Helicobacter pylori and serum kynurenine-tryptophan ratio in patients with colorectal cancer (original) (raw)
Prospective Study Open Access
Copyright ©The Author(s) 2015. Published by Baishideng Publishing Group Inc. All rights reserved.
World J Gastroenterol. Mar 28, 2015; 21(12): 3636-3643
Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3636
Helicobacter pylori and serum kynurenine-tryptophan ratio in patients with colorectal cancer
Ayse Basak Engin, Bensu Karahalil, Ali Esat Karakaya, Department of Toxicology, Faculty of Pharmacy, Gazi University, TR 06330 Hipodrom, Ankara, Turkey
Atilla Engin, Department of General Surgery, Faculty of Medicine, Gazi University, TR 06500 Beşevler, Ankara, Turkey
ORCID number: $[AuthorORCIDs]
Author contributions: Engin AB performed the majority of the experiments, designed the study and wrote the manuscript; Karahalil B and Karakaya AE edited the manuscript; and Engin A co-ordinated and provided the collection of human material, designed the study and edited the manuscript.
Ethics approval: The study was approved by Gazi University, Local Ethics Committee.
Informed consent: All participants’ rights were protected and informed consents were obtained according to the Helsinki Declaration.
Conflict-of-interest: Authors declare that there is no conflict of interest.
Data sharing: Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Correspondence to: Ayse Basak Engin, PhD, Department of Toxicology, Faculty of Pharmacy, Gazi University, TR 06330 Hipodrom, Ankara, Turkey. abengin@gmail.com
Telephone: +90-312-2023084 Fax: +90-312-2222326
Received: September 11, 2014
Peer-review started: September 12, 2014
First decision: October 29, 2014
Revised: December 1, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: March 28, 2015
Processing time: 199 Days and 17.9 Hours
Abstract
AIM: To evaluate how Helicobacter pylori (H. pylori) is able to evade the immune response and whether it enhances systemic immune tolerance against colorectal cancer.
METHODS: This prospective randomized study involved 97 consecutive colorectal cancer patients and 108 cancer-free patients with extra-digestive diseases. Colorectal cancer and cancer-free patients were assigned into subgroups according to H. pylori IgG seropositivity. Exposure to H. pylori was determined by IgG seropositivity which was detected by enzyme linked immunoassay (ELISA). Serum neopterin levels were measured by ELISA. Serum tryptophan, kynurenine, and urinary biopterin concentrations were measured by high performance liquid chromatography. Serum nitrite levels were detected spectrophotometrically. Serum indoleamine 2,3-dioxygenase activity was estimated by the kynurenine to tryptophan ratio and by assessing the correlation between serum neopterin concentrations and the kynurenine to tryptophan ratio. The frequencies of increased serum kynurenine to tryptophan ratio of H. pylori seronegative and seropositive colorectal cancer subgroups were estimated by comparing them with the average kynurenine to tryptophan ratio of H. pylori seronegative tumor-free patients.
RESULTS: Compared with respective controls, in both H. pylori seronegative and seropositive colorectal cancer patients, while serum tryptophan levels were decreased (controls vs patients; seronegative: 20.37 ± 0.89 μmol/L vs 15.71 ± 1.16 μmol/L, P < 0.05; seropositive: 20.71 ± 0.81 μmol/L vs 14.97 ± 0.79 μmol/L, P < 0.01) the kynurenine to tryptophan ratio was significantly increased (controls vs patients; seronegative: 52.85 ± 11.85 μmol/mmol vs 78.91 ± 8.68 μmol/mmol, P < 0.01, seropositive: 47.31 ± 5.93 μmol/mmol vs 109.65 ± 11.50 μmol/mmol, P < 0.01). Neopterin concentrations in cancer patients were significantly elevated compared with controls (P < 0.05). There was a significant correlation between serum neopterin levels and kynurenine/tryptophan in control and colorectal cancer patients groups (rs = 0.494, P = 0.0001 and rs = 0.293, P = 0.004, respectively). Serum nitrite levels of H. pylori seropositive cancer cases were significantly decreased compared with seropositive controls (controls vs patients; 26.04 ± 2.39 μmol/L vs 20.41 ± 1.48 μmol/L, P < 0.05) The decrease in the nitrite levels of H. pylori seropositive cancer patients may be attributed to excessive formation of peroxynitrite and other reactive nitrogen species.
CONCLUSION: A significantly high kynurenine/tryptophan suggested that H. pylori may support the immune tolerance leading to cancer development, even without an apparent upper gastrointestinal tract disease.
Core tip: Persistent inflammation of the stomach induced by Helicobacter pylori (H. pylori) can have consequences on the rest of the body. Despite the vigorous innate and adaptive immune response against the bacterium, H. pylori escape and evade host responses by a variety of mechanisms. Low tryptophan levels and increased concentrations of its degradation product, kynurenine, may be directly involved in diminished T-cell responsiveness to antigenic stimulation in cancer. H. pylori seropositive colorectal cancer patients with significantly higher kynurenine/tryptophan and reduced nitric oxide suggested that H. pylori might support immune tolerance leading to cancer development, even in patients without an apparent upper gastrointestinal tract disease.
- Citation: Engin AB, Karahalil B, Karakaya AE, Engin A. Helicobacter pylori and serum kynurenine-tryptophan ratio in patients with colorectal cancer. World J Gastroenterol 2015; 21(12): 3636-3643
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3636
INTRODUCTION
Persistent inflammation of the stomach induced by Helicobacter pylori (H. pylori) can have consequences on the rest of the body. Recent studies showed that the immunological response against H. pylori is not only a locally but also a systemically evoked phenomena in the host. Particularly in the last few years, many studies have been performed on the role of H. pylori in the pathogenesis of extra-gastric diseases[1]. It is now known that exposure to H. pylori and subsequent seropositivity is associated with an increased number of cardiovascular, respiratory, extra-gastroduodenal digestive, neurological and miscellaneous autoimmune disorders[2].
It has previously been shown that the risk of colon adenomas is increased in _H. pylori_-infected subjects. Two recent studies from Japan, based on a large number of patients, added important evidence for the association between H. pylori infection and colorectal neoplasia. Previously, Fujimori _et al_[3] showed a positive relationship between H. pylori infection and the risk of adenoma and carcinoma, especially in women [odds ratio (OR); 1.68 and 2.09, respectively]. Later, Mizuno _et al_[4] found that H. pylori infection was associated with the presence of colon adenomatous polyps. A very significant increase in the incidence of adenomas was observed in the seropositive group compared with seronegative controls (44.3% vs 18.9%, P < 0.0001).
In the evaluation of the relationship between H. pylori and risk of colorectal cancer, the estimated OR showed a small increase in the risk of colorectal cancer development because of H. pylori infection. Recently, in one of two different meta-analyses, an OR of 1.49 (95%CI: 1.17-1.91) was found for the association between H. pylori infection and colorectal cancer. In another study, serological investigation demonstrated an OR of 1.56 (95%CI: 1.14-2.14) for the association between immunoglobulin G (IgG) antibody and colorectal cancer risk[5,6]. Likewise, in our previous study, we found a 2.2-fold increase in the risk of colorectal carcinoma in patients with H. pylori IgG seropositivity[7]. Very recently, a significant correlation was found by Popović and colleagues between H. pylori seropositivity and colon cancer (P = 0.002) in a series of 142 patients[8].
Although the human host mounts a vigorous innate and adaptive immune response against the bacterium, H. pylori can escape and evade host responses by a variety of mechanisms that lead to persistent colonization and chronic active inflammation[9]. Surprisingly, most people infected with H. pylori are asymptomatic, which suggests that additional factors are necessary for the development of _H. pylori_-associated diseases[10].
Thomas and Stocker proposed a sequential defense mechanism for the immune response, in which indoleamine 2,3-dioxygenase (IDO) activity is the first-line of defense against invading cells[11]. Interferon-gamma (IFN-γ)-induced IDO activity mediates an antimicrobial effect. During the first phase of infection, IDO-mediated tryptophan (Trp) depletion is predominantly antimicrobial whereas in the further stage, it is an inhibitor of T-cell growth[12].
Despite the phenomenon of immune activation against cancer cells, low Trp levels and increased concentrations of its degradation products may be directly involved in diminished T-cell responsiveness to antigenic stimulation in cancer patients[13]. This new mechanism proposed for tumoral immune resistance involves the expression of IDO by tumor cells. It rapidly degrades Trp, and resultant Trp depletion causes a strong inhibitory effect on the development of immune responses[14].
These evidences raise the question of how H. pylori is consistently able to evade these cellular and humoral immune responses. Furthermore, it is not known whether H. pylori enhance systemic immune tolerance against colorectal cancer or not. Regarding the mentioned assumptions, the present study was designed to determine the effects of the serum kynurenine (Kyn)/Trp, serum Trp, Kyn and neopterin levels, which are sustained in persisting H. pylori seropositivity in colorectal cancer patients.
MATERIALS AND METHODS
This prospective randomized study involved 97 consecutive colorectal cancer patients; 61.7 ± 1.3 (mean ± SE) years of age, and body mass index (BMI) of 25.5 ± 0.4 kg/m2, and 108 cancer-free patients with extra-digestive diseases; 55.5 ± 1.3 years of age, and BMI of 27.1 ± 0.5 kg/m2, referred to Gazi University, Faculty of Medicine, Department of General Surgery for surgical evaluation. Diagnosis was made by colonoscopy and histological examination of tumor biopsies in all colorectal cancer patients, and stratified according to the TNM classification of the American Joint Committee on Cancer Staging. From laboratory findings and clinical staging, the primary disease of all cancer patients was found to be suitable for surgical intervention. Subsequent confirmation of the preoperative diagnosis was made by routine histopathological examination of postoperative specimens regarding the presence of lymphatic invasion, lymph node involvement, peritumoral lymphoid cell infiltration, and tumor grade. However, these histological findings were ignored in our series of patients because of the high seroprevalence of H. pylori in the general population and prevalent asymptomatic infection makes the interpretation of the definite role of H. pylori difficult. Therefore, cancer patients and cancer-free cases were divided simply into two subgroups, based on the presence or absence of H. pylori IgG seropositivity. Exposure to H. pylori in each patient was determined by an IgG seropositivity test (ELISA) according to the manufacturer’s instructions (H. pylori IgG ELISA, Demeditec, Germany). Considering the ELISA kit manual, individuals with an H. pylori IgG titer below 8 U/mL were accepted as H. pylori seronegative, while a value above 12 U/mL was interpreted as H. pylori seropositive. The patients whose titers were between 8 and 12 U/mL appeared in the grey zone and were not included in the study. The 13C-urea breath test (UBT) is an accurate, non-invasive test to diagnose gastric viable H. pylori colonization in adults and it is also used to monitor the outcome of eradication therapy in patients[15]. However, findings suggest that infections with H. pylori may have a long-lasting impact on the cell-mediated immune system even after viable bacteria are eradicated. Therefore, we did not perform UBT in our series of patients but determined H. pylori IgG seropositivity in our study. There were 37 H. pylori IgG seronegative and 71 H. pylori IgG seropositive control patients, while 19 of the colorectal cancer patients were H. pylori IgG seronegative and 78 were H. pylori IgG seropositive.
Cancer-free individuals were selected from the patient population admitted to the hospital for hernioraphy, hemorroidectomy or breast biopsies. These patients had undergone diagnostic endoscopic evaluation whenever their complaints were suggestive of digestive diseases. Patients who did not have any pathological finding in either the upper or lower gastrointestinal system were included in the cancer-free control group. No patient had cardio-pulmonary or metabolic risks that could be an obstacle for surgery.
The exclusion criteria were immune system disorders, not able to receive surgical intervention or treatment with neoadjuvant chemotherapy because of late stage carcinoma, or having malnutrition, autoimmune diseases, systemic inflammatory response syndrome, chronic granulomatosis, collagen tissue or neurodegenerative diseases. None of the patients who received traditional triple eradication therapy for H. pylori infection within the last two years were included in the study groups.
All participants’ rights were protected and informed consents were obtained according to the Helsinki Declaration. Gazi University, Local Ethics Committee approved the study protocol.
Peripheral venous blood samples from each individual was collected and used for serum separation. Urine specimens were collected coincidentally. All samples were obtained in the early morning, and kept from direct light at -20 °C until assay.
Biopterin and creatinine levels in urine were analyzed by high performance liquid chromatography (HPLC), as previously described[16]. Serum neopterin concentrations were determined according to the manufacturer’s instructions by a commercially available enzyme immunoassay kit (ELISA, Tani Medical Laboratories, Ankara, Turkey). The optical density was measured at 450 nm. Trp and Kyn concentrations in serum were determined simultaneously by reversed-phase HPLC. In order to estimate Trp degredation, the Kyn to Trp ratio (Kyn/Trp) was calculated by dividing Kyn concentrations (μmol/L) by Trp concentrations (mmol/L)[17]. The frequency of increased serum Kyn/Trp of H. pylori seronegative and seropositive colorectal cancer groups was estimated by comparing each of them with the mean of seronegative cancer-free controls. Serum nitrite concentrations were measured by the Griess method[18]. Serum nitrate measurements were not included in this study, because nitrates are not only released as the final products of nitric oxide (NO) oxidation via nitrites, but could also be produced from peroxynitrites formed during the reaction of NO with the free oxygen radicals. A conclusive profile of NO concentration is reflected by serum nitrite level[19]. Measurement of serum creatinine levels was performed by an auto analyzer.
Statistical analysis
Data were analyzed using SPSS, version 13.0 (SPSS Inc., Chicago, IL, United States). All results are expressed as mean ± SE. After checking the data with the Kolmogorov-Smirnov test, non-parametric data of two independent groups were compared with the Mann-Whitney U test and P < 0.05 was considered statistically significant. Correlations were assessed using the Spearman rank test.
RESULTS
Mean serum neopterin levels of the cancer patients were significantly lower than in the non-tumor group (Table 1). In the evaluation of subgroups, there was a significant increase in serum neopterins in both H. pylori seronegative (Table 2) and seropositive (Table 3) cancer patients (u = 202, P = 0.01 and u = 1265, P = 0.0001, respectively) compared with their matched controls. However, the highly significant increase in serum neopterin levels in H. pylori seropositive cancer patients suggested that H. pylori seropositivity enhanced the immune response of macrophages against colorectal cancer. Considering all colorectal cancer patients, although the reduction in serum nitrite concentrations was not significant (u = 5538.5, P = 0.107) (Table 1), serum nitrite levels of H. pylori seropositive cancer patients were significantly lower compared with H. pylori seropositive cancer-free matched controls (2110, P = 0.01) (Table 3). Serum nitrite levels did not differ between the H. pylori seronegative cancer group and their matched controls (u = 347, P = 0.938) (Table 2). These findings may be consistent with the evidence that the released reactive oxygen and nitrogen species are produced by the phagocytic leukocytes which are recruited to the colorectal tumor site and by H. pylori itself. However, it was not investigated whether H. pylori colonization is still positive or not in 78 seropositive cases with colorectal cancer. Unchanged urinary biopterin, oxidized product of tetrahydrobiopterine (H4-bip) in H. pylori seropositive colorectal cancer patients (1385.5, P = 0.607) (Table 3) showed that despite the increased consumption of NO, supportive NO synthesis in these patients was not evident.
Table 1 Comparison of neopterin, tryptophan, kynurenine, nitrite, biopterin levels (mean ± SE) in controls without malignancy and in gastric cancer patients.
| Control group(n = 108) | Colorectal cancer group(n = 97) | P value | |
|---|---|---|---|
| Serum neopterin (nmol/L) | 7.68 ± 0.56 | 21.18 ± 2.45 | 0.021a |
| Tryptophan (nmol/L) | 20.6 ± 0.61 | 15.38 ± 0.64 | 0.000a |
| Kynurenine (nmol/L) | 0.89 ± 0.09 | 1.24 ± 0.09 | 0.000a |
| Kynurenine/tryptophan (μmol/mmol) | 49.10 ± 5.52 | 99.93 ± 8.70 | 0.000a |
| Nitrite (μmol/L) | 24.60 ± 1.69 | 23.05 ± 1.74 | 0.107 |
| Urinary biopterin/creatinine (μmol/mol) | 121.65 ± 7.79 | 122.70 ± 7.69 | 0.926 |
Table 2 Comparison of neopterin, tryptophan, kynurenine, nitrite, biopterin levels (mean ± SE) in Helicobacter pylori seronegative controls and Helicobacter pylori seronegative colorectal cancer patients.
| H. pylori seronegativecontrol group(n = 37) | H. pylori seronegativecolorectal cancer group (n = 19) | P value | |
|---|---|---|---|
| Serum neopterin (nmol/L) | 8.10 ± 1.11 | 20.89 ± 6.27 | 0.010a |
| Tryptophan (nmol/L) | 20.37 ± 0.89 | 15.71 ± 1.16 | 0.003a |
| Kynurenine (nmol/L) | 0.89 ± 0.12 | 1.22 ± 0.18 | 0.007a |
| Kynurenine/tryptophan (mmol/mmol) | 52.85 ± 11.85 | 78.91 ± 8.68 | 0.000a |
| Nitrite (mmol/L) | 21.86 ± 1.81 | 25.51 ± 4.34 | 0.938 |
| Urinary biopterin/creatinine (mmol/mol) | 116.20 ± 12.87 | 119.1 ± 11.6 | 0.573 |
Table 3 Comparison of neopterin, tryptophan, kynurenine, nitrite, biopterin levels (mean ± SE) in Helicobacter pylori seropositive controls and Helicobacter pylori seropositive colorectal cancer patients.
| H. pylori seropositivecontrol group(n = 71) | H. pylori seropositivecolorectal cancer group (n = 78) | P value | |
|---|---|---|---|
| Serum neopterin (nmol/L) | 7.44 ± 0.61 | 21.8 ± 3.05 | 0.0001a |
| Tryptophan (nmol/L) | 20.71 ± 0.81 | 14.97 ± 0.79 | 0.0001a |
| Kynurenine (nmol/L) | 0.89 ± 0.13 | 1.28 ± 0.11 | 0.0001a |
| Kynurenine/tryptophan (μmol/mmol) | 47.31 ± 5.93 | 109.65 ± 11.50 | 0.0001a |
| Nitrite (μmol/L) | 26.04 ± 2.39 | 20.41 ± 1.48 | 0.017a |
| Urinary biopterin/creatinine (μmol/mol) | 124.65 ± 9.84 | 120.69 ± 9.98 | 0.607 |
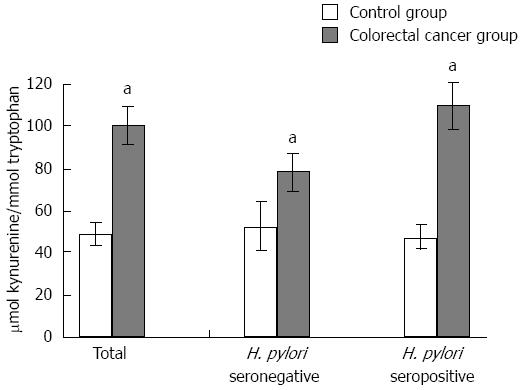
Trp concentrations in cancer patients were significantly lower than that of cancer-free controls (u = 2876, P = 0.0001) (Table 1). As a result of increased IDO activity, levels of Kyn, the toxic product of the Trp degradation pathway, significantly increased in colorectal cancer cases (u = 2931, P = 0.0001). Thus, colorectal cancer patients showed a highly significant rise in Kyn/Trp (u = 2272, P = 0.0001) (Table 1, Figure 1). To elucidate the contribution of H. pylori seropositivity to the frequency of increased Kyn/Trp, the data of H. pylori seronegative or seropositive subgroups were evaluated. H. pylori seronegative or seropositive colorectal cancer patients had significantly higher Kyn/Trp compared with their matched cancer-free controls. Although no statistical difference was found between IDO activities of H. pylori seronegative and seropositive cancer patients, the frequency of increased Kyn/Trp was estimated as 46% and 57% for seronegative and seropositive subgroups, respectively. This means that H. pylori seropositivity might add 11% to the frequency of increased Kyn/Trp in the colorectal cancer group and enhance immune tolerance against cancer cells. There was a significant correlation between the serum neopterin levels and Kyn/Trp in control and colorectal cancer patients groups (rs = 0.494, P = 0.0001 and rs = 0.293, P = 0.004, respectively), while a positive correlation existed in both H. pylori seronegative and seropositive control individuals (rs = 0.652, P = 0.0001 and rs =0.381, P = 0.002, respectively). Similarly, the correlation of the same parameters in H. pylori seropositive cancer patients was also significant (rs = 0.374, P = 0.002, respectively).
Figure 1 Comparison between kynurenine to tryptophan ratios in controls and colorectal cancer patients, in Helicobacter pylori seronegative or seropositive groups. a_P_ < 0.05, controls vs colorectal cancer patients, Helicobacter pylori (H. pylori) seronegative controls vs H. pylori seronegative colorectal cancer patients, H. pylori seropositive controls vs H. pylori seropositive colorectal cancer patients, statistically significant.
DISCUSSION
Neopterin is mainly synthesized by activated monocytes/macrophages in response to induction by IFN-γ. Measurement of neopterin concentrations in body fluids provides information about T helper cell 1 (Th1)-derived cellular immune activation[20]. An increase in neopterin concentrations during cancer growth indicates a chronic cellular immune response; however, it is not specific for malignant cell proliferation[21]. The mean serum neopterin level in the non-tumor group was below the standard cut-off value, 10 nmol/L[22], while there was a significant increase in serum neopterins in cancer patients. Thus in our study, a highly significant increase in serum neopterin concentration of H. pylori seropositive colorectal cancer patients suggested that H. pylori seropositivity induced activation of cell-mediated immunity, in addition to cancer-induced chronic cellular immune response.
On the other hand, chronic stimulation of Th1-mediated immunity may also cause enhanced IDO activity in malignant diseases[23]. IDO is an enzyme ubiquitously distributed in mammalian cells, and converts Trp to N-formylkynurenine. This substance is further catabolized to Kyn[13]. Trp depletion as well as the accumulation of its metabolites results in a strong inhibitory effect on the development of immune responses[24]. IDO-induced Trp depletion from the tumor microenvironment could be the result of enhanced activation of the enzyme and augmented Trp consumption by both tumor cells and antigen-presenting cells of the host[25]. Recent data obtained from tumor models demonstrated that IDO inhibition could significantly enhance the antitumor activity of various chemotherapeutic and immunotherapeutic agents. These results were consistent with data showing that increased IDO expression was an independent prognostic variable for reduced overall survival in cancer patients[24]. In colorectal cancer patients, significantly accelerated degradation of Trp, with lowered serum concentrations of Trp and increased Kyn, as well as an increased Kyn/Trp has previously been reported[26]. Indeed, in our study, the highly significant correlation between neopterin concentrations with increased Kyn/Trp clearly indicated that the formation of Kyn is related to IDO activity by IFN-γ stimulation. It is postulated that IDO limits immune cell proliferation by depleting locally available Trp and/or producing its cytotoxic metabolites[27]. However, enhanced IDO activity and further serum Trp degradation due to _H. pylori_-seropositivity in colorectal cancer patients was demonstrated for the first time in our study. In addition to reduced serum Trp concentrations and raised serum Kyn levels in the H. pylori seropositive colorectal cancer group, a significant increase in serum Kyn/Trp (u = 1026, P = 0.0001) and highly significant correlation between the serum neopterin and Kyn/Trp suggested that IDO activities may be induced by H. pylori seropositivity. A frequency of increased Kyn/Trp of 57% indicated that enhanced IDO activity may be an important additional factor in the development of _H. pylori_-associated colorectal cancer. As a result, H. pylori seropositivity may have an effect on the enhancement of immune tolerance against cancer cells. The number of advanced cancer cases in the H. pylori seropositive group may support this statement.
In our study, while serum nitrite levels were significantly lower in H. pylori seropositive cancer patients, no difference was found between H. pylori seronegative cancer patients and their H. pylori seronegative matched controls. Thus, the nitrite-NO pathway may be viewed as a backup system, to ensure sufficient NO generation along the entire physiological oxygen gradient[28]. Actually, NO regulates IDO activity biphasically, in a dose-dependent manner such that, while high NO production inactivates IDO enzyme and favors the immune response, low concentrations of NO increase IDO activity, resulting in immune tolerance[29]. A decrease in serum NO in H. pylori seropositive cancer patients may be attributed to the relatively effective scavenging of the radicals by NO. NO and superoxide can antagonize each other’s biological actions regarding the NO/superoxide balance in cytoplasmic fractions[30]. The relative NO production rates have an impact on the NO-mediated toxic vs protective effects[31]. Peroxynitrite formed in vivo from superoxide and NO can mediate oxidative nitration or nitrosation reactions, leading to tissue injury. Consequently, a reduction in NO may be due to the formation of peroxynitrite and other reactive nitrogen species (RNS). It was demonstrated that excessive reactive oxygen species (ROS)/RNS production in the _H. pylori_-infected stomach by activated neutrophils and H. pylori itself can damage DNA in gastric epithelial cells, implying its involvement in gastric carcinogenesis[32]. A negative correlation between the amount of superoxide radicals and nitrites suggests that NO has antioxidative effects at the site of injury[33]. However, the vast majority of the studies have employed serological surveillance rather than isolation of H. pylori from the target disease site, as serological testing for indirectly detecting H. pylori is quick, relatively cheap and specific. Moreover, it is well known that there is an ongoing risk of developing cancer even after the eradication of H. pylori infection[34]. These data claimed that H. pylori may induce oxidative stress by different mechanisms. Most probably NO combines with reactive oxygen metabolites to form RNS, such as nitrogen dioxide and peroxynitrite, whereby NO bioavailability was decreased.
As we could not find any difference between the serum nitrite levels of H. pylori seronegative colorectal cancer cases and their matched controls; it seems that the presence of H. pylori may have caused a significant increase in NO consumption by formation of redox active radicals in cancer cases. H4-bip is an indispensable cofactor for NO generation by inducible nitric oxide synthase (iNOS)[35]. The quantity of urinary biopterin excretion is a determinant of the amount of intracellular H4-bip which is critical for iNOS-dependent generation of NO[36]. However, we did not observe an increase in urinary biopterin excretion of H. pylori seropositive cancer group.
In our study, a significant increase in serum neopterin concentrations suggested that IFN-γ-induced guanosine triphosphate (GTP) cyclohydrolase I activities largely supported neopterin synthesis in either H. pylori seronegative or H. pylori seropositive colorectal carcinoma patients. GTP cyclohydrolase I activities correlate with the sum of neopterin plus biopterin rather than with neopterin or biopterin alone[37]. As a conclusion, H. pylori seropositive colorectal cancer patients with significantly higher Kyn/Trp and reduced NO suggest that H. pylori may support immune tolerance leading to cancer development, even in patients without an apparent upper gastrointestinal tract disease. There is little in the literature related to this subject, thus further studies in larger populations are warranted in order to support these findings.
COMMENTS
Background
Persistent inflammation of the stomach induced by Helicobacter pylori (H. pylori) can have consequences on the rest of the body. Despite the vigorous innate and adaptive immune response against the bacteria, it is still not known how H. pylori escape and evade host responses. Low tryptophan levels and increased concentrations of its degradation product, kynurenine, may be directly involved in diminished T-cell responsiveness to antigenic stimulation in cancer.
Research frontiers
An increased kynurenine to tryptophan ratio that is correlated with neopterin concentrations may be directly involved in T-cell unresponsiveness to cancer cells. The present study was designed to determine the effects of serum kynurenine/tryptophan, serum tryptophan, kynurenine and neopterin levels with persistent H. pylori seropositivity in colorectal cancer patients.
Innovations and breakthroughs
H. pylori seropositive colorectal cancer patients with significantly higher kynurenine/tryptophan and reduced nitric oxide suggested that H. pylori may support immune tolerance leading to cancer development, even in patients without an apparent upper gastrointestinal tract disease.
Applications
H. pylori seropositivity may have consequences not only in the stomach but also in the colon and rectum, and this may lead to the increased incidence of colorectal cancer. Significantly higher kynurenine/tryptophan and reduced nitric oxide may be an indicator of immune tolerance related to H. pylori seropositivity that supports colorectal cancer development.
Terminology
The presence of H. pylori immunglobulin G is associated with previous infection. Indolamine-2,3-dioxygenase is an enzyme, ubiquitously distributed in mammalian cells, which converts tryptophan into kynurenine. The correlation of kynurenine/tryptophan with neopterin indicates indolamine-2,3-dioxygenase activity. Thus, tryptophan depletion as well as the accumulation of its metabolites results in a strong inhibitory effect on the development of immune responses against cancer cells.
Peer-review
This is a good descriptive study in which the authors analyzed consequences of H. pylori on the body other than stomach. The results are interesting and suggest that increased kynurenine/ tryptophan- may be directly involved in diminished T-cell responsiveness to antigenic stimulation in cancer and H. pylori might support immune tolerance leading to cancer development, even in patients without an apparent upper gastrointestinal tract disease.
Footnotes
P- Reviewer: Ikematsu H S- Editor: Qi Y L- Editor: Cant MR E- Editor: Wang CH
References
| 1. | Richy F, Mégraud F. [Helicobacter pylori infection as a cause of extra-digestive diseases: myth or reality?]. Gastroenterol Clin Biol. 2003;27:459-466. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 3. | Fujimori S, Kishida T, Kobayashi T, Sekita Y, Seo T, Nagata K, Tatsuguchi A, Gudis K, Yokoi K, Tanaka N. Helicobacter pylori infection increases the risk of colorectal adenoma and adenocarcinoma, especially in women. J Gastroenterol. 2005;40:887-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
|---|
| 8. | Popović N, Nikolić V, Karamarković A, Blagojević Z, Sijacki A, Surbatović M, Ivancević N, Gregorić P, Ilić M. Prospective evaluation of the prevalence of Helicobacter pylori in abdominal surgery patients. Hepatogastroenterology. 2010;57:167-171. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 10. | Lacy BE, Rosemore J. Helicobacter pylori: ulcers and more: the beginning of an era. J Nutr. 2001;131:2789S-2793S. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 14. | Van den Eynde B. [A new mechanism of tumor resistance to the immune system, based on tryptophan breakdown by indoleamine 2,3-dioxygenase]. Bull Mem Acad R Med Belg. 2003;158:356-63; discussion 364-5. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 15. | Peeters M. Urea breath test: a diagnostic tool in the management of Helicobacter pylori-related gastrointestinal diseases. Acta Gastroenterol Belg. 1998;61:332-335. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 17. | Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Göbel G. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
|---|
| 20. | Murr C, Bergant A, Widschwendter M, Heim K, Schröcksnadel H, Fuchs D. Neopterin is an independent prognostic variable in females with breast cancer. Clin Chem. 1999;45:1998-2004. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 34. | Tsang KW, Lam SK. Extragastroduodenal conditions associated with Helicobacter pylori infection. Hong Kong Med J. 1999;5:169-174. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 37. | Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Wachter H. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J. 1989;262:861-866. [PubMed] [DOI] [Cited in This Article: ] |
|---|