metabolism (Brand et al., 1991, 1994; Hulbert, 2007). Meanwhile, the evolution and diversification of flowering plants led to increases in the _n_−3 content of terrestrial plants, especially in their fruits, nuts, and seeds, and permitted the co-evolution of many new species of herbivorous insects with high levels of ALA.
The first primates were insectivores occupying a nocturnal, arboreal niche, and their enhanced feeding skills and diet permitted the further expansion of the primate brain. (Chimpanzee females continue to invest considerable amounts of time feeding on insects as shown by McGrew, 1979.) Frugivorous primates also obtain substantial amounts of insects in the fruit they eat (Redford et al., 1984) as well as higher concentrations of _n_−3 in nuts and seeds. Folivorous primates, like gorillas, have relatively smaller brains compared to frugivores (Clutton-Brock and Harvey, 1980; Harvey et al., 1980), and must still ingest a large volume of plants to provide the necessary _n_−3.
As the hominid brain expanded to a size seven times larger than expected from the brain:body size relationship in mammals, the need for _n_−3 and DHA increased proportionately. Because human synthesis of DHA from ALA remains very limited, like that of other terrestrial animals, sources of preformed DHA in the diet are important. Potential sources include the meat, organs, and eggs of herbivores and birds, and especially flesh from aquatic animals, which provides larger amounts of DHA. Some have argued that exploitation of aquatic nutritional resources was essential for the evolution of the large hominid brain (Broadhurst et al., 2002), and there is evidence for significant amounts of aquatic foods in hominid diets from two million years ago (Braun et al., 2010; Stewart, 2010).
In order to grow their very large brains, human fetuses, and nursing infants require much larger amounts of DHA than can be reliably obtained from maternal daily intake, and most of the DHA they receive comes from maternal fat stores. Studies using radioisotope-labeled fatty acids show that approximately 80% of the DHA and other essential long-chain fatty acids provided in human milk come from maternal fat rather than from the current diet (Sauerwald et al., 2000). These fatty acids are stored mainly in women’s gluteofemoral fat, and these depots are protected except during the third trimester and lactation when their fatty acids are mobilized (Lassek and Gaulin, 2006). Because men do not make these physiological investments in offspring, women’s need for these fatty acids greatly exceeds that of men, a fact that probably explains the unique human sex difference in body fat.
There is no facilitated transport of DHA into adipose, so the proportion of DHA in fat stores depends on the concentration of DHA in the blood. Because this concentration is relatively low compared with other fatty acids, the percentage in adipose is also relatively low (0.2–0.3%). Developing human females must therefore have substantial amounts of adipose tissue in order to store sufficient amounts of DHA to support the growth of large brains in their children. In a study of Dutch children, female fat increased from 14.8 to 25.5% of body weight during puberty, while male fat decreased from 10.5 to 9.3% (Boot et al., 1997). In a sample of young American women, there was a mean of 16.2 kg of adipose tissue at the end of puberty (Lassek and Gaulin, 2006). Assuming a DHA percentage of 0.2%, this amount of adipose would contain 32 g of DHA which would become available when adipose is mobilized during late pregnancy and lactation, when the fetal and infant DHA requirement is 100–200 mg/day (Clandinin et al., 1980a,b; Haggarty, 2004). A female child must store this DHA at the same time that she requires substantial amounts of DHA to support her own growth and development. Because she must allocate some of her limited dietary _n_−3 to storing DHA for her future children, there is a competition between her need for DHA for her own body and brain and her need to store DHA for future reproduction. Girls with proportionately larger amounts of gluteofemoral fat and lower waist-hip ratios have earlier menarche (Lassek and Gaulin, 2007). While human males usually have much less adipose than females, they have substantially more than typical primates (Pond and Mattacks, 1987).
The optimal amount of DHA per day in children has not been established, but participants in a 1999 workshop sponsored by National Institutes of Health recommended that at least 0.1% of calories should be DHA (Simopoulos and Leaf, 1999), which would be 220 mg for a 2000 calorie (8.4 MJ) diet (assuming 9 calories/g of fat). Similar amounts are recommended for pregnant and nursing women (Koletzko et al., 2007.) The per capita amount of DHA and ALA in the American food supply in 1990 was 70 mg and 2.4 g respectively (Gerrior et al., 2004).
While _n_−3 fatty acids are known to have positive effects on cognition, less is known about the effects of _n_−6 fatty acids, such as linoleic (LA, 18:2n−6) and arachidonic acid (AA, 20:4n−6). As with _n_−3, some form of _n_−6 must come from the diet, and there is no interconversion of _n_−3 and _n_−6 fatty acids. In the terrestrial synthetic pathway that evolved to elongate ALA, the LA-to-arachidonic conversion competes for the same enzymes used to synthesize DHA from ALA (Rubin and Laposata, 1992; Emken et al., 1994; Innis et al., 2004; Hibbeln et al., 2006; Harnack et al., 2009; Gibson et al., 2011). Because of this metabolic competition, higher _n_−6 fatty acid intake might be expected to have negative effects on cognition; and four studies have shown this (Agostoni et al., 1997; Whalley et al., 2004; Novak et al., 2008; Neggers et al., 2009).
Amounts of _n_−3 fatty acids have declined in the American diet during the twentieth century while _n_−6 have increased (Blasbalg et al., 2011). Reconstructions of the paleolithic diet suggest that over most of human evolution, there was more _n_−3 than _n_−6 in the diet (Kuipers et al., 2010). Per capita linoleic acid in the American food supply was 29.3 g in 1990 and the ratio of total _n_−6 to _n_−3 was 12.3 (Gerrior et al., 2004). Because of their metabolic competition for necessary enzymes, the very high amount of _n_−6 linoleic acid compared with _n_−3 in the diet of American children is likely to decrease the conversion rate of ALA to DHA.
Larger, population-based samples may help to clarify the relationships between fatty acid consumption and cognitive performance. Cognitive and dietary data collected in the Third National Health and Nutrition Examination Survey (NHANES III) conducted in 1988–1994 provides an opportunity to examine the relationship between dietary _n_−3 and _n_−6 fatty acid intake and cognitive outcomes in a large sample of American children, as well as the possible effects of other nutrients. We recently found a relationship between lower maternal waist–hip ratios and cognitive performance which may be mediated by _n_−3 fatty acids (Lassek and Gaulin, 2008). This leads us to predict that dietary _n_−3 will also be positively related to cognitive outcomes in this sample, whereas dietary _n_−6 will be inversely related to the same outcome measures. Because of the much greater requirement for _n_−3 fatty acids in human females, we predict that their cognitive performance will be more sensitive to the amount of _n_−3 in the diet and to the competing effects of dietary _n_−6.
Materials and Methods
Detailed dietary histories based on 24-h recall were obtained by skilled interviewers for 13,923 males and 15,182 females aged 0–90 in the NHANES III sample, 1988–1994. From this larger sample we restricted our focus to children 6–16 years old. The child sample included 26% non-Hispanic whites, 35% non-Hispanic blacks, 35% Mexican-Americans, and 5% other. Because of the oversampling of blacks and Hispanics, this sample is not representative of the American population.
Twenty-four hour dietary recall was used to estimate individual intake. Specific fatty acid content of the foods consumed was estimated using the food database of the University of Minnesota’s Nutrition Coordinating Center, and for other nutrients, the USDA National Nutrient Database for Standard Reference (USDA). Nutrients used in the analysis which were estimated by conjoining the dietary histories and food composition databases in this way included vitamins A, B6, B12, C, and E, iron, folate, riboflavin, niacin, and thiamine, serum electrolytes, specific sugars, saturated fats with 10, 12, 14, 17, 18, 20, and 22 carbons, monounsaturated fats with 14, 16, 18, 20, and 22 carbons, the _n_−6 fatty acids LA and AA (summed for total _n_−6), the _n_−3 fatty acids ALA, EPA, DPA, and DHA (added together for total _n_−3), and total saturated, monounsaturated, and polyunsaturated fats.
Four cognitive tests were administered to 2253 males and 2309 females aged 6–16 in the NHANES III sample, including the math and reading tests from the Wide Range Achievement Test-Revised and the digit span and block design tests from the Wechsler Intelligence Scale for Children-Revised. The mean scaled score across these four tests was used as a measure of cognitive performance for each of the 6–16 year-olds in this study; both cognitive scores and dietary data were available for 2103 females and 2051 males, and these subgroups thus comprise our primary sample.
Other (non-dietary) measures included in the analyses as possible confounding variables include race/ethnicity, family income, family size, and years of education of the householder parent. Serum lead was also included because it is known to have a significant negative relationship with cognitive performance in children (Wasserman et al., 1997; Needleman and Landrigan, 2004). Multiple linear regression was performed using SPSS-17. Sample weights and complex adjustments for the sampling methodology were not used; this is in accordance with the recommendations of Korn and Graubard (1991).
Results
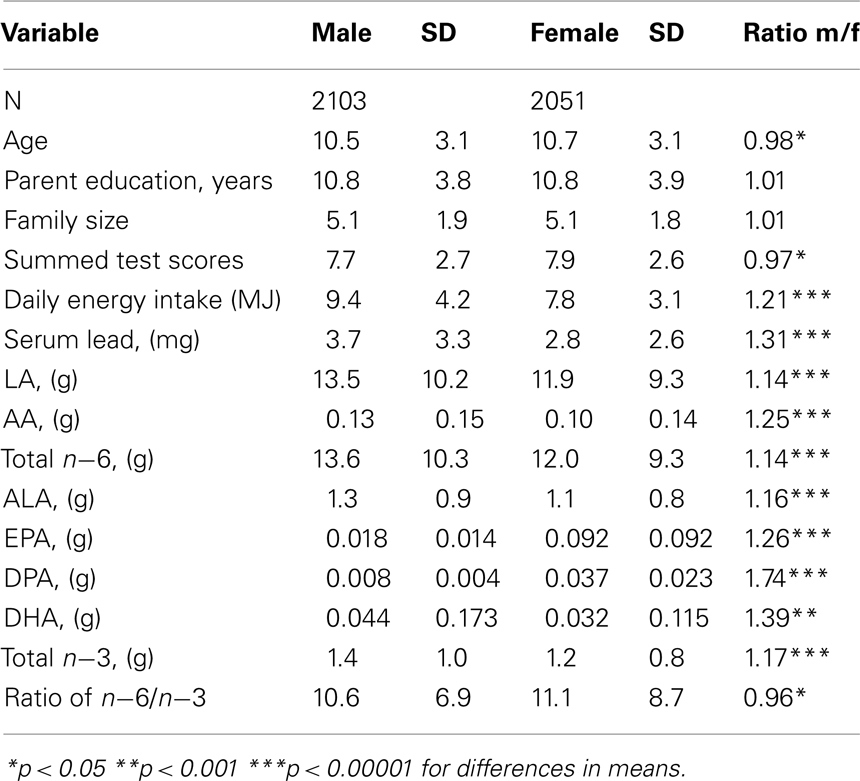
Table 1 provides descriptive statistics for the children in the study sample. Mean energy intake was 20% higher in males and dietary fatty acid intakes showed similar differences; the mean test score and ratio of _n_−6 to _n_−3 was higher in females. Dietary intakes of fatty acids show a high degree of individual variation.
Table 1. Mean and SD by sex (data from NHANES III, ages 6–16).
Based on stepwise multiple regression, Table 2 shows the effect of significant nutrients on the cognitive performance of the children in the sample, controlling for race/ethnicity, family income and size, years of education of the householder parent, and serum lead. For both sexes, total dietary _n_−3 fatty acids are significantly positively related to test scores, while for females, total _n_−6 fatty acids are negatively related. None of the other 33 nutrients are significantly related, including iron and folate and total dietary saturated, monounsaturated, and polyunsaturated fatty acids. Based on its unstandardized regression coefficient, an increase in daily _n_−3 intake of 1 g increases the average test score by 0.38 points (±0.09 SD) in females and by 0.19 points (±0.10 SD) in males; this represents 0.14 and 0.07 of the SDs for the test scores. In females, but not males, when the ratio of _n_−6 to _n_−3 was used in place of the separate _n_−3 and _n_−6 intakes in the regression, it was significantly and negatively related to the cognitive score (beta = −0.071, p = 0.001). When the regression was run with both sexes, sex was not a significant variable.
Table 2. Standardized regression coefficients for the effect of dietary fatty acids on performance on four cognitive tests in youth 6–16, NHANES III.
National Health and Nutrition Examination Survey may sample more than one member in participating households; this creates a lack of independence among cases that is potentially problematic. To examine whether this lack of independence may have favored our hypothesis, we repeated the analysis using both sexes of children and limiting the sample to one child per household (1581 boys; 1682 girls), and found that there was no difference in the results of the regression.
Discussion
Using a large sample drawn from NHANES III, dietary _n_−3 fatty acids are positively related to cognitive performance in children 6–16 years of age, while _n_−6 fatty acids are negatively related to cognitive performance in females in the same sample. As predicted, the contribution of dietary _n_−3 to cognitive performance is much greater (two-fold) in females, and females also show a significant negative effect for _n_−6 fatty acids which compete with _n_−3 for enzymes needed in the biosynthesis of DHA.
This result controls for other relevant variables known to affect cognitive outcomes. The special effects of _n_−3 and _n_−6 are apparent because dietary consumption of 33 other fatty acids and nutrients are not related to cognitive outcome measures based on the same dietary data set and the same children. The positive cognitive effect of dietary _n_−3 fatty acids, as measured by the imperfect method of 24-h recall, is of greater magnitude in girls than the negative effect of serum lead, a well known influence on cognition in children. Dietary iron and folate, which have also been found to relate to cognitive performance in some studies of children (Arija et al., 2006), were not significant when added to this regression.
Because of the complex sampling method used in the NHANES, these results should be viewed with caution and should not be considered representative of the American population. Also, the diet estimates used in this study were based on a single 24-h recall; and while this type of assessment is related to the long-term diet, it is not a highly accurate measure (Knutsen et al., 2003; Sekula et al., 2005; Slater et al., 2010). In addition, the children in the sample are past the ages of maximal brain growth, a period when the effects of dietary fatty acids would be expected to be greater. However, despite these limitations, their cognitive ability is still related to the amount of dietary _n_−3 fatty acids, and of the 40 nutritional variables used in the analysis, only _n_−3 and _n_−6 fatty acids were significantly related to cognitive ability.
The stronger effect of _n_−3 and significantly negative effect of _n_−6 in girls may reflect their greater need for _n_−3 fatty acids to sustain future pregnancy and lactation, as explained above. Because stored maternal fat is selectively used to support the development of the fetal and infant brain – via the placenta and breast milk – females must prepare for these demands by storing DHA in fat at a much higher rate than males during their childhood and adolescence, while their own brains and bodies are still growing. This competition between growth and reproductive goals, absent in boys, may make girls more subject to the antagonism between the _n_−6 and _n_−3 fatty acid families in commandeering necessary synthetic enzymes.
The effect of dietary fatty acids on cognition demonstrated here is relatively small – but of similar magnitude to the negative effect of lead. Both are environmental variables that can be altered, and clear steps have been taken in the case of lead. Since more than half of the variance in cognition is heritable (Plomin et al., 2000), and other environmental variables are often intractable (e.g., family income), dietary fatty acid intake may be of significant pragmatic importance.
These findings on the relationship between dietary fatty acids and cognitive performance are of particular interest in relation to current American food consumption patterns. The cognitive effects of dietary fatty acids in American children may be greater than in other populations because of the limited amount of _n_−3 fatty acids in the American diet combined with unusually high levels of _n_−6 (Blasbalg et al., 2011). Based on the energy intake of the sample children, the recommended amount of 0.1% of energy for DHA would be 250 in males and 210 mg in females per day. Assuming a very generous conversion rate of 2% for ALA to DHA, the mean total daily amount of DHA for the sample children would be just 70 mg for males and 54 mg for females, considerably short of the recommended amounts.
A coordinated increase in _n_−6 and decrease in _n_−3 supplies are the hallmarks of modern American industrial food production, and reflected in the high _n_−6/_n_−3 ratios in the NHANES III sample. Corn, which increased by 78% in the US diet from 1970 to 2000 (Putnam et al., 2002), has an _n_−6/_n_−3 ratio of 34 to 1 (USDA). Soybean oil, especially when partially hydrogenated or made from prevalent low-ALA varieties of soybeans, has a similarly high ratio. Moreover, _n_−3 in processed food is often removed because it is prone to spoil and thus reduces product shelf life (Holman, 1998).
An experimental study in rats using feeds corresponding to the Japanese diet (high _n_−3, low _n_−6) and American diet (high _n_−6, low _n_−3) found both better learning and many more synapses in the hippocampus in the rats fed the “Japanese” diet (Yoshida et al., 1997). It thus seems possible that the high _n_−6/_n_−3 ratio in the American diet might contribute to the relatively low ranking of American children in international testing (NCES, 2005) compared to children in countries with lower _n_−6/_n_−3 ratios, like Japan. Thus, evolutionary considerations may help lead to findings with considerable potential public health significance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Dr. Lassek was primarily responsible for the design of the analysis, data analysis, and the writing of the paper. Dr. Gaulin assisted in the design and data analysis and in the writing of the paper. We appreciate the helpful comments of the two reviewers.
References
Aberg, M. A. L., Aberg, N., Brisman, J., Sundberg, R., Winkvist, A., and Toren, K. (2009). Fish intake of Swedish male adolescents is a predictor of cognitive performance. Acta Paediatr. 98, 555–560.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Agostoni, C., Trojan, S., Bell, R., Riva, E., Bruzzese, M. G., and Giovannini, M. (1997). Developmental quotient at 24 months and fatty acid composition of diet in early infancy: a follow up study. Arch. Dis. Child. 76, 421–424.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Arija, V., Esparo, G., Fernandez-Ballart, J., Murphy, M. M., Biarnes, E., and Canals, J. (2006). Nutritional status and performance in test of verbal and non-verbal intelligence in 6 year old children. Intelligence 34, 141–149.
Auestad, N., and Innis, S. M. (2000). Dietary n-3 fatty acid restriction during gestation in rats: neuronal cell body and growth-cone fatty acids. Am. J. Clin. Nutr. 71, 312S–314S.
Pubmed Abstract | Pubmed Full Text
Blasbalg, T. L., Hibbeln, J. R., Ramsden, C. E., Majchrzak, S. F., and Rawlings, R. R. (2011). Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 93, 950–962.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bongiovanni, K. D., Depeters, E. J., and Van Eenennaam, A. L. (2007). Neonatal growth rate and development of mice raised on milk transgenically enriched with omega-3 fatty acids. Pediatr. Res. 62, 412–416.
Pubmed Abstract | Pubmed Full Text
Boot, A. M., Bouquet, J., Ridder, M. A. J. D., Krenning, E. P., and Keizer-Shrama, S. M. (1997). Determinants of body composition measured by dual-energy x-ray absorptiometry in Dutch children and adolescents. Am. J. Clin. Nutr. 66, 232–238.
Pubmed Abstract | Pubmed Full Text
Bourre, J. M., Francois, M., Youyou, A., Dumont, O., Piciotti, M., Pascal, G., and Durand, G. (1989). The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J. Nutr. 119, 1880–1892.
Pubmed Abstract | Pubmed Full Text
Brand, M. D., Couture, P., Else, P. L., Withers, K. W., and Hulbert, A. J. (1991). Evolution of energy metabolism. Proton permeability of the inner membrane of liver mitochondria is greater in a mammal than in a reptile. Biochem. J. 275, 81–86.
Pubmed Abstract | Pubmed Full Text
Brand, M. D., Couture, P., and Hulbert, A. J. (1994). Liposomes from mammalian liver mitochondria are more polyunsaturated and leakier to protons than those from reptiles. Comp. Biochem. Physiol. Biochem. Mol. Biol. 108, 181–188.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Braun, D. R., Harris, J. W. K., Levin, N. E., Mccoy, J. T., Herries, A. I. R., Bamford, M. K., Bishop, L. C., Richmond, B. G., and Kibunjia, M. (2010). Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proc. Natl. Acad. Sci. U.S.A. 107, 10002–10007.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Broadhurst, C. L., Wang, Y., Crawford, M. A., Cunnane, S. C., Parkington, J. E., and Schmidt, W. F. (2002). Brain specific lipids from marine, lacustrine, or terrestrial food resources: potential impact on early African Homo sapiens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 131B, 653–673.
Cansev, M., and Wurtman, R. J. (2007). Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, but not arachidonic acid, alone or in combination with uridine, increases brain phosphatide and synaptic protein levels in gerbils. Neuroscience 148, 421–431.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Cao, D., Kevala, K., Kim, J., Moon, H.-S., Jun, S. B., Lovinger, D., and Kim, H.-Y. (2009). Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 111, 510–521.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chang, C.-Y., Ke, D.-S., and Chen, J.-Y. (2009). Essential fatty acids and human brain. Acta Neurol. Taiwan. 18, 231–241.
Pubmed Abstract | Pubmed Full Text
Chung, W. L., Chen, J. J., and Su, H. M. (2008). Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr. 138, 1165–1171.
Pubmed Abstract | Pubmed Full Text
Clandinin, M. T., Chappell, J. E., Leong, S., Heim, T., Swyer, P. R., and Chance, G. W. (1980a). Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum. Dev. 4, 131–138.
Clandinin, M. T., Chappell, J. E., Leong, S., Heim, T., Swyer, R., and Chance, G. W. (1980b). Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum. Dev. 4, 121–129.
Clutton-Brock, T. H., and Harvey, P. H. (1980). Primates, brains and ecology. J. Zool. (Lond.) 190, 309–323.
Cockburn, F. (1994). Neonatal brain and dietary lipids. Arch. Dis. Child. 70, F1–F2.
Coluccia, A., Borracci, P., Renna, G., Giustino, A., Latronico, T., Riccio, P., and Carratu, M. R. (2009). Developmental omega-3 supplementation improves motor skills in juvenile-adult rats. Int. J. Dev. Neurosci. 27, 599–605.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Coti Bertrand, P., O’usky, J. R., and Innis, S. M. (2006). Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J. Nutr. 136, 1570–1575.
Pubmed Abstract | Pubmed Full Text
Da Costa, K.-A., Rai, K. S., Craciunescu, C. N., Parikh, K., Mehedint, M. G., Sanders, L. M., Mclean-Pottinger, A., and Zeisel, S. H. (2009). Dietary docosahexaenoic acid supplementation modulates hippocampal development in the Pemt-/- mouse. J. Biol. Chem. 285, 1008–1015.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Dalton, A., Wolmarans, P., Witthuhn, R. C., Van Stuijvenberg, M. E., Swanevelder, S. A., and Smuts, C. M. (2009). A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins Leukot. Essent. Fatty Acids 80, 143–149.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
De Groot, R. H., Hornstra, G., and Jolles, J. (2007). Exploratory study into the relation between plasma phospholipid fatty acid status and cognitive performance. Prostaglandins Leukot. Essent. Fatty Acids 76, 165–172.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Eilander, A., Hundscheid, D. C., Osendarp, S. J., Transler, C., and Zock, P. L. (2007). Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins Leukot. Essent. Fatty Acids 76, 189–203.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Emken, E. A., Adlof, R. O., and Gulley, R. M. (1994). Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young males. Biochim. Biophys. Acta 1213, 277–288.
Pubmed Abstract | Pubmed Full Text
Fedorova, I., Hussein, N., Baumann, M. H., Di Martino, C., and Salem, N. Jr. (2009). An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behav. Neurosci. 123, 196–205.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Fontaneto, D., Tommaseo-Ponzetta, M., Galli, C., Risé, P., Glew, R. H., and Paoletti, M. G. (2011). Differences in fatty acid composition between aquatic and terrestrial insects used as food in human nutrition. Ecol. Food Nutr. 50, 351–367.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Garcia-Calatayud, S., Redondo, C., Martin, E., Ruiz, J. I., Garcia-Fuentes, M., and Sanjurjo, P. (2005). Brain docosahexaenoic acid status and learning in young rats submitted to dietary long-chain polyunsaturated fatty acid deficiency and supplementation limited to lactation. Pediatr. Res. 57, 719–723.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Gerrior, S., Bente, L., and Hiza, H. (2004). Nutrient content of the US food supply, 1909–2000 (Home Economics Res. Report Number 56). USDA Center for Nutrition Policy and Promotion, Alexandria.
Gibson, R. A., Muhlhausler, B., Makrides, M., Gibson, R. A., Muhlhausler, B., and Makrides, M. (2011). Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 7(Suppl. 2), 17–26.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Greiner, R. S., Moriguchi, T., Hutton, A., Slotnick, B. M., and Salem, N. Jr. (1999). Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory-based and spatial learning tasks. Lipids 34(Suppl.), S239–S243.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Harnack, K., Andersen, G., and Somoza1, V. (2009). Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutr. Metab. (Lond.) 6, 8.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
He, C., Qu, X., Cui, L., Wang, J., and Kang, J. X. (2009). Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. U.S.A. 106, 11370–11375.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Hibbeln, J. R., Nieminen, L. R. G., Blasbalg, T. L., Riggs, J. A., and Lands, W. E. M. (2006). Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am. J. Clin. Nutr. 83, 1483S–1493S.
Pubmed Abstract | Pubmed Full Text
Holguin, S., Huang, Y., Liu, J., and Wurtman, R. (2008). Chronic administration of DHA and UMP improves the impaired memory of environmentally impoverished rats. Behav. Brain Res. 191, 11–16.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Holman, R. T. (1998). The slow discovery of the importance of omega-3 essential fatty acids in human health. J. Nutr. 128, 427s–433s.
Pubmed Abstract | Pubmed Full Text
Hooijmans, C. R., Van Der Zee, C. E., Dederen, P. J., Brouwer, K. M., Reijmer, Y. D., Van Groen, T., Broersen, L. M., Lutjohann, D., Heerschap, A., Kiliaan, A. J., and Van Der Zee, C. E. E. M. (2009). DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol. Dis. 33, 482–498.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Innis, S. M., Vaghri, Z., and King, D. J. (2004). N-6 docosapentaenoic acid is not a predictor of low docosahexaenoic acid status in Canadian school children. Am. J. Clin. Nutr. 80, 768–773.
Pubmed Abstract | Pubmed Full Text
Issa, A. M., Moijica, W. A., Morton, S. C., Traina, S., Newberry, S. J., Hilton, L. G., Garland, R. H., and Maclean, C. H. (2006). The efficacy of omega-3 fatty acids on cognitive function in aging and dementia: a systematic review. Dement. Geriatr. Cogn. Disord. 21, 88–96.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Jamieson, E. C., Farquharson, J., Logan, R. W., Howatson, A. G., Patrick, W. J. A., Weaver, L. T., and Cockburn, F. (1999). Infant cerebellar gray and white matter fatty acids in relation to age and diet. Lipids 34, 1065–1071.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Jerde, R. S., Joel, P., Stroemer, J., Haight, R., and Joel, C. (1975). Comparison of nervous-tissue lipid fatty acid patterns of various animal species with particular reference to docosahexaenoic acid. Biochem. Soc. Trans. 3, 727–730.
Pubmed Abstract | Pubmed Full Text
Kennedy, D. O., Jackson, P. A., Elliott, J. M., Scholey, A. B., Robertson, B. C., Greer, J., Tiplady, B., Buchanan, T., Haskell, C. F., Kennedy, D. O., Jackson, P. A., Elliott, J. M., Scholey, A. B., Robertson, B. C., Greer, J., Tiplady, B., Buchanan, T., and Haskell, C. F. (2009). Cognitive and mood effects of 8 weeks’ supplementation with 400 mg or 1000 mg of the omega-3 essential fatty acid docosahexaenoic acid (DHA) in healthy children aged 10–12 years. Nutr. Neurosci. 12, 48–56.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kirby, A., Woodward, A., Jackson, S., Wang, Y., and Crawford, M. A. (2010). Childrens’ learning and behaviour and the association with cheek cell polyunsaturated fatty acid levels. Res. Dev. Disabil. 31, 731–742.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kitajka, K., Puskas, L. G., Zvara, A., Hackler, L. H. Jr., Barcelo-Coblijn, G., Yoo, Y. K., and Farkas, T. (2002). The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc. Natl. Acad. Sci. U.S.A. 99, 2619–2624.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Knutsen, S. F., Fraser, G. E., Beeson, W. L., Lindsted, K. D., and Shavlik, D. J. (2003). Comparison of adipose tissue fatty acids with dietary fatty acids as measured by 24-hour recall and food frequency questionnaire in black and white Adventists: the Adventist Health Study. Ann. Epidemiol. 13, 119–127.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kuipers, R. S., Luxwold, M. F., Dijck-Brouwer, D. A. J., Eaton, S. B., Crawford, M. A., Cordain, L., and Muskiet, F. A. J. (2010). Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br. J. Nutr. 104, 1666–1687.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lassek, W. D., and Gaulin, S. J. C. (2006). Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am. J. Phys. Anthropol. 131, 295–302.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lassek, W. D., and Gaulin, S. J. C. (2008). Waist-hip ratio and cognitive ability: is gluteofemoral fat a privileged store? Evol. Hum. Behav. 29, 26–34.
Lim, S.-Y., Hoshiba, J., Moriguchi, T., and Salem, N. Jr. (2005). N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr. Res. 58, 741–748.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Liu, J.-W., Almaguel, F. G., Bu, L., De Leon, D. D., and De Leon, M. (2008). Expression of E-FABP in PC12 cells increases neurite extension during differentiation: involvement of n-3 and n-6 fatty acids. J. Neurochem. 106, 2015–2029.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
McCann, J. C., and Ames, B. N. (2005). Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 82, 281–295.
Pubmed Abstract | Pubmed Full Text
McGrew, W. C. (1979). “Evolutionary implications of sex differences in chimpanzee predation and tool use,” in The Great Apes, eds D. A. Hamburg, and E. R. McCown (London: Benjamin Cummings), 441–463.
Mitchell, T. W., Ekroos, K., Blanksby, S. J., Hulbert, A. J., and Else, P. L. (2007). Differences in membrane acyl phospholipid composition between an endothermic mammal and an ectothermic reptile are not limited to any phospholipid class. J. Exp. Biol. 210, 3440–3450.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Mukherjee, P. K., Chawla, A., Loayza, M. S., and Bazan, N. G. (2007). Docosanoids are multifunctional regulators of neural cell integrity and fate: significance in aging and disease. Prostaglandins Leukot. Essent. Fatty Acids 77, 233–238.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
NCES. (2005). Highlights From the Trends in International Mathematics, and Science Study: TIMSS. (2003). Department of Education, National Center for Educational Statistics, 2005–2005, Washington, DC.
Neggers, Y. H., Kim, E.-K., Song, J.-M., Chung, E.-J., Um, Y.-S., and Park, T. (2009). Mental retardation is associated with plasma omega-3 fatty acid levels and the omega-3/omega-6 ratio in children. Asia Pac. J. Clin. Nutr. 18, 22–28.
Pubmed Abstract | Pubmed Full Text
Nichols, P. D., Danaher, K. T., and Koslow, J. A. (2003). Occurrence of high levels of tetracosahexaenoic acid in the jellyfish Aurelia sp. Lipids 11, 1207–1210.
Niemoller, T. D., Stark, D. T., and Bazan, N. G. (2009). Omega-3 fatty acid docosahexaenoic acid is the precursor of neuroprotectin D1 in the nervous system. World Rev. Nutr. Diet. 99, 46–54.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Novak, E. M., Dyer, R. A., and Innis, S. M. (2008). High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 1237, 136–145.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Osendarp, S. J., Baghurst, K. I., Bryan, J., Calvaresi, E., Hughes, D., and Hussaini, M. (2007). Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am. J. Clin. Nutr. 86, 1082–1093.
Pubmed Abstract | Pubmed Full Text
Ouellet, M., Emond, V., Chen, C., Julien, C., Bourasset, F., Oddo, S., Laferla, F., Bazinet, R. P., and Calon, F. (2009). Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: an in situ cerebral perfusion study. Neurochem. Int. 55, 476.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pawlosky, R. J., Hibbeln, J. R., Novotny, J. A., and Salem, N. (2001). Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J. Lipid Res. 42, 1257–1265.
Pubmed Abstract | Pubmed Full Text
Pifferi, F., Jouin, M., Alessandri, J. M., Haedke, U., Roux, F., Perriere, N., Denis, I., Lavialle, M., and Guesnet, P. (2007). N-3 fatty acids modulate brain glucose transport in endothelial cells of the blood-brain barrier. Prostaglandins Leukot. Essent. Fatty Acids 77, 279–286.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Plomin, R., DeFries, J. C., McClearn, G. E., and McGuffin, P. (2000). Behavioral Genetics, 4th Edn. New York: Worth.
Putnam, J., Allshouse, J., and Kantor, L. S. (2002). U.S. per capita food supply trends: more calories, refined carbohydrates, and fats. Food Rev. 25, 1–15.
Putnam, N. H., Srivastava, M., Hellsten, U., Dirks, B., Chapman, J., Salamov, A., Terry, A., Shapiro, H., Lindquist, E., Kapitonov, V. V., Jurka, J., Genikhovich, G., Grigoriev, I. V., Lucas, S. M., Steele, R. E., Finnerty, J. R., Technau, U., Martindale, M. Q., and Rokhsar, D. S. (2007). Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Redford, K. H., Bouchardet Da Fonseca, G. A., and Lacher, T. E. J. (1984). The relationship between frugivory and insectivory in primates. Primates 25, 433–440.
Richardson, A. J., and Montgomery, P. (2005). The Oxford-Durham Study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics 115, 1360–1366.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Rubin, D., and Laposata, M. (1992). Cellular interactions between n-6 and n-3 fatty acids: a mass analysis of fatty acid elongation/desaturation, distribution among complex lipids, and conversion to eicosanoids. J. Lipid Res. 33, 1431–1440.
Pubmed Abstract | Pubmed Full Text
Ryan, A. S., Astwood, J. D., Gautier, S., Kuratko, C. N., Nelson, E. B., and Salem, N. Jr. (2010). Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot. Essent. Fatty Acids 82, 305–314.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Ryan, A. S., and Nelson, E. B. (2008). Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: a randomized, placebo-controlled, double-blind study. Clin. Pediatr. (Phila) 47, 355–362.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Sakamoto, T., Cansev, M., and Wurtman, R. J. (2007). Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 1182, 50–59.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Salem, N. Jr., Moriguchi, T., Greiner, R. S., Mcbride, K., Ahmad, A., Catalan, J. N., and Slotnick, B. (2001). Alterations in brain function after loss of docosahexaenoate due to dietary restriction of n-3 fatty acids. J. Mol. Neurosci. 16, 299–307.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Sauerwald, T. U., Demmelmair, H., Fidler, N., and Koletzko, B. (2000). Polyunsaturated fatty acid supply with human milk. Adv. Exp. Med. Biol. 478, 261–270.
Pubmed Abstract | Pubmed Full Text
Schuchardt, J. P., Huss, M., Stauss-Grabo, M., and Hahn, A. (2010). Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur. J. Pediatr. 169, 149–164.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Sekula, W., Nelson, M., Figurska, K., Oltarzewski, M., Weisell, R., and Szponar, L. (2005). Comparison between household budget survey and 24-hour recall data in a nationally representative sample of Polish households. Public Health Nutr. 8, 430–439.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Shanker, K., Shireesha, K. K. S., Kumar, S. V., Srinivas, C., Rao, J. V., and Prasad, R. B. (2006). Isolation and characterization of neutral lipids of desilked eri silkworm pupae grown on castor and tapioca leaves. J. Agric. Food Chem. 2006, 3305–3309.
Simopoulos, A. P., Leaf, A., and Salem, N. S. Jr. (1999). Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J. Am. Coll. Nutr. 18, 487–489.
Pubmed Abstract | Pubmed Full Text
Slater, B., Enes, C. C., Lopez, R. V., Damasceno, N. R., Voci, S. M., Slater, B., Enes, C. C., Lopez, R. V. M., Damasceno, N. R. T., and Voci, S. M. (2010). Validation of a food frequency questionnaire to assess the consumption of carotenoids, fruits and vegetables among adolescents: the method of triads. Cad. Saude Publica 26, 2090–2100.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Stark, W. S., Lin, T. N., Brackhahn, D., Christianson, J. S., and Sun, G. Y. (1993). Fatty acids in the lipids of Drosophila heads: effects of visual mutants, carotenoid deprivation and dietary fatty acids. Lipids 28, 345–350.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Stewart, K. M. (2010). “The case for exploitation of wetlands by pre-sapiens hominins,” in Human Brain Evolution: The Influence of Freshwater and Marine Food Resources, eds S. C. Cunnane, and K. M. Stewart (Hoboken: John Wiley and Sons), 137–172.
Stoknes, I. S., Økland, H. M. W., Falch, E., and Synnes, M. (2004). Fatty acid and lipid class composition in eyes and brain from teleosts and elasmobranchs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 138, 183–191.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Svennerholm, L. (1968). Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 9, 570–579.
Pubmed Abstract | Pubmed Full Text
Takeuchi, T., Fukumoto, Y., and Harada, E. (2002). Influence of a dietary n-3 fatty acid deficiency on the cerebral catecholamine contents, EEG and learning ability in rat. Behav. Brain Res. 131, 193–203.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Turner, N., Else, P. L., and Hulbert, A. J. (2003). Docosahexaenoic acid (DHA) content of membranes determines molecular activity of the sodium pump: implications for disease states and metabolism. Naturwissenschaften 90, 521–523.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Wasserman, G. A., Liu, X., Lolacono, N. J., Factor-Litvak, P., Kline, J. K., Popovac, D., Morina, N., Musabegovic, A., Vrenezi, N., Capuni-Paracka, S., Lekic, V., Preteni-Redjepi, E., Hadzialjevic, S., Slavkovich, V., and Graziano, J. (1997). Lead exposure and intelligence in 7-year-old children: the Yugoslavia Prospective Study. Environ. Health Perspect. 105, 956–962.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Whalley, L. J., Fox, H. C., Wahle, K. W., Starr, J. M., and Deary, I. J. (2004). Cognitive aging, childhood intelligence, and the use of food supplements: possible involvement of n-3 fatty acids. Am. J. Clin. Nutr. 80, 1650–1657.
Pubmed Abstract | Pubmed Full Text
Wu, A., Ying, Z., and Gomez-Pinilla, F. (2008). Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 155, 751–759.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Wurtman, R. J., Cansev, M., and Ulus, I. H. (2009). Synapse formation is enhanced by oral administration of uridine and DHA, the circulating precursors of brain phosphatides. J. Nutr. Health Aging 13, 189–197.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Yoshida, S., Yasuda, A., Kawazato, H., Sakai, K., Shimada, T., Takeshita, M., Yuasa, S., Kobayashi, T., Watanabe, S., and Okuyama, H. (1997). Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of α-linolenate deficiency and a learning task. J. Neurochem. 68, 1261–1268.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Keywords: essential fatty acids, DHA, cognition, diet, brain, sex differences, evolution
Citation: Lassek WD and Gaulin SJC (2011) Sex differences in the relationship of dietary fatty acids to cognitive measures in American children. Front. Evol. Neurosci. 3:5. doi: 10.3389/fnevo.2011.00005
Received: 15 August 2011; Accepted: 14 October 2011;
Published online: 02 November 2011.
Copyright: © 2011 Lassek and Gaulin. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: William D. Lassek, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 527A Parran Hall, 130 DeSoto Street, Pittsburgh, PA 15261, USA. e-mail: will.lassek@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.