Induction of the unfolded protein response and cell death pathway in Alzheimer's disease, but not in aged Tg2576 mice (original) (raw)
- Article
- Open access
- Published: 05 April 2010
- Sun Mi Won1,2,3 na1,
- Jaehong Suh1,2,3,
- Sun Joo Son4,
- Gyeong Joon Moon1,2,3,4,
- Ui-Jin Park1,2,3,4 &
- …
- Byoung Joo Gwag1,2,3,4
Experimental & Molecular Medicine volume 42, pages 386–394 (2010)Cite this article
Abstract
The endoplasmic reticulum (ER) stress results from disrupted protein folding triggered by protein mutation or oxidation, reduced proteasome activity, and altered Ca2+ homeostasis. ER stress is accompanied by activation of the unfolded protein response (UPR) and cell death pathway. We examined if the UPR and cell death pathway would be activated in Alzheimer's disease (AD). RT-PCR experiments revealed increased splicing of X-box binding protein-1 (XBP-1), an UPR transcription factor, in AD compared with age-matched control. Among target genes of XBP-1, expression of protein disulfide isomerase (PDI), but not glucose-regulated protein 78 (GRP78), was increased in AD, suggesting disturbed activation of the UPR in AD. C/EBP homologous protein (CHOP), caspase-3, caspase-4, and caspase-12, downstream mediators of cell death pathway, were activated in AD. Neither the UPR nor cell death pathway was induced in aged Tg2576 mice, a transgenic mouse model of Alzheimer's disease that reveals both plaque pathology and some cognitive deficits. The present study suggests that disturbed induction of the UPR and activation of the pro-apoptotic proteins contribute to neuropathological process in AD irrespective of amyloid β and senile plaque.
Similar content being viewed by others
Introduction
ER stress, accumulation of unfolded protein in the endoplasmic reticulum, can be provoked primarily by imbalance in homeostasis, proteasome activity during degeneration and differentiation (Kozutsumi et al., 1988; Wong et al., 1993; Friedlander et al., 2000; Cho et al., 2009). ER stress induces an adaptive signaling pathway called the UPR that involves activation of transcription factors, XBP-1 and activating transcription factor 6 (ATF6) (Yoshida et al., 2000; Lee et al., 2003). Activation of these transcription factors induces the UPR genes (e.g., GRP78, PDI). The coordinated activation of the UPR alleviates accumulation of unfolded proteins in the lumen of ER (Cox and Walter, 1996; Yoshida et al., 2001). However, prolonged activation of ER stress can turn on cell death pathway through activation of CHOP, caspase-4, and caspase-12 (Wang et al., 1996; Nakagawa et al., 2000; Hitomi et al., 2004).
ER stress has been implicated in abnormal protein processing and neuronal death in AD. Administration of amyloid β induced activation of ER stress in cultured neurons and in rabbit in vivo (Ghribi et al., 2001; Ferreiro et al., 2006). Caspase-4 and caspase-12 were shown to mediate ER stress-mediated apoptosis by amyloid β (Nakagawa et al., 2000; Kim et al., 2006). In a recent study, increased levels of GRP78 and p-PERK (RNA-dependent protein kinase-like endoplasmic reticulum kinase) were observed in the cortex and hippocampus from patients with AD, suggesting that ER stress induces activation of the UPR in AD (Hoozemans et al., 2005; Hoozemans, et al., 2009). Contrarily, Western blot analysis revealed that expression of GRP78 was decreased or maintained in the cortical tissues of AD patients (Katayama et al., 1999; Sato et al., 2000). In the present study, activation of the UPR in AD was further examined by analyzing XBP-1 mRNA splicing and PDI expression as well as GRP78. In addition, activation of ER stress-induced cell death pathway in AD patients was investigated by analyzing activation of CHOP, caspase-3, caspase-4, and caspase-12. A putative role of amyloid β and plaques for ER stress was examined in aged Tg2576 transgenic mice that developed widespread plaque pathology and cognitive deficit.
Results
Induction of the unfolded protein response by ER stress in AD
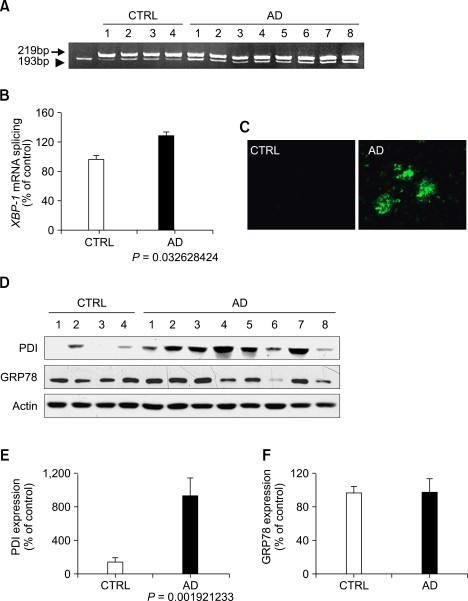
We analyzed processing of mRNA encoding the transcription factor XBP-1 to determine if the UPR would be induced in the temporal cortex of AD patients. RT-PCR experiments showed overall increase in the spliced form (193 bp) of XBP-1 mRNA in the temporal cortex of AD brain (Figure 1A). The relative splicing ratio of XBP-1 mRNA was significantly increased compared to the age-matched control (Figure 1B). Extensive amyloid plaques were observed in the cortex of AD brain (Figure 1C).
Figure 1
Induction of the unfolded protein response by ER stress in AD. (A, B) RT-PCR analysis of XBP-1 mRNA splicing in the temporal cortex of control (CTRL) and AD brain (A). XBP-1 mRNA splicing was analyzed by scaling intensity of splicing band (193bp) to total band (193 bp + 219 bp) (B). (C) Representative images of thioflavin S staining in the temporal cortex of control (CTRL) and AD brain. (D-F) Western blot analysis of PDI, GRP78, and actin (D). Levels of PDI (E) and GRP78 (F) were measured and normalized to the level of relevant actin (n = 4 for control and n = 8 for AD).
We next examined the expression pattern of PDI and GRP78, the target UPR genes of XBP-1. Levels of PDI, a family of enzymes that catalyze disulfide bond formation, reduction, or isomerization of newly synthesized proteins in the lumen of the ER, were markedly increased up to 9.49 fold in AD brain (P < 0.01) compared to the control (Figure 1). However, the expression of GRP78 that promotes proper folding of proteins in the ER was not increased in AD brain (Figure 1D).
PDI is induced in tangle-bearing neurons in AD
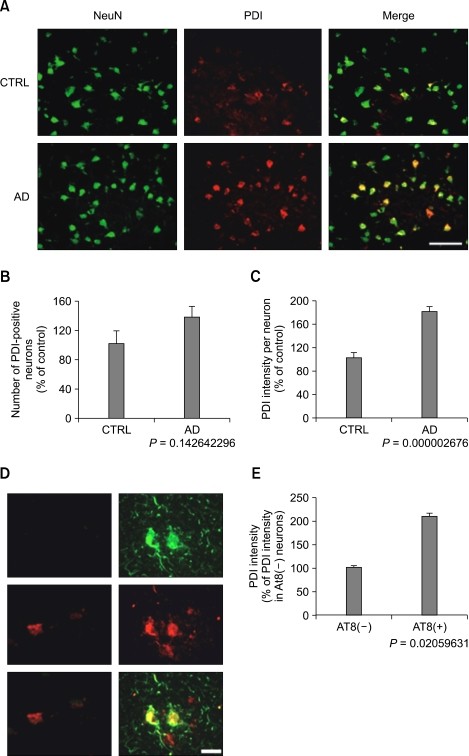
Immunohistochemistry revealed that PDI was expressed primarily in neurons in the temporal cortex from AD and the age-matched control brain. The number of neurons immunoreactive to PDI tended to be slightly increased in the temporal cortex of AD patients (P = 0.1426, Figure 2B). The expression of PDI was significantly increased in the cortical neurons from AD (Figure 2).
Figure 2
PDI is induced in tangle-bearing neurons in AD. (A-C) Fluorescence photomicrographs of cortical sections from control (CTRL) and AD immunolabeled with anti-NeuN antibody (left panel, green) and anti-PDI antibody (middle panel, red). Note that PDI expression is increased in neurons (right panel, yellow) (A). Analysis of PDI-positive neurons (B) and PDI intensity per neuron (C) collected from 400-450 neurons in the temporal cortex from 4 control and 8 AD patients. Bar, 100 µm. (D, E) Fluorescence photomicrographs of cortical neurons immunolabeled with anti-AT8 antibody (green) and anti-PDI antibody (red) in sections of AD and control (D). Note that AT8-positive neurons (AT8+) show higher levels of PDI than AT8-negative neurons (AT8-) (E). Analysis of PDI levels in AT8 (-) and (+) neurons collected from 250 to 320 neurons in the temporal cortex of AD. Bar, 20 µm.
Additional experiments were performed to examine if induction of the UPR would be correlated with the neurofibrillary tangle. Several neurons immunoreactive to AT8 antibody recognizing the phosphorylated forms of tau at Ser202 residue were observed in the temporal cortex of AD patients, but not from the control group (Figure 2D). Interestingly, all AT8-positive neurons showed higher levels of PDI than AT8-negative neurons (Figure 2E). This implies that ER stress is induced primarily in the tangle-bearing cortical neurons from AD.
Activation of ER stress-induced cell death pathway in AD
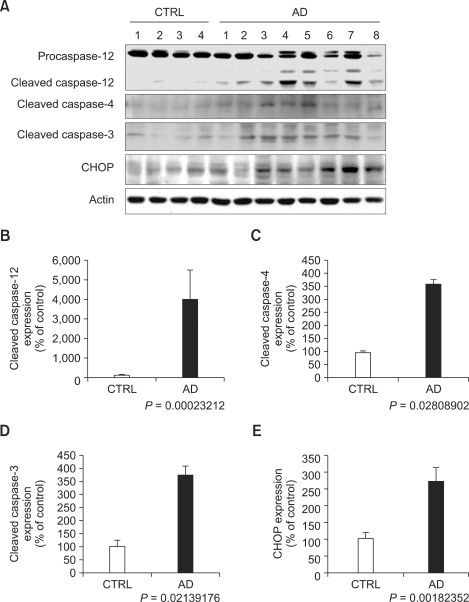
ER stress-mediated cell death pathway was examined by Western blot analysis of caspase-3, caspase-4, caspase-12, and CHOP. Cleaved forms of caspase-3, caspase-4, and caspase-12 were barely detectable in the cortex of age-matched control group. The cleaved forms of caspase-3, caspase-4, and caspase-12 were significantly increased in AD brain (Figure 3). The expression of CHOP was also increased in the temporal cortex of AD brain compared to the control group.
Figure 3
Activation of ER stress-induced cell death pathway in AD. Western blot analysis of caspase-12, caspase-4, caspase-3, CHOP, and actin in the temporal cortex of control (CTRL) and AD brain (A). Levels of cleaved caspase-12 (B), cleaved caspase-4 (C), cleaved caspase-3 (D), and CHOP (E) were measured and normalized to the level of relevant actin (n = 4 for control and n = 8 for AD).
Neither the UPR nor cell death pathway is induced in aged Tg2576 mice
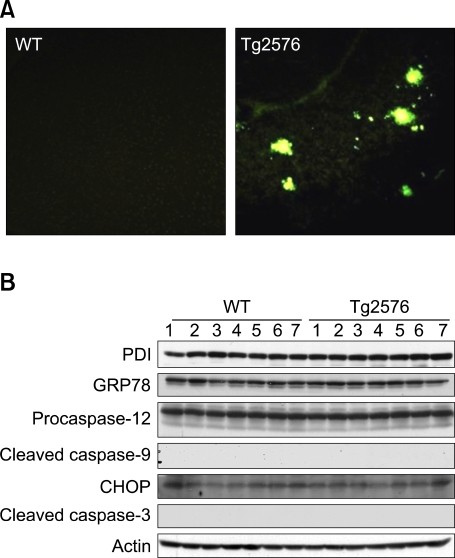
We finally examined the possibility that the induction pattern of the UPR and cell death pathway by ER stress in AD brain would be related to amyloid burden. Levels of soluble or insoluble amyloid β were increased in the cortical areas of Tg2576 mice at the age of 17 months as previously reported (Holcomb et al., 1998; Yang et al., 2000; Kawarabayashi et al., 2001; Lim et al., 2001). Thioflaivin-S staining revealed widespread senile plaques in the cortical area of aged Tg2576 mice (Figure 4A). Levels of PDI and GRP78 were similar in the cortex of Tg2576 mice and wild type. Cleaved caspase-3, 9, 12, and CHOP expression were not altered in Tg2576 mice compared to the control (Figure 4B).
Figure 4
Neither the UPR nor cell death pathway is induced in aged Tg2576 mice. (A) Fluorescence photomicrographs of cortical sections from wild type (WT) and Tg2576 mice at the age of 17 months after thioflavin-S staining. (B) Western blot analysis of PDI, GRP78, cleaved caspase-3, 9, 12, CHOP, and actin in the cortex of wild type and Tg2576 mice.
Discussion
ER stress-induced UPR and cell death pathway appear to play a role in the pathogenesis of AD. Disturbed activation of the UPR is evident by splicing of XBP-1 mRNA and induction of PDI presumably in tangle-bearing neurons in the absence of up-regulation of GRP78. Cleavages of caspase-3, 4, 12 and upregulation of CHOP suggest activation of ER stress-induced proapoptotic pathway in AD. However, neither the UPR nor cell death pathway by ER stress is induced in aged Tg2576 mice loaded with amyloid β and plaques, raising a possibility that the ER stress-mediated events in AD can be induced irrespective of amyloid burden.
The UPR seems to be incompletely activated in the brain of AD patients. In particular, expression of GRP78, a key regulator of protein folding and assembly, is not induced but rather reduced although ER is under stressed condition in AD (Katayama et al., 1999; Sato,et al., 2000). The expression of GRP78 is also reduced by familial AD-linked presenilin-1 mutations that interfere with IRE1 function (Katayama et al., 1999). Hosoi et al (2010) found that systemic application of homocysteine increases XBP-1 splicing in various brain areas including hippocampus, hypothalamus and cortex in mice without inducing expression of GRP78 (Hosoi et al., 2010). Target genes of XBP-1 have been identified including p58IPK, ERdj4, HEDJ, and protein disulfide isomerase PDI-P5 (Lee et al., 2003). In this study, GRP78 did not depend upon XBP-1s expression. The disturbed activation of the UPR likely renders neurons sensitive to apoptosis induced by ER stress or amyloid β (Guo et al., 1997; Yoneda et al., 2001). PDI is localized primarily in neurons and its expression is increased in AT8-positive neurons in AD, suggesting that ER stress is prominent in tangle-bearing neurons. Neurons lacking compensatory activation of the UPR are expected to undergo misfolding and abnormal aggregation and processing of various proteins such as tau, amyloid β-protein precursor, and neurofilament. It is conceivable to reason that the disturbed protein homeostasis may contribute to the formation of neurofibrillary tangle in AD.
Prolonged activation of the UPR can trigger apoptosis through activation of caspase-4, caspase-12, and CHOP, key mediators of ER stress-induced cell death pathway. We provide first evidence that cleavages of caspase-4 and caspase-12, and expression of CHOP are increased in the brain of AD patients. CHOP, a transcription factor induced by ATF6 or PERK, acts as a proapoptotic protein that suppresses transcription of Bcl-2 (Wang et al., 1996; McCullough et al., 2001). Moreover, ER stress reduces interactions of procaspase-12 and tumor necrosis factor receptor-associated factor 2, an adaptor protein linked to the cytoplasmic portion of IRE1 at ER membrane (Yoneda et al., 2001). Dissociated procaspase-12 is cleaved by the calcium-activated neutral protease calpain that is activated by ER stress (Siman et al., 2001) and in AD patients (Saito et al., 1993). Activated caspase-12 then induces cytochrome c-independent activation of caspase-9, which will lead to activation of caspase-3 in vulnerable neurons in the brain of AD patients (Morishima et al., 2002; Rohn et al., 2002; Kang et al., 2005). Taken together, ER stress-induced cell death pathway is activated and expected to participate in caspase-3-mediated neuronal loss in AD.
Mechanisms underlying induction of ER stress in AD remain largely unknown. Amyloid β has been proposed as a potential trigger of ER stress in AD based upon the findings that the UPR and cell death pathway are induced in cultured neurons exposed to amyloid β or over-expressing mutant PS1 (Yu et al., 1999; Nakagawa et al., 2000; Ferreiro et al., 2006; Seyb et al., 2006). However, none of ER stress markers have been detected in the cortex of aged Tg2576 mice undergoing extensive amyloid β burden and cognitive deterioration. The lack of the UPR and cell death pathway in aged Tg2576 indicates that amyloid β and plaques alone are not sufficient for induction of ER stress in aged AD patients, suggesting that neuronal injury and neurofribrillary tangles as well as amyloid plaques contribute to ER stress induced in AD. Moreover, other pathological events such as inflammation may influence induction of ER stress in AD which is accompanied by pneumonia or urosepsis.
Glutamate or free radicals, triggers of neuronal injury in AD (Smith et al., 2000; Li et al., 2004; Moreira et al., 2005; Cosman et al., 2007), may play a central role in inducing ER stress in AD. Activation of glutamate receptors sensitive to NMDA results in excess accumulation of Ca2+ in neurons that can induce free radical production and ER stress (Dugan et al., 1995; Reynolds and Hastings, 1995; Liu et al., 1998). It is of note that memantine, a partial NMDA receptor antagonist, is approved for treating moderate-to-severe AD patients (Li et al., 2004; Cosman et al., 2007).
ER stress induces activation of the UPR to reduce the accumulation of unfolded proteins. The UPR likely fails to remove unfolded proteins due to disturbed activation of the UPR and turns on ER stress-specific cell death pathway in the brain of AD patients. The pathological activation of the UPR is expected to contribute to accumulation and aggregation of misfolded proteins and neuronal death progressively evolving in AD and other neurodegenerative diseases including Parkinson's disease and amyotrophic lateral sclerosis (Bence et al., 2001; Forloni et al., 2002; Ryu et al., 2002; Soto, 2003; Paschen and Mengesdorf, 2005; Kikuchi et al., 2006; Kanekura et al., 2009). Further study will be needed to delineate a potential role of excitotoxicity, oxidative stress, and dysfunction of the ubiquitin proteasome system in inducing ER stress in neurodegenerative diseases.
Methods
Human brain tissue
Frozen tissues from 8 cases of AD and 4 cases of age-matched control were obtained from Boston University Alzheimer's Disease Center (Dr. Ann C. Mckee). AD was diagnosed according to clinical and neuropathological criteria (Supplemental data Table S1).
Transgenic mice
Tg2576 mice and their corresponding wild type controls were purchased from Taconic Farms, Inc. (Germantown, NY). Tg2576 mice overexpress human APP695, containing the double-mutation Lys670-Asn and Met 671-Leu (K670N, M671L) (Hsiao et al., 1996). Animals were handled in accordance with a protocol approved by our institutional animal care committee. Tg2576 mice (n = 7) and their non-transgenic littermates (n = 7) at the age of 17 months were used in this study.
Isolation of total RNA and RT-PCR
Total RNA from tissues of AD human brain and transgenic mice was isolated according to manufacturer's instruction (iNtRON Bio technology co., Ltd). RNA integrity was confirmed by detection of 28S and 18S rRNA band. RNA was confirmed to be free of genomic DNA contamination by PCR in the absence of reverse transcriptase. The RNA samples were reverse transcribed in 20 µl of a reaction mixture containing 0.5 mM of mixed dNTP, 100 ng of oligo dT (5'-TTTTTTTTTTTTTTT-3'), 0.5 unit RNAsin, and 40 unit of MMLV reverse transcriptase at 42℃ for 30 min. The samples were then incubated at 95℃ for 5 min and transferred to 4℃. 4 µl of RT product were subjected to PCR amplification with 20 pmole XBP-1 primer, 20 mM dNTP, and 1 unit Taq polymerase in 20 µl of 1 × reaction buffer (Promega). PCR primers were used as follows (5'-3'): for human XBP-1, GAAGCCAAGGGGAATGAAGT (forward) and GGGAAGGGCATTTGAAGAAC (reverse): for mouse XBP-1, CCATGGGAAGATGTTCTGGG (forward) and TCTGGTTGGCGGATCTACTC (reverse). PCR mixtures were heated to 94℃ for 5 min and cycled 31 times for XBP-1 gene; 31 cycles consisted of 94℃ for 15 s, 60℃ for 30 s, and 72℃ for 45 s. After additional incubation at 72℃ for 10 min, PCR products were subjected to electrophoresis in 8% polyacrylamide gel and visualized with ethidium bromide staining. Relative intensity of PCR band was analyzed using Gel Doc 1000 video-imaging system (Bio-Rad, Hercules, CA).
Western blot analysis
Brain tissues were lysed in a lysis buffer containing 10 mM Tris-HCl pH 7.5, 50 mM NaCl, 1% Triton X-100, 30 mM sodium pyrophosphate, 50 mM NaF, 5 µM ZnCl2, 2 mM PMSF, and 100 µg/ml leupeptin, 10 µg/ml pepstatin A, and 1 mM DTT. Lysates were centrifuged at 13,000 g for 10 min, the supernatants collected, subjected to electrophoresis on 12-15% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. The blot was incubated in 5% nonfat dry milk, reacted with primary antibodies at 4℃ for overnight. The primary antibodies used and the dilutions for each were rabbit polyclonal anti-caspase-12 antibody (New England Biolabs, Beverly, MA) recognizes both procaspase-12 of 54 kDa and active caspase-12 of 42 kDa at 1:2500, mouse polyclonal anti-PDI antibody (Pharmingen, San Diego, CA) at 1:2000, mouse polyclonal anti-GRP78 antibody (Pharmingen, San Diego, CA) at 1:5000, rabbit polyclonal anti-cleaved caspase-3 antibody (New England Biolabs, Beverly, MA), goat polyclonal anti-cleaved caspase-4 antibody (Santa Cruz Biotechnology), and rabbit polyclonal anti-CHOP antibody (Santa Cruz Biotechnology) at 1:2000. The blot was incubated with a horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody (Cell Signaling, Beverly, MA). Target proteins were detected with enhanced chemiluminescence reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK) on X-ray film or with an LAS 1000 image analyzer (Fuji Photo Film Co.). The image was analyzed using the Image Gauge 3.12 (Fuji Photo Film Co.) according to parameters defined by the softerware algolithms for band and lane depiction. Net band intensities are defined as the sum of pixels within the area of the band limited by performed rectangular area after subtraction of the background pixels. To assess for protein degradation and to normalize protein load among samples, the membranes were incubated with antibody against β-actin after stripping the blots. No tissue extracts exhibited degradation of β-actin.
Immunohistochemistry
Sections at a thickness of 25 µm on a cryostat were incubated in 10% normal horse serum for 1 h, reacted with antibody recognizing NeuN (CHEMICON International, Inc., 1:200), rabbit polyclonal antibody recognizing PDI (Abcam Ltd. 332 Cambridge, UK, 1:50), mouse monoclonal antibody recognizing AT8 (Pierce Biotechnology, Inc., 1:200) overnight, and then reacted with FITC- or Texas-red-labeled secondary antibodies (Vector, Burlingame, CA, 1:200). In case of human, autofluorescence eliminator reagent (CHEMICON International, Inc.) was used to remove the autofluorescent pigment lipofusin. Finally, sections were washed with distilled water, air dried, and mounted with Vectashield (Vector, Burlingame, CA). All images were collected and analyzed with a fluorescence microscope (Zeiss, Germany) equipped with the REAL-14k precision digital camera (Apogee Instruments, Tucson, AZ) and Image Pro Plus Plug-in.
Thioflavin S staining
Brain sections (25 µm) were mounted on gelatin-coated slides. Slides were rinsed with distilled water and incubated with thioflavin-S (1% w/v, Sigma) for 10 min. Slides were incubated in 70% ethanol for 5 min and briefly washed in distilled water.
Statistical analysis
Data were analyzed with the SPSS software package (version 12.0 for Windows SPSS, Chicago, Ill.). Statistical analysis of the data was performed using an independent sample t test.
Abbreviations
AD:
Alzheimer's disease
ATF6:
activating transcription factor 6
CHOP:
C/EBP homologous protein
ER:
endoplasmic reticulum
GRP78:
glucose-regulated protein 78
PDI:
protein disulfide isomerase
p-PERK:
RNA-dependent protein kinase-like endoplasmic reticulum kinase
UPR:
unfolded protein response
XBP-1:
X-box binding protein-1
References
- Bence NF, Sampat RM, Kopito RR . Impairment of the ubiquitin-proteasome system by protein aggregation . Science 2001 ; 292 : 1552 - 1555
Article CAS PubMed Google Scholar - Cho YM, Jang YS, Jang YM, Chung SM, Kim HS, Lee JH, Jeong SW, Kim IK, Kim JJ, Kim KS, Kwon OJ . Induction of unfolded protein response during neuronal induction of rat bone marrow stromal cells and mouse embryonic stem cells . Exp Mol Med 2009 ; 41 : 440 - 452
Article CAS PubMed PubMed Central Google Scholar - Cosman KM, Boyle LL, Porsteinsson AP . Memantine in the treatment of mild-to-moderate Alzheimer's disease . Expert Opin Pharmacother 2007 ; 8 : 203 - 214
Article CAS PubMed Google Scholar - Cox JS, Walter P . A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response . Cell 1996 ; 87 : 391 - 404
Article CAS PubMed Google Scholar - Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW . Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate . J Neurosci 1995 ; 15 : 6377 - 6388
Article CAS PubMed PubMed Central Google Scholar - Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CM . An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity . Neurobiol Dis 2006 ; 23 : 669 - 678
Article CAS PubMed Google Scholar - Forloni G, Terreni L, Bertani I, Fogliarino S, Invernizzi R, Assini A, Ribizzi G, Negro A, Calabrese E, Volonte MA, Mariani C, Franceschi M, Tabaton M, Bertoli A . Protein misfolding in Alzheimer's and Parkinson's disease: geneticsand molecular mechanisms . Neurobiol Aging 2002 ; 23 : 957 - 976
Article CAS PubMed Google Scholar - Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T . A regulatory link between ER-associated protein degradation and the unfolded-protein response . Nat Cell Biol 2000 ; 2 : 379 - 384
Article CAS PubMed Google Scholar - Ghribi O, Herman MM, DeWitt DA, Forbes MS, Savory J . Abeta(1-42) and aluminum induce stress in the endoplasmic reticulum in rabbit hippocampus, involving nuclear translocation of gadd 153 and NF-kappaB . Brain Res Mol Brain Res 2001 ; 96 : 30 - 38
Article CAS PubMed Google Scholar - Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP . Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals . J Neurosci 1997 ; 17 : 4212 - 4222
Article CAS PubMed PubMed Central Google Scholar - Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M . Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death . J Cell Biol 2004 ; 165 : 347 - 356
Article CAS PubMed PubMed Central Google Scholar - Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K . Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes . Nat Med 1998 ; 4 : 97 - 100
Article CAS PubMed Google Scholar - Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W . The unfolded protein response is activated in Alzheimer's disease . Acta Neuropathol(Berl) 2005 ; 110 : 165 - 172
Article CAS Google Scholar - Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W . The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus . Am J Pathol 2009 ; 174 : 1241 - 1251
Article CAS PubMed PubMed Central Google Scholar - Hosoi T, Ogawa K, Ozawa K . Homocysteine induces X-box-binding protein 1 splicing in the mice brain . Neurochem Int 2010 ; 56 : 216 - 220
Article CAS PubMed Google Scholar - Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G . Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice . Science 1996 ; 274 : 99 - 102
Article CAS PubMed Google Scholar - Kanekura K, Suzuki H, Aiso S, Matsuoka M . ER stress and unfolded protein response in amyotrophic lateral sclerosis . Mol Neurobiol 2009 ; 39 : 81 - 89
Article CAS PubMed Google Scholar - Kang HJ, Yoon WJ, Moon GJ, Kim DY, Sohn S, Kwon HJ, Gwag BJ . Caspase-3-mediated cleavage of PHF-1 tau during apoptosis irrespective of excitotoxicity and oxidative stress: an implication to Alzheimer's disease . Neurobiol Dis 2005 ; 18 : 450 - 458
Article CAS PubMed Google Scholar - Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano Y, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St George-Hyslop P, Takeda M, Tohyama M . Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response . Nat Cell Biol 1999 ; 1 : 479 - 485
Article CAS PubMed Google Scholar - Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG . Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease . J Neurosci 2001 ; 21 : 372 - 381
Article CAS PubMed PubMed Central Google Scholar - Kikuchi H, Almer G, Yamashita S, Guegan C, Nagai M, Xu Z, Sosunov AA, McKhann GM, Przedborski S . Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model . Proc Natl Acad Sci USA 2006 ; 103 : 6025 - 6030
Article CAS PubMed PubMed Central Google Scholar - Kim SJ, Zhang Z, Hitomi E, Lee YC, Mukherjee AB . Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL . Hum Mol Genet 2006 ; 15 : 1826 - 1834
Article CAS PubMed Google Scholar - Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J . The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins . Nature 1988 ; 332 : 462 - 464
Article CAS PubMed Google Scholar - Lee AH, Iwakoshi NN, Glimcher LH . XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response . Mol Cell Biol 2003 ; 23 : 7448 - 7459
Article CAS PubMed PubMed Central Google Scholar - Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K . Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration . FEBS Lett 2004 ; 566 : 261 - 269
Article CAS PubMed Google Scholar - Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao-Ashec K, Frautschy SA, Cole GM . Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice . Neurobiol Aging 2001 ; 22 : 983 - 991
Article CAS PubMed Google Scholar - Liu H, Miller E, van de WB, Stevens JL . Endoplasmic reticulum stress proteins block oxidant-induced Ca2+ increases and cell death . J Biol Chem 1998 ; 273 : 12858 - 12862
Article CAS PubMed Google Scholar - McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ . Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state . Mol Cell Biol 2001 ; 21 : 1249 - 1259
Article CAS PubMed PubMed Central Google Scholar - Moreira PI, Smith MA, Zhu X, Nunomura A, Castellani RJ, Perry G . Oxidative stress and neurodegeneration . Ann N Y Acad Sci 2005 ; 1043 : 545 - 552
Article CAS PubMed Google Scholar - Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y . An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12 . J Biol Chem 2002 ; 277 : 34287 - 34294
Article CAS PubMed Google Scholar - Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J . Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta . Nature 2000 ; 403 : 98 - 103
Article CAS PubMed Google Scholar - Paschen W, Mengesdorf T . Endoplasmic reticulum stress response and neurodegeneration . Cell Calcium 2005 ; 38 : 409 - 415
Article CAS PubMed Google Scholar - Reynolds IJ, Hastings TG . Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation . JNeurosci 1995 ; 15 : 3318 - 3327
Article CAS Google Scholar - Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E . Caspase-9 activation and caspase cleavage of tau in the Alzheimer's disease brain . Neurobiol Dis 2002 ; 11 : 341 - 354
Article CAS PubMed Google Scholar - Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA . Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease . JNeurosci 2002 ; 22 : 10690 - 10698
Article CAS Google Scholar - Saito K, Elce JS, Hamos JE, Nixon RA . Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration . Proc Natl Acad Sci U S A 1993 ; 90 : 2628 - 2632
Article CAS PubMed PubMed Central Google Scholar - Sato N, Urano F, Yoon LJ, Kim SH, Li M, Donoviel D, Bernstein A, Lee AS, Ron D, Veselits ML, Sisodia SS, Thinakaran G . Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression . Nat Cell Biol 2000 ; 2 : 863 - 870
Article CAS PubMed Google Scholar - Seyb KI, Ansar S, Bean J, Michaelis ML . beta-Amyloid and endoplasmic reticulum stress responses in primary neurons: effects of drugs that interact with the cytoskeleton . J Mol Neurosci 2006 ; 28 : 111 - 123
Article CAS PubMed Google Scholar - Siman R, Flood DG, Thinakaran G, Neumar RW . Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer's disease-linked presenilin-1 knock-in mutation . J Biol Chem 2001 ; 276 : 44736 - 44743
Article CAS PubMed Google Scholar - Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G . Oxidative stress in Alzheimer's disease . Biochim Biophys Acta 2000 ; 1502 : 139 - 144
Article CAS PubMed Google Scholar - Soto C . Unfolding the role of protein misfolding in neurodegenerative diseases . Nat Rev Neurosci 2003 ; 4 : 49 - 60
Article CAS PubMed Google Scholar - Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM, Ron D . Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) . Mol Cell Biol 1996 ; 16 : 4273 - 4280
Article CAS PubMed PubMed Central Google Scholar - Wong WL, Brostrom MA, Kuznetsov G, Gmitter-Yellen D, Brostrom CO . Inhibition of protein synthesis and early protein processing by thapsigargin in cultured cells . Biochem J 1993 ; 289 : 71 - 79
Article CAS PubMed PubMed Central Google Scholar - Yang F, Ueda K, Chen P, Ashe KH, Cole GM . Plaque-associated alpha-synuclein (NACP) pathology in aged transgenic mice expressing amyloid precursor protein . Brain Res 2000 ; 853 : 381 - 383
Article CAS PubMed Google Scholar - Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M . Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress . J Biol Chem 2001 ; 276 : 13935 - 13940
Article CAS PubMed Google Scholar - Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K . ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response . Mol Cell Biol 2000 ; 20 : 6755 - 6767
Article CAS PubMed PubMed Central Google Scholar - Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K . XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor . Cell 2001 ; 107 : 881 - 891
Article CAS PubMed Google Scholar - Yu Z, Luo H, Fu W, Mattson MP . The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis . Exp Neurol 1999 ; 155 : 302 - 314
Article CAS PubMed Google Scholar
Acknowledgements
We thank Boston University Alzheimer's Disease Center (Dr. Ann C. Mckee) for human brain tissues. This work was supported by Cell Transformation and Regeneration Research Center of the BK-21 project and Anti-Alzheimer's Drug Development Research Center of the Korean Ministry of Health and Welfare.
Author information
Author notes
- Jin Hwan Lee and Sun Mi Won: These authors contributed equally to this work.
Authors and Affiliations
- Department of Neuroscience, Ajou University School of Medicine, Suwon 442-749, Korea.,
Jin Hwan Lee, Sun Mi Won, Jaehong Suh, Gyeong Joon Moon, Ui-Jin Park & Byoung Joo Gwag - Department of Pharmacology, Ajou University School of Medicine, Suwon 442-749, Korea.,
Jin Hwan Lee, Sun Mi Won, Jaehong Suh, Gyeong Joon Moon, Ui-Jin Park & Byoung Joo Gwag - Research Institute for Neural Science and Technology, Ajou University School of Medicine, Suwon 442-749, Korea.,
Jin Hwan Lee, Sun Mi Won, Jaehong Suh, Gyeong Joon Moon, Ui-Jin Park & Byoung Joo Gwag - Department of Pharmacology, Neurotech Pharmaceuticals Co., Suwon 442-749, Korea.,
Jin Hwan Lee, Sun Joo Son, Gyeong Joon Moon, Ui-Jin Park & Byoung Joo Gwag
Authors
- Jin Hwan Lee
You can also search for this author inPubMed Google Scholar - Sun Mi Won
You can also search for this author inPubMed Google Scholar - Jaehong Suh
You can also search for this author inPubMed Google Scholar - Sun Joo Son
You can also search for this author inPubMed Google Scholar - Gyeong Joon Moon
You can also search for this author inPubMed Google Scholar - Ui-Jin Park
You can also search for this author inPubMed Google Scholar - Byoung Joo Gwag
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toByoung Joo Gwag.
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lee, J., Won, S., Suh, J. et al. Induction of the unfolded protein response and cell death pathway in Alzheimer's disease, but not in aged Tg2576 mice.Exp Mol Med 42, 386–394 (2010). https://doi.org/10.3858/emm.2010.42.5.040
- Accepted: 24 March 2010
- Published: 05 April 2010
- Issue Date: May 2010
- DOI: https://doi.org/10.3858/emm.2010.42.5.040