Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma (original) (raw)
Colorectal Cancer Open Access
Copyright ©The Author(s) 2004. Published by Baishideng Publishing Group Inc. All rights reserved.
World J Gastroenterol. Jun 1, 2004; 10(11): 1569-1573
Published online Jun 1, 2004. doi: 10.3748/wjg.v10.i11.1569
Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma
Xiang-Tao Ma, Shan Wang, Ying-Jiang Ye, Ru-Yu Du, Department of Surgery, Peking University People’s Hospital, Beijing 100044, China
Zhi-Rong Cui, Division of Surgical Oncology, Peking University People’s Hospital, Beijing 100044, China
Ma Somsouk, Gastrointestinal Unit, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 32 Fruit Street, Boston, MA 02114, USA
ORCID number: $[AuthorORCIDs]
Author contributions: All authors contributed equally to the work.
Supported by the National Natural Science Foundation of China, No. 30271269
Correspondence to: Dr. Shan Wang, Department of Surgery, Peking University People’s Hospital, Beijing 100044, China. shwang60@sina.com
Telephone: +86-10-68792772 Fax: +86-10-68318386
Received: October 24, 2003
Revised: December 4, 2003
Accepted: December 8, 2003
Published online: June 1, 2004
Abstract
AIM: Signal transducers and activators of transcription (STATs) are a family of transcription factors activated in response to cytokines and growth factors. Constitutive activation of Stat3 has been observed in a growing number of tumor-derived cell lines, as well as tumor specimens from human cancers. The purpose of this study was to investigate the expression of p-Stat3, activated form of Stat3, and its downstream mediators including cyclin D1 and Bcl-xL in colorectal carcinoma (CRC), and to explore the possible mechanism of Stat3 signaling pathway in the tumorigenesis of colorectal carcinoma.
METHODS: Tissue samples from 45 patients of primary colorectal carcinoma were selected for studying Stat3 signaling pathway protein expression. Western blot analysis was used to measure the expression of p-Stat3, cyclin D1, and Bcl-xL proteins in colorectal carcinomas. Furthermore, the expression patterns of these proteins were analyzed for their distribution at the cellular level by immunohistochemical staining of the tissues.
RESULTS: Protein levels of p-Stat3, cyclin D1, and Bcl-xL were increased in colorectal carcinomas compared with adjacent normal mucosae (P < 0.05). Elevated levels of p-Stat3 were correlated with the nodal metastasis and the stage (P < 0.05). Overexpression of cyclin D1 was associated with the nodal metastasis (P < 0.05). There was also a significant correlation between the expressions of p-Stat3 and cyclin D1 (r = 0.382, P < 0.05).
CONCLUSION: Constitutive activation of Stat3 may play an important role in the tumorigenesis of colorectal carcinoma, and the detailed mechanism of Stat3 signaling pathway in CRC deserves further investigation.
Key Words: $[Keywords]
- Citation: Ma XT, Wang S, Ye YJ, Du RY, Cui ZR, Somsouk M. Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma. World J Gastroenterol 2004; 10(11): 1569-1573
- URL: https://www.wjgnet.com/1007-9327/full/v10/i11/1569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i11.1569
INTRODUCTION
Colorectal carcinoma (CRC) is a very common malignancy in developed countries and the incidence of CRC has been increasing rapidly in the latter part of the twentieth century in urban China[1,2]. Although there have been advances in surgical and cytotoxic treatments of colorectal carcinoma, the overall survival percentage has not changed in recent years. While significant progresses have been achieved in identifying oncogenes and tumor suppressor genes involved in the tumorigenesis of colorectal carcinoma, the molecular mechanisms in colorectal carcinoma are still poorly understood. Recently, with the delineation of important signal transduction cascades, it has become clear that the signal transducers and activators of transcription (STATs) signaling pathway may play an important role in the malignant transformation of a number of human malignancies[3].
STATs are transcription factors activated in response to cytokines and growth factors. At present, seven STATs have been identified in mammals: Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5 b, and Stat6. Stat5 a and Stat5 b are encoded by distinct genes whereas Stat1 and Stat3 exhibit two isoforms, each resulting from alternative splicing[4]. These proteins have a conserved structural organization and range in size from 750 to 900 amino acids. Activated STATs rapidly translocate into nuclei, bind to recognition sequences in the promoter region of target genes, and regulate their transcription. Recent studies have demonstrated the essential roles of STATs proteins in modulating the process of cell proliferation, differentiation, and apoptosis[5-7].
Constitutively activated STAT proteins have been observed in a wide variety of human tumor cell lines and primary tumors including leukemia, multiple myeloma, breast cancer, prostate cancer, and other cancers[8-13]. Further investigation demonstrated that activation of Stat3 was associated with the transformation by v-Src and other viral oncoproteins[13-15]. Stat3 has been classified as an oncogene because constitutively activated Stat3 was found to mediate oncogenic transformation in cultured cells and tumor formation in nude mice[16,17]. Stat3 activation may not only provide a growth advantage, but also confer resistance to conventional therapies that rely on the mechanism of apoptosis to eliminate tumor cells[18]. The events downstream from constitutive activation of Stat3 that promote tumorigenesis are unclear but could include deregulation of cell cycle progression and/or providing protection against apoptosis. Recent studies showed that constitutive activation of Stat3 correlated with cyclin D1 expression and might provide a prognostic marker in head and neck cancer[19], and activated Stat3 contributed to the process of apoptosis in ovarian cancer cells by regulating the expression of Bcl-xL[20]. These findings suggest that constitutive activation of Stat3 participates in the development of different human malignancies. However, the expression and activation of Stat3 protein in human colorectal carcinomas have not been studied. It is important to know whether or not constitutive activation of Stat3 signaling pathway plays a central role in human colorectal carcinomas.
In the present study, we examined the expression of p-Stat3, the activated form of Stat3, cyclin D1, and Bcl-xL in 45 primary tumor samples obtained from patients with CRC. Our results demonstrate that constitutive activation of Stat3 signaling pathway may play an important role in the tumorigenesis of colorectal carcinoma. Furthermore, activation of Stat3 is correlated with the overexpression of cyclin D1 in colorectal carcinoma.
MATERIALS AND METHODS
Materials
PVDF membranes for Western blot analysis were purchased from Millipore (Bedford, MA), and x-ray film was from Eastman Kodak (Rochester, NY). All antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Prestained molecular mass markers were from GIBCO/BRL (Grand Island, NY). The enhanced chemiluminescence (ECL) system for Western blot analysis was from Amersham (Arlington Heights, IL). Concentrated protein assay dye reagents were from Bio-Rad Laboratories (Hercules, CA). All other reagents were of molecular biology grade and were purchased from either Sigma (St. Louis, MO) or Amresco (Solon, OH).
Patients and tissue samples
Primary colorectal adenocarcinomas and adjacent normal mucosae distant from the tumor (5-10 cm away) were obtained from 45 patients undergoing colorectal cancer resection at the Department of Surgery, Peking University People’s Hospital from February, 1999 to February, 2000. No patient had received chemotherapy or radiation therapy before surgery. The samples were collected after informed consent was obtained from the patients at the time of surgery. Malignant tissues and adjacent normal mucosae were immediately snap-frozen in liquid nitrogen within 15-20 min after surgical removal to ensure preservation of Stat3 activities. Detailed clinicopathological parameters including gender, age, site of primary tumor, stage, and degree of differentiation are shown in Table 1. Staging of the tumors was conducted according to the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) TNM Classification after brief histological studies.
Table 1 Clinicopathological parameters of 45 patients with colorectal carcinoma.
| Clinicopathological parameters | Numbers (%) | |
|---|---|---|
| Gender | Male | 24 (53.3) |
| Female | 21 (46.7) | |
| Age (yr) | Range | 35-81 |
| Mean | 61.7 | |
| Median | 66.0 | |
| Primary site | Colon | 25 (55.6) |
| Rectum | 20 (44.4) | |
| I | 1 (0.22) | |
| II | 24 (53.3) | |
| III | 14 (31.1) | |
| IV | 6 (13.3) | |
| Depth of invasion and Lymph node involvement | T1-T2 N0 | 7 (15.6) |
| T3-T4 N0 | 18 (40.0) | |
| T1-T2 N1-2N2 | 12 (26.7) | |
| T3-T4 N1-N2 | 8 (17.8) | |
| Distant Metastasis | M0 | 39 (86.7) |
| M1 | 6 (13.3) | |
| Histological grade | G1 | 12 (26.7) |
| G2 | 22 (48.9) | |
| G3 | 11 (24.4) | |
| Tumor size | > 5 cm | 22 (48.9) |
| ≤ 5 cm | 23 (51.1) |
Western blot analysis
Tissues were lysed with lysis buffer (150 mmol/L NaCl, 10 g/L sodium deoxycholate, 10 g/L Triniton X-100, 1 g/LSDS, 10 mmol/L Tris, pH7.2, 1 mmol/L Na3 VO4, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L NaF, 0.1 mmol/L aprotinin, and 1 mmol/L leupeptin). After centrifugation at 13000 g for 30 min at 4 °C, the protein concentrations in the cell lysates were determined by the Bradford assay. For Western blot analysis, whole cell extracts were mixed with 2 × sodium dodcyl sulfate (SDS) sample buffer (125 mmol/L Tris·HCl, pH6.8, 40 g/L SDS, 200 mL/L glycerol, 100 mL/L 2-mercaptoethanol) at 1:1 ratio and were heated for 5 min at 100 °C. Proteins (50 mg/lane) were separated by electrophoresis on 7.5%-10% gradient SDS-polyacrylamide gel and transferred onto a PVDF membrane. Prestained molecular weight markers were included in each gel. Membranes were blocked for 30 min in Tris-buffered saline (TBS: 10 mmol/L Tris·HCl, pH7.5 and 150 mmol/L NaCl) with 5 g/L Tween-20 (TBST) and 50 g/L BSA. After blocking, membranes were incubated at 4 °C overnight with Stat3 (C-20) phospho-independent, phospho-specific (Tyr-705) p-Stat3 (B-7) ; cyclin D1 (M-20), and Bcl-xL (H-62) antibody in TBST and 10 g/L BSA respectively. Additionally, anti-glyceraldehydes-3-phosphate dehydrogenase (GAPDH) antibody was used to determine the amount of endogenous GAPDH protein to serve as an internal control. After the membranes were washed three times with TBST (5 min each), they were incubated with horseradish peroxidase-conjugated secondary antibody in TBST and 10 g/L BSA for 30 min. Subsequently, membranes were washed three times with TBST and developed by using the enhanced chemiluminescence (ECL) detection system. The optical density (OD) was measured by densitometry using a Storm PhosphoImager (Molecular Dynamics, Sunnyvale, CA) and the result was shown as relative expression for tumor (T) versus normal mucosae (N).
Immunohistochemical staining
Tissue samples were fixed in 40 g/L buffered formaldehyde, and embedded in paraffin. Five-micrometer sections of normal mucosa and colorectal carcinoma were cut and mounted onto poly-L-lysine-coated glass slides, air-dried, and heated for 2 h at 60 °C in an oven. The sections were dewaxed in xylene, rehydrated in descending alcohols, and endogenous peroxidase activity was blocked using 3 mL/L H2O2-methanol solution. These sections were then subjected to an antigen retrieval procedure; slides were heated in citrate buffer 10 mmol/L (pH 6.0) for 10 min. The sections were then cooled and washed in phosphate-buffered saline (pH 7.4) and nonspecific binding sites were blocked by incubating with 50 mL/L goat serum for 30 min in a humidified chamber at room temperature. The slides were incubated at 4 °C overnight with appropriate primary antibody [Stat3 (C-20), dilution 1:75; p-Stat3 (B-7), dilution 1:150; cyclin D1 (M-20), dilution 1:100; Bcl-xL (H-62), dilution 1:100]. Immunologic reaction was developed using 3-3-diaminobenzidine in TBS containing 0.2 mL/L hydrogen peroxide. The slides were counterstained with hematoxylin. Negative controls were performed by substituting the primary antibody with Tris-buffered saline.
Statistical analysis
Statistical analysis was performed with SPSS software version 10.0 (SPSS Inc., Chicago, IL). Data were presented as mean ± SD. The relationship between levels of p-Stat3, cyclin D1 or Bcl-xL and various clinicopathological parameters was determined by Student’s t test or one-way ANOVA. The association between p-Stat3, cyclin D1 and Bcl-xL expression was analyzed by Pearson’s correlation coefficient. P < 0.05 was considered statistically significant.
RESULTS
Activated Stat3 was constitutively expressed in colorectal carcinoma
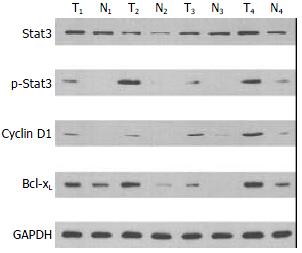
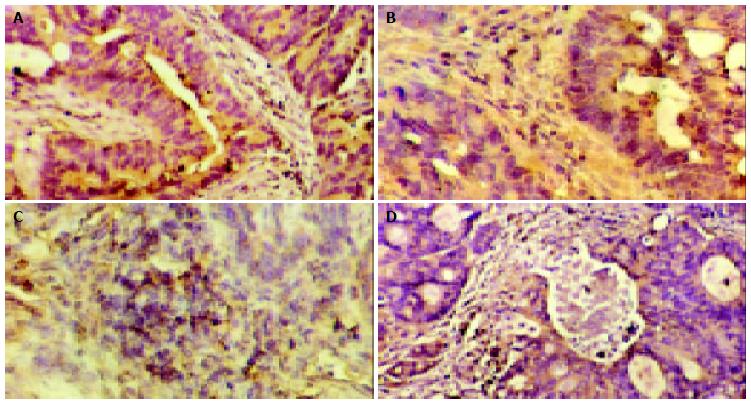
One objective was to determine whether Stat3 was constitutively activated in human colorectal carcinoma and whether activation of Stat3 correlated with various clinicopathological parameters in patients with CRC. To determine whether Stat3 was activated in CRC, we performed Western blot analysis using antibody to p-Stat3, activated form of Stat3. Representative cases are shown in Figure 1. Of the 45 CRC samples examined, 57.8% (26 of 45) of the samples showed strong p-Stat3 expression. Quantitative evaluation of the relative expression (tumor versus normal mucosae) of these experiments demonstrated an average 2.6-fold increase in the level of p-Stat3 protein in cancers compared with adjacent normal mucosae (P = 0.002, Table 2). Both cytoplasmic and nuclear localizations of the Stat3 (p-Stat3) were detected in CRC primary tumors (Figure 2). When we examined possible correlations with various clinicopathological parameters, we found that increased levels of p-Stat3 were significantly correlated with the existence of nodal metastasis (P = 0.018). We also found that the levels of p-Stat3 were increased in stages III and IV, whereas its levels were decreased in stages I and II (P = 0.026). No statistically significant correlation was observed between p-Stat3 expression and gender, age, primary site, size, and grade of tumors (Table 3).
Figure 1 Expressions of Stat3, p-Stat3, cyclin D1, and Bcl-xL in colorectal carcinoma. Lysates were made as described under Materials and Methods. GAPDH represents the internal pro-tein control. Elevated levels of Stat3, p-Stat3 (Tyr-705), cyclin D1, and Bcl-xL in tumor (T) tissues were compared to adjacent normal mucosae (N).
Table 2 Expressions of p-Stat3, cyclin D1, and Bcl-xL in colorectal carcinoma (mean ± SD).
| Items | n | Percentage (%) | A Value | t | P | |
|---|---|---|---|---|---|---|
| Tumor | Normal | |||||
| p-Stat3 | 45 | 57.8 (26/45) | 114263 ± 53598 | 55971 ± 28762 | 3.573 | 0.002 |
| cyclin D1 | 45 | 64.4 (29/45) | 58321 ± 24872 | 22563 ± 11160 | 5.191 | 0.0001 |
| Bcl-xL | 45 | 68.9 (31/45) | 71032 ± 43425 | 37281 ± 14622 | 4.627 | 0.0001 |
Figure 2 Expressions of Stat3, p-Stat3, cyclin D1, and Bcl-xL in colorectal carcinoma. A: Cytoplasmic staining of Stat3 in CRC (original magnification × 200) ; B: Nuclear staining of p-Stat3 in CRC (original magnification × 200) ; C: Nuclear staining of cyclin D1 in CRC (original magnification × 200) ; D: Cytoplasmic staining of Bcl-xL (original magnification × 200).
Table 3 Correlations between p-Stat3, cyclin D1, Bcl-xL and clinicopathological parameters in colorectal carcinoma (mean ± SD).
| Item | n | p-Stat3 | P | cyclin D1 | P | Bcl-xL | P |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 24 | 2.7 ± 0.6 | 0.846 | 4.1 ± 1.6 | 0.913 | 3.7 ± 1.2 | 0.746 |
| Female | 21 | 2.5 ± 0.5 | 4.3 ± 1.8 | 3.3 ± 1.1 | |||
| Age (yr) | |||||||
| ≥ 65 | 22 | 2.8 ± 0.7 | 0.736 | 4.8 ± 2.1 | 0.825 | 3.4 ± 1.5 | 0.870 |
| < 65 | 23 | 2.4 ± 0.4 | 3.6 ± 1.3 | 3.6 ± 0.8 | |||
| Stage | |||||||
| III + IV | 20 | 3.6 ± 0.6 | 0.026 | 5.2 ± 1.9 | 0.065 | 4.2 ± 1.3 | 0.235 |
| I + II | 25 | 1.8 ± 0.5 | 3.4 ± 1.5 | 3.1 ± 1.0 | |||
| Histological | |||||||
| Grade | |||||||
| G1 | 12 | 2.3 ± 0.5 | 0.778 | 4.7 ± 1.8 | 0.732 | 3.9 ± 1.3 | 0.894 |
| G2 | 22 | 2.4 ± 0.6 | 0.645 | 4.2 ± 1.4 | 0.627 | 3.3 ± 1.0 | 0.771 |
| G3 | 11 | 3.4 ± 0.6 | 0.530 | 3.6 ± 2.2 | 0.565 | 3.6 ± 1.2 | 0.832 |
| Node | |||||||
| Metastasis | |||||||
| Positive | 27 | 3.8 ± 0.8 | 0.018 | 5.5 ± 1.9 | 0.041 | 4.4 ± 1.4 | 0.162 |
| Negative | 18 | 1.6 ± 0.4 | 3.3 ± 1.5 | 2.9 ± 1.0 | |||
| Distant | |||||||
| Metastasis | |||||||
| M1 | 6 | 3.6 ± 0.8 | 0.638 | 5.4 ± 2.2 | 0.612 | 5.2 ± 1.6 | 0.324 |
| M0 | 39 | 2.5 ± 0.5 | 4.0 ± 1.6 | 3.3 ± 1.1 | |||
| Tumor Size | |||||||
| ≥ 5 cm | 22 | 3.0 ± 0.7 | 0.582 | 4.5 ± 1.8 | 0.776 | 3.8 ± 1.3 | 0.735 |
| < 5 cm | 23 | 2.2 ± 0.4 | 3.9 ± 1.6 | 3.2 ± 1.0 |
Expression of downstream mediators of Stat3 in colorectal carcinoma
We next investigated the expression of cyclin D1 and Bcl-xL, which could be potential downstream mediators of Stat3 in CRC. We found that cyclin D1 and Bcl-xL were overexpressed in CRC tissues (P < 0.05, Table 2). When we examined possible correlations with various clinicopathological parameters, we found that increased expression of cyclin D1 correlated with the nodal metastasis (_P_ = 0.041), whereas increased expression of Bcl-xL did not significantly correlate with any of these parameters (Figure 1, Table 3). The expression pattern of cyclin D1 and Bcl-xL was also checked with immunohistochemistry. Representative examples of immunohistochemical staining are shown in Figure 2. Furthermore, we studied the possible correlations between expression of p-Stat3 and downstream mediators. When these data were analyzed by Pearson’s correlation coefficient, we found a significant association between expressions of p-Stat3 and cyclin D1 (_r_ = 0.382, _P_ < 0.05). No statistically significant correlation was observed between p-Stat3 and Bcl-xL expressions (_r_ = 0.162, _P_ > 0.05).
DISCUSSION
Though significant progresses have been achieved in delineating the molecular mechanisms of colorectal carcinoma tumorigenesis, specific signal transduction pathways involved in CRC have not been fully characterized[21]. Increasing reports suggested that Stat3 signaling pathway played a critical role in malignant transformation and tumor progression[3]. Constitutive activation of Stat3 has been detected in a wide variety of human primary tumors and tumor cell lines including blood malignancies, breast cancer, and other cancers[8-13]. In the current study, we provided evidences that constitutive activation of Stat3 signaling might play an important role in human colorectal carcinoma.
Western blot analysis with p-Stat3 antibody showed that the activated form of Stat3 was elevated in the majority (57.8%) of CRC samples. Both cytoplasmic and nuclear localizations of Stat3 (p-Stat3) were also detected in CRC primary tumors. Nagpal _et al_[22] reported in their work on head and neck carcinomas that 58.9% (53/90) of HNSCC tumors showed very high Stat3 protein accumulation, and none of the normal epithelium samples showed Stat3 absence. Campbell _et al_[23] found that in 51 human primary tissues from normal prostate, benign prostatic hyperplasia, and prostate cancer, p-Stat3 was observed more prominently in the nuclei of cells residing in malignant glands compared to those in nonmalignant samples. As previously discussed, there were a growing number of evidences associating constitutive or aberrant activation of Stat3 with human cancers. The presence of p-Stat3 and its up-regulation in colorectal carcinoma could have important implications in colorectal cancer biology.
Because the above results provided evidence that Stat3 was constitutively activated in colorectal carcinomas, it was of interest to determine whether activation of stat3 correlated with various clinicopathological parameters in patients with CRC. When we examined possible correlations with various parameters, we found that increased levels of p-Stat3 significantly correlated with the existence of nodal metastasis and its stage but not with other parameters. Masuda _et al_[19] indicated in their work on HNSCC, that elevated levels of the activated form of Stat3 had a significant association with the clinical stage of HNSCC. Ni _et al_[24,25] demonstrated that Stat3 DNA binding activity was correlated with malignant potential in both human prostate cancer cell lines and a large series of rat Dunning prostate cancer cell lines. The most aggressive cell lines were found to have the highest Stat3 DNA binding activities. Blockade of activated Stat3 by dominant-negative Stat3 constructs significantly suppressed their growth in vitro and tumorigenicity in vivo. This specific activation of Stat3 in the tumorigenesis of colorectal carcinoma could qualify it as a potential diagnostic marker.
Tumor progression could be facilitated by activation of genes that regulate proliferation and/or apoptosis. However, the downstream events from constitutively activated Stat3 are not fully understood. Cyclin D1, an important cell cycle regulator, was overexpressed and associated with the poor prognosis in colorectal carcinoma[26]. We investigated the expression of cyclin D1 in CRC tissues. When we examined possible correlations with various parameters, we found that increased levels of cyclin D1 correlated with the nodal metastasis, and there was a significant correlation between overexpressions of p-Stat3 and cyclin D1. However, the precise mechanism underlying the overexpression of cyclin D1 in CRC is unclear. In the majority of the cases of CRC, cyclin D1 gene was not amplified, suggesting that the increased expression of cyclin D1 was due to defects at the level of gene transcription[27]. The similar discrepancy between cyclin D1 overexpression and gene amplification was also reported in breast and HNSCC cancers[28,29]. Constitutive activation of Stat3 constructs has been shown to activate the cyclin D1 promoter in rodent fibroblast cell lines[16], and overexpression of cyclin D1 was associated with increased activation of Stat3 in ovarian carcinoma and HNSCC[20,30]. Therefore, frequent overexpression of cyclin D1 in CRC might be attributable, at least in part, to increased levels of p-Stat3.
Another important downstream mediator is Bcl-xL. Bcl-xL is a member of Bcl-2 family that could play a critical role in apoptosis[31]. Previous studies showed that Bcl-xL was overexpressed in colorectal carcinomas, and Bcl-xL might be a useful prognostic marker in CRC[32,33]. We also found that Bcl-xL was overexpressed in CRC tissues, but the increased protein level did not significantly correlate with the clinicopathological parameters in CRC. No statistically significant correlation was observed between p-Stat3 and Bcl-xL expressions. Bromberg _et al_[16] observed that Stat3 could transcriptionally up-regulate the expression of Bcl-xL in Stat3 transformed cell lines. Evidence suggested that blocking of the activated Stat3 in multiple myeloma cells and ovarian cancer cells could down-regulate the expression of Bcl-xL and enhance the apoptosis, and the cells regained the sensitivity to chemotherapy[9,20]. The precise mechanism by which activation of Stat3 enhances the transcription of Bcl-xL in CRC remains to be determined.
Our results demonstrated that in comparison with normal mucosae, p-Stat3 protein was overexpressed in human colorectal carcinomas. Expression of p-Stat3 was associated with the presence of nodal metastasis and its stage. Our clinical data also provides evidence that there is a strong association of p-Stat3 and cyclin D1 overexpression in CRC. These results suggest that constitutive activation of Stat3 signaling pathway may play an important role in the tumorigenesis of colorectal carcinoma, and the detailed mechanism of Stat3 signaling pathway in CRC deserves further investigation.
ACKNOLEDGEMENTS
We appreciate the technical assistance of Dr. Li-Mei Ma at Cornell University Weill Medical College and Dr. Cong-Rong Yu at Columbia University.
Footnotes
Edited by Wang XL and Xu CT Proofread by Xu FM
References
| 7. | Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659-6666. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 13. | Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33-36. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 14. | Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553-2558. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 19. | Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351-3355. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 25. | Ni Z, Lou W, Leman ES, Gao AC. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res. 2000;60:1225-1228. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 29. | Quon H, Liu FF, Cummings BJ. Potential molecular prognos-tic markers in head and neck squamous cell carcinomas. Head Neck. 2001;23:147-159. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 30. | Kijima T, Niwa H, Steinman RA, Drenning SD, Gooding WE, Wentzel AL, Xi S, Grandis JR. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002;13:355-362. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 32. | Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422-2427. [PubMed] [DOI] [Cited in This Article: ] |
|---|