Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells (original) (raw)
Abstract
Aims/hypothesis
Recent studies have suggested resveratrol (RSV) as a new natural therapeutic agent to treat type 2 diabetes and lipid-induced insulin resistance. Here, we investigated whether RSV could reverse palmitate-induced insulin resistance in human primary muscle cells.
Methods
Myotubes obtained from six healthy men (54 ± 3 years (mean ± SE), BMI 25.0 ± 1.7 kg/m2, fasting plasma glucose concentration (fP-glucose) 5.47 ± 0.09 mmol/l) were treated for 4 h with 100 μmol/l RSV and/or 0.2 mmol/l palmitate, and stimulated with or without 100 nmol/l insulin. Assays of glucose uptake, glycogen synthesis, palmitate oxidation, intracellular signalling and AMP-activated protein kinase (AMPK) activity were performed.
Results
RSV did not reverse palmitate-induced impairment of glucose metabolism. Surprisingly, RSV decreased glucose uptake and glycogen synthesis in human skeletal muscle cells. Palmitate oxidation and phosphorylation of AMPK and its downstream target acetyl-CoA carboxylase β (ACCβ) were inhibited by RSV, and RSV completely blocked the activity of AMPK isoform complexes α1/β2/γ1 and α2/β2/γ1 in in-vitro kinase activity assays. Endoplasmic reticulum (ER) stress was increased in response to RSV, as indicated by increased phosphorylation of eukaryotic initiation factor 2α (eIF2α) and increased expression of CCAAT/enhancer binding protein homologous protein (CHOP).
Conclusions/interpretation
Acute exposure to RSV inhibits AMPK activity, fatty-acid oxidation and glucose metabolism in human myotubes.
Similar content being viewed by others
Introduction
Type 2 diabetes and obesity are characterised by defects in insulin signalling, glucose transport, glycogen synthesis and lipid metabolism. Elevated non-esterified fatty acids (NEFA) are regarded as one of the major causes of insulin resistance [1–3]. Exposure to NEFAs impairs insulin signalling and affects glycogen synthesis in cultured cells and rodent muscle tissue [4–7] and acute exposure of isolated muscle strips to palmitate causes insulin resistance in human skeletal muscle from obese people [8]. Elevated NEFA levels are postulated to lead to the accumulation of active intramuscular lipid metabolites, such as diacylglycerol (DAG), long-chain Acyl-CoAs:s and ceramides, which interfere with insulin signalling via activation of serine/threonine kinases such as protein kinase C (PKC) and c-Jun N-terminal kinase (JNK) [9–11]. Therefore, interventions that impact intracellular levels of these active lipid metabolites have been suggested to improve insulin sensitivity.
Resveratrol (RSV), a natural polyphenol found in grapes and peanuts, has been proposed as a potential therapeutic agent to treat type 2 diabetes and lipid-induced insulin resistance. In obese men, treatment with RSV for 30 days is associated with reduced sleeping and resting metabolic rates and improved insulin action [12]. However, the mechanisms of RSV action on metabolism are not fully understood. RSV reduces weight and concentration of intramuscular lipid metabolites and reactive oxygen species (ROS) in a mouse model [13], and it induces mitochondrial biogenesis via activation of the sirtuin 1 (SIRT1)– peroxisome proliferator-activated receptor γ co-activator 1-α (PGC-1α) pathway [14]. Deacetylation (and activation) of PGC-1α by SIRT1 requires AMP-activated protein kinase (AMPK) activation, which is directly regulated by RSV [13, 15]. AMPK improves glucose metabolism in an insulin-independent fashion [16] and is a critical regulator of lipid metabolism [17]. Given that RSV activates AMPK in animal and cell models [13], we tested the hypothesis that RSV could protect primary human muscle cells from palmitate-induced insulin resistance.
Methods
Materials
The sources of antibodies and reagents are described in the electronic supplementary materials (ESM) Methods.
Participants
Experimental protocols were approved by the Ethical Committee of Department of Medicine, Helsinki University Central Hospital and written informed consent was obtained from all participants. The principles of the Declaration of Helsinki were followed. Six healthy nonsmoking men (54 ± 3 years, BMI 25.0 ± 1.7 kg/m2) were studied. All had normal glucose tolerance according to WHO criteria (fasting plasma glucose concentration [fP-glucose] 5.47 ± 0.09 mmol/l, 2 h P-glucose 5.33 ± 0.54 mmol/l).
Primary human muscle cells
A small (∼100 mg) muscle biopsy from the vastus lateralis was taken under local anaesthesia (15 ml of lidocaine hydrochloride 10 mg/ml) [18, 19]. Satellite cells were isolated, plated on collagen-coated plates and grown in the proliferating medium (ESM Methods). Magnetic cell separation with CD56 antibody was used to select primary myoblasts, which were differentiated into myotubes. The cells were starved of serum for 2 h in low-glucose DMEM before experiments. Myotubes were exposed to 100 μmol/l RSV, 1 mmol/l 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside (AICAR), 20 μmol/l Compound C (a potent reversible inhibitor of AMPK [23]) or 0.2 mmol/l palmitate, or their combination, for 4 h. In dose–response experiments 0.1–200 μmol/l RSV was used. For the time course experiments, the cells were incubated with 100 μmol/l RSV for up to 24 h.
Immunocytochemistry
Myogenin staining was performed on cells grown on glass coverslips to determine the differentiation of myoblasts into myotubes (ESM Methods). The cells were permeabilised with −20°C methanol, blocked in 5% BSA–0.01% Triton-X 100, and incubated with anti-myogenin. After incubation with secondary antibody the coverslips were mounted with Mowiol. The images were acquired using an AXIOVERT 200 M microscope (Carl Zeiss, Tuusula, Finland).
Glucose uptake and glycogen synthesis
Glucose uptake into muscle cells and glucose incorporation into glycogen were measured in triplicate, as described [20, 21].
Fatty-acid oxidation
Serum-starved myotubes were exposed for 4 h to 100 μmol/l RSV, 100 nmol/l insulin, 1 mmol/l AICAR and 20 μmol/l Compound C, with only a trace amount of 9,10-[3H]palmitic acid in the medium (5 μCi/ml). In separate experiments, we also performed fatty-acid oxidation assay in the presence of 0.2 mmol/l cold palmitate. Palmitate oxidation was analysed by measuring 3H-labelled water, as described [22].
Western blot
Protein detection was performed by western blotting using enhanced chemiluminescence. Proteins were quantified by densitometry using the ImageJ Software (NIH, USA, http://rsbweb.nih.gov/ij/).
PGC-1α acetylation
Human primary myotubes were exposed to 1 or 100 μmol/l RSV for 4 h, lysed in ice-cold immunoprecipitation lysing buffer, and 250 μg total protein was incubated with anti-PGC-1α antibodies overnight at 4°C (ESM Methods). Protein A-agarose was added, and the agarose-immunocomplex was pelleted and dissolved in 1× Laemmli Buffer. PGC-1α acetylation was analysed by western blotting with anti-acetyl-lysine antibodies.
AMPK kinase activity assay
Activity of both AMPK isoforms (α1/β2/γ1 and α2/β2/γ1) was measured according to the manufacturer’s protocol (SignalChem Pharmaceuticals Inc, Richmond, BC), as described in ESM Methods. Briefly, 300 ng of AMPK was incubated with 1 or 100 μmol/l RSV, 1 mmol/l AICAR, 1 mmol/l 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′monophosphate (ZMP) or 20 μmol/l Compound C in kinase assay buffer in the presence of synthetic peptide substrate for AMPK (SAMS) and AMP for 2 h at 30°C. Then 5 μl of 250 μmol/l 33P-labelled ATP (Perkin Elmer, Waltham, MA, USA) Assay Cocktail (SignalChem Pharmaceuticals, Richmond, BC, Canada) was added and the reaction mixture was incubated for 15 min. The reaction was terminated by spotting 20 μl into the P81 paper strip, and radioactivity was counted in a scintillation counter.
Cell viability assay
Human primary or L6 myotubes were treated with different doses of RSV (0.1–200 μmol/l) for 4 h to assess the toxicity of RSV using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) test (ESM Methods). MTT compound, which is reduced from yellow tetrazole to purple formazone in intact and living cells, was added and the absorbance was measured at 560 nm.
Statistics
All results are presented as means ± SE. One-way ANOVA with repeated measurements was used to analyse the data, unless noted otherwise (Prism 4; GraphPad Software, http://www.graphpad.com). A p value of <0.05 was considered significant.
Results
Differentiation of the human primary muscle cells
The levels of the muscle-specific transcription factor myogenin was analysed using immunocytochemistry (ESM Fig. 1). Myogenin levels were transiently increased with a maximum at 3 days and return to basal at 7 days (ESM Fig. 1 and data not shown). The formation of myotubes was significantly increased 3 days after the induction of differentiation, with further differentiation by 7 days. To confirm formation of myotubes we also assessed the abundance of desmin, a structural protein specific for muscle cells. Desmin levels progressively increased during the differentiation period, with the maximum reached at day 7 (data not shown). Thus, we chose 7 days of differentiation for further experiments.
Glucose uptake
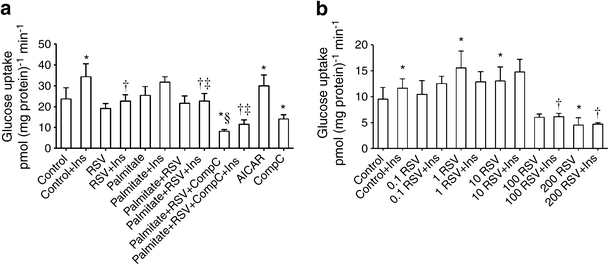
Insulin stimulated glucose uptake 1.5 fold in human primary muscle cells (Fig. 1a). RSV decreased the insulin-stimulated glucose uptake by 34% (p < 0.05). Basal and insulin-stimulated glucose uptake were not significantly affected by palmitate. AICAR increased basal glucose uptake 1.3 fold (p < 0.05) whereas Compound C reduced glucose uptake (p < 0.05, Fig. 1a). We next exposed primary human myotubes to increasing RSV concentrations (0.1, 1, 10, 100 and 200 μmol/l, Fig. 1b). RSV at 1 or 10 μmol/l increased basal glucose uptake whereas exposure to higher RSV concentrations (100 and 200 μmol/l) led to a decrease in glucose uptake. Insulin-stimulated glucose uptake was decreased by 100 or 200 μmol/l RSV and not affected by lower concentrations. To account for species differences in RSV action, we analysed the dose–response characteristics of RSV in a rat muscle cell line, L6 myotubes (ESM Fig. 2). Insulin-stimulated glucose uptake was reduced by 100 or 200 μmol/l RSV. Basal glucose uptake was reduced by 200 μmol/l RSV and unaffected by lower RSV concentrations in L6 cells. To exclude the possibility that our results could have been due to any toxic effects of RSV, the viability of the cells was verified by MTT test. RSV up to 100 μmol/l for 4 h did not affect the viability of the human (ESM Fig. 3a) or L6 myotubes (ESM Fig. 3b).
Fig. 1
Glucose uptake. (a) The effect of 4 h pre-exposure to 0.2 mmol/l palmitate, 100 μmol/l RSV, 1 mmol/l AICAR or 20 μmol/l Compound C (CompC) on glucose uptake in human primary muscle cells. Insulin (Ins) concentration was 100 nmol/l. Results are mean ± SE from six men. (b) The dose–response effect of 4 h exposure to RSV (in μmol/l) on glucose uptake in human primary myotubes from three men. Results are mean ± SE. *p < 0.05 vs Control; † p < 0.05 vs Control + Ins; ‡ p < 0.05 vs Palmitate + Ins; § p < 0.05 vs Palmitate
Glycogen synthesis
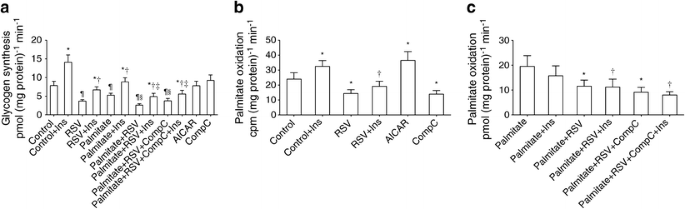
Insulin increased glycogen synthesis twofold (p < 0.05, Fig. 2a). Pre-exposure of muscle cells to 100 μmol/l RSV for 4 h decreased basal as well as insulin-stimulated glucose incorporation into glycogen. Palmitate impaired basal and insulin-stimulated glycogen synthesis. RSV did not improve glycogen synthesis in cells pre-exposed to palmitate but further reduced glucose incorporation into glycogen (Fig. 2a).
Fig. 2
Glycogen synthesis and palmitate oxidation. The effect of RSV on glycogen synthesis (a) and palmitate oxidation (b, c) in human primary muscle cells. The concentration of the compounds is the same as in Fig. 1a. Results are mean ± SE from six (a) or five (b, c) men. (b) The palmitate oxidation assay was performed without cold palmitate throughout the experiment, with only a trace amount of labelled palmitate in the media. (c) Cold palmitate (0.2 mmol/l) was present in every condition. In (a, b) *p < 0.05 vs respective basal (Control) without insulin (Ins); † p < 0.05 vs Control + Ins; ‡ p < 0.05 vs Palmitate + Ins; § p < 0.05 vs Palmitate; ¶ p < 0.05 vs Control. In (c), *p < 0.05 vs Palmitate; † p < 0.05 vs Palmitate + Ins. CompC, Compound C
Fatty-acid oxidation
Palmitate oxidation was first determined using only a trace amount of radiolabelled palmitate in the media (without any cold palmitate, Fig. 2b). Insulin stimulated palmitate oxidation, most likely due to increased palmitate uptake into cells [24]. However, in the presence of RSV, palmitate oxidation was reduced by 40% (p < 0.05). AICAR increased palmitate oxidation whereas in the presence of Compound C palmitate oxidation was inhibited. Next, palmitate oxidation was analysed at physiological palmitate concentration (0.2 mmol/l). As expected, in this experimental set-up, insulin tended to decrease palmitate oxidation (Fig. 2c). Exposure of human muscle cells to RSV led to decreased palmitate oxidation rate.
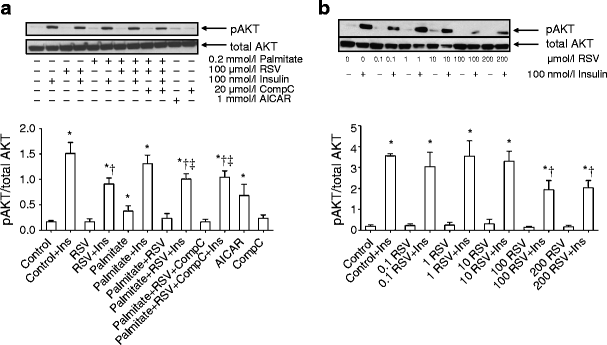
Effect of RSV on AKT phosphorylation
Insulin increased AKT Ser473 phosphorylation (Fig. 3a). Exposure to RSV attenuated insulin-stimulated AKT Ser473 phosphorylation in cells incubated with or without palmitate (p < 0.05). To determine whether the effect of RSV on AKT phosphorylation was concentration dependent, we exposed human and L6 myotubes to 0.1–200 μmol/l RSV. RSV at lower concentrations (0.1–10 μmol/l) did not affect, whereas at 100 or 200 μmol/l RSV decreased, insulin-stimulated AKT Ser473 phosphorylation in human primary myotubes (Fig. 3b). Similar to human myotubes, lower RSV concentrations did not affect insulin-stimulated AKT Ser473 phosphorylation, whereas RSV at 100 or 200 μmol/l resulted in impaired AKT Ser473 phosphorylation in L6 myotubes (ESM Fig. 4a).
Fig. 3
Phosphorylation of AKT at Ser473. (a) Human myotubes were exposed to RSV and/or palmitate for 4 h, whereafter cells were stimulated with or without 100 nmol/l insulin (Ins) for 10 min. Phosphorylation of AKT Ser473 was determined with western blotting. Results are reported as mean ± SE from six men. (b) The dose–response effect of RSV (in μmol/l) on AKT Ser473 phosphorylation in human myotubes from three men. Results are mean ± SE *p < 0.05 vs respective basal (Control) without Ins; † p < 0.05 vs Control + Ins; ‡ p < 0.05 vs Palmitate + Ins. CompC, Compound C
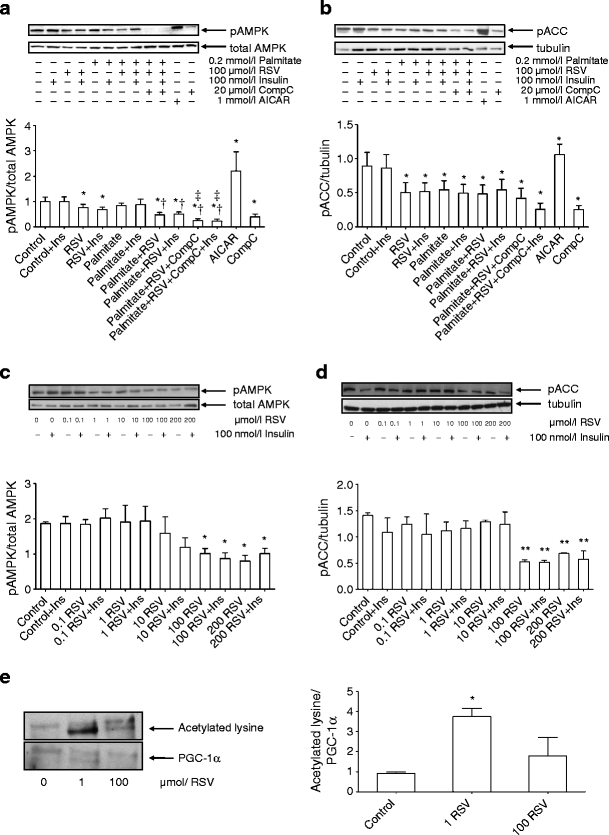
Effect of RSV on AMPK signalling pathway
RSV inhibited, whereas AICAR stimulated, AMPK phosphorylation in human primary muscle cells (p < 0.05, Fig. 4a). Treatment with RSV impaired phosphorylation of ACCβ, the downstream target of AMPK [16] (Fig. 4b). To determine the dose–response effect of RSV on the AMPK signalling pathway, myotubes were exposed to 0.1–200 μmol/l RSV. The phosphorylation of AMPK or ACCβ was unaffected in human primary myotubes at lower RSV concentrations. However, treatment with 100 or 200 μmol/l RSV significantly decreased phosphorylation of both AMPK (Fig. 4c) and ACCβ (Fig. 4d) in human myotubes. In contrast, in L6 myotubes all RSV concentrations activated AMPK, as indicated by increased ACCβ phosphorylation (ESM Fig. 4b).
Fig. 4
Phosphorylation of AMPK at Thr172 and ACCβ at Ser79. Human primary myotubes were treated with 100 μmol/l RSV and/or 0.2 mmol/l palmitate for 4 h and phosphorylation of AMPK (a), as well as its downstream target ACCβ (b), was assessed. Data are expressed as mean ± SE from six men. AICAR and Compound C (CompC) were used as positive and negative controls, respectively. (c) Dose–response effect of 4 h exposure to RSV (in μmol/l) on AMPK phosphorylation in human primary myotubes from three men. Results are mean ± SE, (d) Dose–response effect of RSV (in μmol/l) on ACCβ phosphorylation in human primary myotubes from three men. Results are mean ± SE (e) The effect of 1 or 100 μmol/l of RSV on sirtuin-1 activation was determined by immunoprecipitating PGC-1α and immunoblotting with anti-acetyl-lysine antibody in human myotubes from three men. Total PGC-1α was determined with anti-PGC-1α antibody. Results are mean ± SE *p < 0.05 vs respective Control; **p < 0.01 vs respective Control; † p < 0.05 vs respective Palmitate; ‡ p < 0.05 vs respective condition with Palmitate + RSV. One-way ANOVA with repeated measurements (a,b,d,e), Student´s paired t test (c)
RSV increases PGC-1α acetylation in human myotubes
Activation of SIRT1 by RSV requires AMPK activation and leads to deacetylation (and activation) of transcriptional coactivator PGC-1α in nonhuman cell and animal models [13, 14]. To study SIRT1 activation in primary human myotubes, we exposed cells to 1 or 100 μmol/l RSV for 4 h and determined PGC-1α acetylation. Acute exposure to 1 μmol/l RSV significantly increased acetylation of PGC-1α in human primary myotubes, suggesting inhibition of SIRT1 activity and increased activation of acetyltransferases by RSV (Fig. 4e). The change in PGC-1α acetylation in response to 100 μmol/l RSV was not statistically significant.
In-vitro effect of RSV on AMPK activity
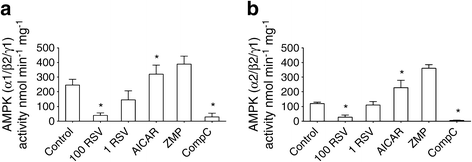
To confirm the inhibitory action of RSV on AMPK activity we performed in-vitro kinase activity assays. Although AMPK possesses 12 different isoform combinations, only three of them have been identified in human skeletal muscle, with the highest content of combinations of α1/β2/γ1 and α2/β2/γ1 isoforms [25]. Thus, we measured the activity of both α1/β2/γ1 and α2/β2/γ1 complexes in the presence of 1 or 100 μmol/l RSV, 1 mmol/l AICAR, 1 mmol/l ZMP and 20 μmol/l Compound C. RSV at 1 μmol/l inhibited AMPK-α1 activity by 50% and AMPK-α2 activity by 35% (n = 2) whereas 100 μmol/l RSV totally blocked the activity of both AMPK isoform complexes in the in-vitro assays (p < 0.05, Fig. 5). Compound C had a similar effect to RSV, whereas AICAR and ZMP increased the activity of both α1/β2/γ1 and α2/β2/γ1 isoform complexes.
Fig. 5
Effect of RSV on AMPK activity in vitro. The activity of both α1/β2/γ1 (a) and α2/β2/γ1 (b) isoforms of AMPK was measured using in-vitro kinase activity assay. RSV at 1 or 100 μmol/l (1 RSV and 100 RSV, respectively) decreased the activity of both isoforms of AMPK. AICAR (1 mmol/l) and Compound C (CompC, 20 μmol/l) were used as positive and negative controls, respectively. Results are mean ± SE from three independent experiments performed in duplicate (from two experiments for 1 μmol/l RSV and 1 mmol/l ZMP). *p < 0.05 vs control
RSV increases ER stress in human and L6 myotubes
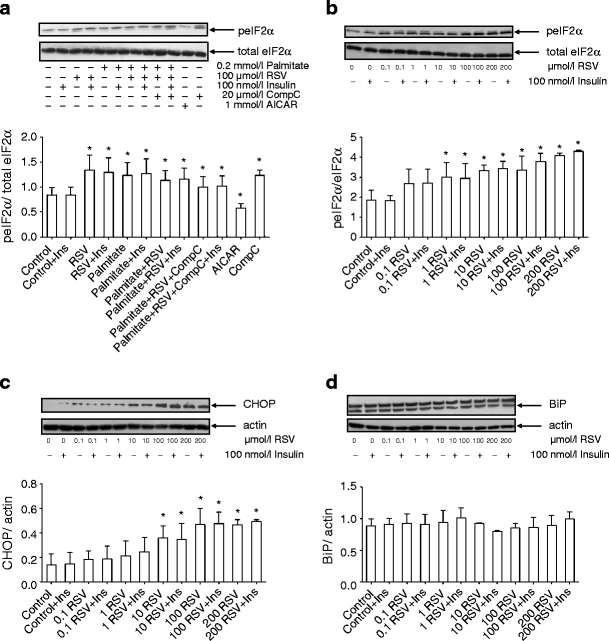
ER stress has recently been described as an important player in insulin resistance [26]. Under conditions of ER stress, an adaptive system called unfolded protein response (UPR) is activated to restore cellular homeostasis. One arm of UPR, eukaryotic initiation factor 2α (eIF2α), is inhibited by phosphorylation, leading to global inhibition of translation [27]. Here, we investigated the effect of our experimental perturbations on eIF2α-Ser52 phosphorylation. Exposure to RSV or palmitate, or their combination, significantly increased eIF2α phosphorylation in primary human myotubes (p < 0.05) (Fig. 6a). Treatment with AICAR decreased eIF2α-Ser52 phosphorylation by 32% (p < 0.05) whereas exposure to compound C increased eIF2α-Ser52 phosphorylation (p < 0.05). Next, human myotubes from three individuals were exposed for 4 h to increasing RSV concentrations (0.1, 1, 10, 100 and 200 μmol/l). While 0.1 μmol/l RSV did not significantly affect eIF2α phosphorylation, at higher concentrations RSV significantly increased eIF2α phosphorylation with the maximum effect at 200 μmol/l (Fig. 6b).
Fig. 6
Activation of ER stress markers. Myotubes were treated with 100 μmol/l RSV and/or palmitate for 4 h. Phosphorylation of eIF2α was measured in myotubes from six healthy male volunteers (a). The dose–response effect of RSV (in μmol/l) on the phosphorylation of eIF2α (b), the expression of CHOP (c) and BiP (d) in human myotubes from three men. Results are mean ± SE. *p < 0.05 vs respective Control. CompC, Compound C; Ins, insulin; peIF2α, phosphorylated eIF2α
Activation of UPR during ER stress also results in induction of CCAAT/enhancer binding protein homologous protein (CHOP) and chaperone protein binding immunoglobulin protein (BiP) [27]. Treatment of human myotubes with RSV increased CHOP levels in a dose-dependent manner up to 100 μmol/l (Fig. 6c). BiP levels were unaffected by RSV in human muscle cells (Fig. 6d). In L6 myotubes, eIF2α phosphorylation was increased in response to 100 or 200 μmol/l RSV but was not affected by lower RSV concentrations (ESM Fig. 5a). The production of CHOP was increased by RSV at a concentration 10 μmol/l or higher (ESM Fig. 5b), whereas BiP levels were increased at all RSV concentrations (from 0.1 μmol/l upwards) in L6 myotubes (ESM Fig. 5c).
Time course effect of RSV on protein phosphorylation
To determine whether the inhibitory action of RSV was time-dependent, we performed a time course experiment where the level of phosphorylation of AKT, AMPK, ACC and eIF2α was analysed in human skeletal muscle cells (ESM Fig. 6). Treatment with 100 μmol/l RSV decreased insulin-mediated phosphorylation of AKT-Ser473 and this inhibition was maintained for 24 h (ESM Fig. 6a). There was a nonsignificant trend for decreased AMPK phosphorylation after 2–12 h incubation with RSV, with a return to basal after 24 h (ESM Fig. 6b). Exposure to RSV reduced phosphorylation of ACCβ and this effect was maintained for 12 h (p < 0.05, ESM Fig. 6c). ACCβ phosphorylation was restored to basal levels after 24 h exposure to RSV. eIF2α-Ser52 phosphorylation was increased by RSV at all time points up to 24 h (p < 0.05, ESM Fig. 6d).
Discussion
The natural polyphenolic compound RSV has recently attracted considerable scientific interest due to its glucose-lowering properties. In rodent models, RSV improves the action of insulin and improves whole-body glucose homeostasis [13, 14, 28–30]. RSV has also been shown to be involved in the reduction of fat accumulation and body weight [13, 29, 31]. Since type 2 diabetes is characterised by defects in glucose metabolism and is often associated with obesity, these findings have raised hope that RSV or compounds with similar mode of action could be used to improve metabolic control and alleviate overweight in insulin-resistant patients. Therefore, it is important to understand the mechanisms whereby RSV influences metabolism. Since skeletal muscle is the main site of glucose use under insulin-stimulated conditions [32, 33], we have studied herein the metabolic effects of RSV on glucose and lipid metabolism using cultured primary human skeletal muscle cells.
First, we studied the effect of RSV on glucose metabolism in primary human myotubes. In the basal state, glucose uptake was unaffected by the presence of RSV. However, glucose incorporation into glycogen in the basal state, as well as insulin-stimulated glucose uptake and glycogen synthesis, were significantly reduced by RSV. These data were surprising, since RSV has previously been shown to stimulate glucose uptake in L6 muscle cells through the activation of insulin and AMPK signalling cascades [34, 35]. To determine whether the effects of RSV on glucose uptake were concentration or species dependent, we performed a dose–response experiment using primary human myotubes and a rat-derived cell line, L6 cells. In human myotubes, we observed increased basal glucose uptake with lower RSV concentrations (1 and 10 μmol/l) and an inhibition of basal and insulin-stimulated glucose uptake by higher RSV concentrations (100 and 200 μmol/l). In L6 myotubes, RSV 0.1–100 μmol/l did not affect basal glucose uptake, whereas basal glucose uptake was inhibited by 200 μmol/l RSV. Similar to human myotubes, insulin-stimulated glucose uptake was inhibited by 100 and 200 μmol/l RSV. The reason for different findings is not readily apparent since the concentrations of RSV and duration of exposure have been similar in previous studies [34, 35]. The inhibitory effect of higher RSV concentration on glucose uptake was also similar in human and L6 myotubes in our study, excluding an effect of possible species differences. It is interesting that lower concentrations of RSV enhanced basal glucose uptake in human myotubes, while higher concentrations were inhibitory. These data would be compatible with the concept of hormesis: lower concentrations of RSV seem to exert positive, whereas higher concentrations of RSV seem to exert negative, biological effects, similar to what has been suggested for oxidative stress [36].
Our original working hypothesis was that RSV could reverse palmitate-induced insulin resistance in human muscle cells, as it does in C2C12 muscle cells [30]. However, we observed no such effect. The defect in glucose incorporation into glycogen was more pronounced in the presence of RSV. Thus, exposure to RSV impairs insulin action in human primary myotubes and does not protect cells from lipid-induced insulin resistance. Consistent with the negative effects on glucose metabolism, RSV attenuated insulin-stimulated phosphorylation of AKT, an effect sustained for 24 h. RSV-induced inhibition of insulin action on glucose uptake or insulin signalling has previously been reported also in other studies [37–39], and RSV has been shown to be a direct inhibitor of class IA PI3-kinase [37]. More specifically [37], 100 μmol/l RSV for 30 min fully inhibited insulin-stimulated AKT phosphorylation at Ser473 and Thr308 as well as the activation of PI3-kinase in a dose-dependent fashion (5–100 μmol/l) in primary human myotubes. Insulin-stimulated AKT Ser473 phosphorylation was inhibited by 10–100 μmol/l RSV also in rat L6 and human rhabdomyosarcoma CCL muscle-derived cells. Further analysis revealed that RSV targeted the ATP-binding site of PI3K in a competitive and reversible manner. Insulin-stimulated glucose uptake was reduced 82.7% also in 3T3-L1 adipocytes by 100 μmol/l RSV [37]. RSV decreased basal glucose uptake in two myelocytic cell lines, U937 and HL-60 cells, with a dose-dependent inhibition from 20 to 120 μmol/l and 50% inhibition at approximately 73–75 μmol/l [40]. Zhang reported that RSV, at similar concentrations to ours (1–200 μmol/l), inhibited insulin-stimulated AKT signalling in H4IIE cells (a rat hepatoma cell line), HepG2 cells (a human hepatoma cell line, RSV 100 μmol/l) and primary rat hepatocytes (RSV 100 μmol/l) [38]. Taken together, RSV inhibits glucose metabolism and AKT signalling in several different cell models.
RSV increases mitochondrial biogenesis and physical endurance and reduces weight, fat accumulation and muscle DAG content in rodent models [13, 14, 28]. Since these data suggest that RSV has beneficial effects on lipid metabolism, we investigated whether RSV affects fatty-acid oxidation in human skeletal muscle cells. As expected, exposure of muscle cells to AICAR increased palmitate oxidation. However, in the presence of RSV, palmitate oxidation was severely compromised. The marked inhibition of basal palmitate oxidation by the AMPK inhibitor Compound C points to a pivotal role of AMPK activity in regulation of fatty-acid oxidation, as suggested [17]. Taken together, our data on both glucose and lipid metabolism suggest that RSV acts as a metabolic inhibitor in human skeletal muscle cells and also raise the possibility that RSV inhibits AMPK activity.
Activation of AMPK by 10–100 μmol/l RSV has been reported in several studies [13, 28, 31, 34, 37]. We analysed the effect of RSV on the activation of the AMPK pathway in human primary muscle cells. Acute exposure to 100 or 200 μmol/l RSV inhibited activation of the AMPK pathway in human myotubes, as demonstrated by decreased phosphorylation of AMPK and ACC. The inhibitory effect of RSV on the AMPK signalling pathway was maintained for 12 h with a return to basal by 24 h. The AMPK pathway is known to be the main regulator of fatty-acid oxidation [17] and, thus, the inhibition of AMPK signalling is in line with our metabolic data showing reduced palmitate oxidation by RSV. Interestingly, we found that lower RSV concentrations (0.1 and 10 μmol/l) did not affect AMPK or ACCβ phosphorylation in human myotubes. In L6 myotubes, all RSV concentrations used (0.1–200 μmol/l) activated AMPK (as demonstrated by increased ACC phosphorylation), suggesting some species differences in response to RSV.
NAD+-dependent deacetylase SIRT1 deacetylates and thereby activates transcriptional coactivator PGC-1α; SIRT1 activation by RSV requires intact AMPK activity [13, 14]. To ascertain whether RSV activates SIRT1 in primary human myotubes, PGC-1α acetylation was determined. Acute exposure to 1 μmol/l RSV significantly increased acetylation of PGC-1α in human primary myotubes, which is the total opposite to what we expected. These data suggest that RSV acutely inhibits SIRT1 activity and may increase the activation of acetyltransferases. Given that SIRT1 activation by RSV is downstream of AMPK [13, 14], the observed increase in PGC-1α acetylation and inhibition of the AMPK-ACC signalling pathway suggest that RSV directly inhibits AMPK activity in human myotubes. To confirm this hypothesis, we examined the effect of RSV on activation of both α1/β2/γ1 and α2/β2/γ1 isoform complexes of AMPK in in-vitro kinase assays. RSV blocked the activity of both isoform complexes of AMPK in our study. Taken together, our data suggest that RSV is an inhibitor of AMPK in human skeletal muscle cells.
In contrast to our findings, 30-day treatment with RSV resulted in increased phosphorylation of AMPK in human skeletal muscle [12]. Thus, it is possible that acute and chronic effects of RSV may be different. Alternatively, the increase in AMPK phosphorylation in human muscle following 30-day treatment may be a secondary effect. In accordance, Park et al recently reported that RSV inhibits cAMP phosphodiesterases 1, 3 and 5; resulting elevation of cAMP activates cAMP-regulated guanine nucleotide exchange factor Epac1 and leads secondarily to AMPK activation via increased intracellular Ca2+ concentration and activation of calcium/calmodulin dependent protein kinase β (CamKKβ) via phospholipase C and the ryanodine receptor Ca2+-release channel [41]. Interestingly, despite an increase in AMPK phosphorylation in skeletal muscle, 30-day peroral RSV therapy led to a decrease in sleeping and resting metabolic rate [12]. This might be compatible with RSV-induced inhibition of glucose metabolism and insulin signalling observed in our and other studies [37–39], as well as observed RSV-induced inhibition of fatty-acid metabolism.
ER stress has emerged as an important contributor to the pathogenesis of insulin resistance. Here, we tested the effect of RSV on ER stress using phosphorylation of eIF2α or CHOP levels as readouts. eIF2α is a downstream target of the PKR-like endoplasmic reticulum kinase (PERK) signalling arm of the UPR and its phosphorylation leads to global inhibition of translation, thus protecting cells from accumulation of misfolded proteins that aggravate ER stress [26]. Exposure of muscle cells to palmitate increased eIF2α phosphorylation, which is compatible with previous data and verifies the notion that excess nutrients create ER stress. Interestingly, exposure to RSV also increased eIF2α phosphorylation and CHOP levels in a dose-dependent fashion suggesting that RSV induces ER stress. The effect of RSV on eIF2α phosphorylation and CHOP levels was also observed in L6 myotubes, whereas BiP was induced by RSV in L6 cells but not in human myotubes. Exposure of human muscle cells to AICAR resulted in a decrease in eIF2α phosphorylation, suggesting that AMPK activation alleviates ER stress. It has been reported that AMPK activation improves ER-stress-induced insulin resistance [42]. This may be an essential part of the mechanisms whereby AMPK activation mediates its beneficial effects on metabolism. The effects of AICAR and other AMPK activators on ER stress need to be studied in more detail in the future, as the findings may reveal prospective new targets for drug development.
In conclusion, acute exposure to RSV has a negative impact on glucose and lipid metabolism in human primary muscle cells. RSV inhibits insulin-stimulated AKT Ser473 phosphorylation, an effect maintained for 24 h. Furthermore, RSV increases ER stress and inhibits the AMPK signalling pathway in human skeletal muscle, as demonstrated by diminished phosphorylation of AMPK and ACC as well as direct inhibition of AMPK activity. AICAR reduces ER stress in muscle cells and thus targeting ER stress via AMPK pathway may reveal novel targets to treat metabolic diseases.
Abbreviations
ACC:
Acetyl-CoA carboxylase
AICAR:
5-Aminoimidazole-4-carboxamide-1-β-d-ribonucleoside
AKT:
V-akt murine thymoma viral oncogene (protein kinase B)
AMPK:
AMP-activated protein kinase
BiP:
Binding immunoglobulin protein
CHOP:
CCAAT/enhancer binding protein homologous protein
DAG:
Diacylglycerol
eIF2α:
Eukaryotic initiation factor 2α
ER:
Endoplasmic reticulum
fP-glucose:
Fasting plasma glucose concentration
MTT:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
PGC:
Peroxisome proliferator-activated receptor γ co-activator 1
PKC:
Protein kinase C
ROS:
Reactive oxygen species
RSV:
Resveratrol
SIRT1:
Sirtuin 1
UPR:
Unfolded protein response
ZMP:
5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′monophosphate
References
- Boden G (2011) Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 18:139–143
Article PubMed CAS Google Scholar - McGarry JD (1992) What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258:766–770
Article PubMed CAS Google Scholar - Opie LH, Walfish PG (1963) Plasma free fatty acid concentrations in obesity. N Engl J Med 268:757–760
Article PubMed CAS Google Scholar - Storz P, Doppler H, Wernig A, Pfizenmaier K, Muller G (1999) Cross-talk mechanisms in the development of insulin resistance of skeletal muscle cells. Palmitate rather than tumour necrosis factor inhibits insulin-dependent protein kinase B (PKB)/Akt stimulation and glucose uptake. Eur J Biochem 266:17–25
Article PubMed CAS Google Scholar - Chavez JA, Summers SA (2003) Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419:101–109
Article PubMed CAS Google Scholar - Chavez JA, Knotts TA, Wang LP et al (2003) A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 278:10297–10303
Article PubMed CAS Google Scholar - Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW (1998) Five-hour fatty acid elevation increases muscle lipids and impairs glycogen synthesis in the rat. Metabolism 47:1121–1126
Article PubMed CAS Google Scholar - Thrush AB, Heigenhauser GJ, Mullen KL, Wright DC, Dyck DJ (2008) Palmitate acutely induces insulin resistance in isolated muscle from obese but not lean humans. Am J Physiol Regul Integr Comp Physiol 294:R1205–R1212
Article PubMed CAS Google Scholar - Itani SI, Ruderman NB, Schmieder F, Boden G (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51:2005–2011
Article PubMed CAS Google Scholar - Schmitz-Peiffer C (2000) Signalling aspects of insulin resistance in skeletal muscle: mechanisms induced by lipid oversupply. Cell Signal 12:583–594
Article PubMed CAS Google Scholar - Kraegen EW, Cooney GJ (2008) Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol 19:235–241
Article PubMed CAS Google Scholar - Timmers S, Konings E, Bilet L et al (2011) Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14:612–622
Article PubMed CAS Google Scholar - Um JH, Park SJ, Kang H et al (2010) AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59:554–563
Article PubMed CAS Google Scholar - Lagouge M, Argmann C, Gerhart-Hines Z et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122
Article PubMed CAS Google Scholar - Canto C, Jiang LQ, Deshmukh AS et al (2010) Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11:213–219
Article PubMed CAS Google Scholar - Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9:407–416
Article PubMed Google Scholar - Osler ME, Zierath JR (2008) Adenosine 5′-monophosphate-activated protein kinase regulation of fatty acid oxidation in skeletal muscle. Endocrinology 149:935–941
Article PubMed CAS Google Scholar - Kuoppamaa H, Skrobuk P, Sihvo M et al (2008) Globular adiponectin stimulates glucose transport in type 2 diabetic muscle. Diabetes Metab Res Rev 24:554–562
Article PubMed CAS Google Scholar - Skrobuk P, Kuoppamaa H, Hiukka A, Koistinen HA (2010) Acute exposure to rosiglitazone does not affect glucose transport in intact human skeletal muscle. Metabolism 59:224–230
Article PubMed CAS Google Scholar - Al-Khalili L, Chibalin AV, Kannisto K et al (2003) Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cell Mol Life Sci 60:991–998
PubMed CAS Google Scholar - Somwar R, Sweeney G, Ramlal T, Klip A (1998) Stimulation of glucose and amino acid transport and activation of the insulin signaling pathways by insulin lispro in L6 skeletal muscle cells. Clin Ther 20:125–140
Article PubMed CAS Google Scholar - Rune A, Salehzadeh F, Szekeres F, Kuhn I, Osler ME, Al-Khalili L (2009) Evidence against a sexual dimorphism in glucose and fatty acid metabolism in skeletal muscle cultures from age-matched men and post-menopausal women. Acta Physiol (Oxf) 197:207–215
Article CAS Google Scholar - Zhou G, Myers R, Li Y et al (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174
PubMed CAS Google Scholar - Bouzakri K, Austin R, Rune A et al (2008) Malonyl coenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes 57:1508–1516
Article PubMed CAS Google Scholar - Wojtaszewski JF, Birk JB, Frosig C, Holten M, Pilegaard H, Dela F (2005) 5′AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol 564:563–573
Article PubMed CAS Google Scholar - Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Article PubMed CAS Google Scholar - Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61
Article PubMed CAS Google Scholar - Baur JA, Pearson KJ, Price NL et al (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342
Article PubMed CAS Google Scholar - Su HC, Hung LM, Chen JK (2006) Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 290:E1339–E1346
Article PubMed CAS Google Scholar - Sun C, Zhang F, Ge X et al (2007) SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319
Article PubMed CAS Google Scholar - Zang M, Xu S, Maitland-Toolan KA et al (2006) Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55:2180–2191
Article PubMed CAS Google Scholar - DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP (1981) The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007
PubMed CAS Google Scholar - DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J (1985) Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76:149–155
Article PubMed CAS Google Scholar - Breen DM, Sanli T, Giacca A, Tsiani E (2008) Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun 374:117–122
Article PubMed CAS Google Scholar - Minakawa M, Kawano A, Miura Y, Yagasaki K (2011) Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J Clin Biochem Nutr 48:237–244
Article PubMed CAS Google Scholar - Hawley JA, Burke LM, Phillips SM, Spriet LL (2011) Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol 110:834–845
Article PubMed CAS Google Scholar - Fröjdö S, Cozzone D, Vidal H, Pirola L (2007) Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J 406:511–518
Article PubMed Google Scholar - Zhang J (2006) Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J 397:519–527
Article PubMed CAS Google Scholar - Fröjdö S, Durand C, Molin L et al (2011) Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol 335:166–176
Article PubMed Google Scholar - Park JB (2001) Inhibition of glucose and dehydroascorbic acid uptakes by resveratrol in human transformed myelocytic cells. J Nat Prod 64:381–384
Article PubMed CAS Google Scholar - Park S-J, Ahmad F, Philp A et al (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148:421–433
Article PubMed CAS Google Scholar - Hwang S-L, Chang H-W, Lee I-K, Yang B-K, Magae J, Chang Y-C (2010) Ascofuranone prevents ER stress-induced insulin resistance via activation of AMP-activated protein kinase in L6 myotube cells. Biochem Biophys Res Commun 396:967–972
Article PubMed CAS Google Scholar
Funding
This study was supported by grants from the Finnish Academy of Science (grant no. 127093), Finnish Cultural Foundation, Finnish Diabetes Research Foundation, Finnish Foundation for Cardiovascular Research, Finnish Medical Foundation, Liv och Hälsa Foundation, Novo Nordisk Foundation, Paulo Foundation, Sigrid Juselius Foundation, the governmental subsidy for research of Helsinki University Central Hospital (EVO-funding), and the Research Council of University of Helsinki. P. Skrobuk has received grant support from Finska Läkaresällskapet, University of Helsinki and Paulo Foundation.
Contribution statement
PS, SK, MS, AZ and HK designed the study, researched and interpreted the data; PS and HK wrote the manuscript while SK, MS and AZ participated in its revision; all authors have approved the submitted manuscript.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
- Minerva Foundation Institute for Medical Research, Biomedicum 2U, Tukholmankatu 8, Helsinki, 00290, Finland
P. Skrobuk, S. von Kraemer, M. M. Semenova, A. Zitting & H. A. Koistinen - Department of Medicine, Division of Cardiology, Helsinki University Central Hospital, Helsinki, Finland
P. Skrobuk, S. von Kraemer, M. M. Semenova, A. Zitting & H. A. Koistinen - Institute of Clinical Medicine, Medical Faculty, University of Helsinki, Helsinki, Finland
P. Skrobuk, S. von Kraemer, M. M. Semenova, A. Zitting & H. A. Koistinen
Authors
- P. Skrobuk
You can also search for this author inPubMed Google Scholar - S. von Kraemer
You can also search for this author inPubMed Google Scholar - M. M. Semenova
You can also search for this author inPubMed Google Scholar - A. Zitting
You can also search for this author inPubMed Google Scholar - H. A. Koistinen
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toH. A. Koistinen.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM Figure 1
The differentiation of human primary myotubes. The formation of myotubes was monitored up to 7 days. The expression of muscle specific transcription factor myogenin was analyzed using immunocytochemistry. Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI). (PDF 1364 kb)
ESM Figure 2
Glucose uptake in L6 cells. The dose-response effect of 4 h exposure to RSV (in μmol/l) on glucose uptake in L6 myotubes (n = 3). Data are mean ± SE. **p < 0.01 vs respective basal; ***p < 0.001 vs respective basal; †p < 0.05 vs control + insulin; ‡p < 0.05 vs control. (PDF 246 kb)
ESM Figure 3
Cell viability. Cell viability was determined by MTT test in human myotubes (a, n = 3) and in L6 myotubes (b, n = 3). RSV up to 100 μM for 4 h did not affect the viability of the human (a) or L6 myotubes (b). The MTT test revealed a slight effect on viability at 200 μM RSV (revealed by a lower absorbance), however, this was significant only in L6 myotubes (b). Data are mean ± SE. *p < 0.05 vs respective control. (PDF 341 kb)
ESM Figure 4
AKT and ACCβ phosphorylation in L6 myotubes. (a) The dose-response effect of RSV on AKT-Ser473 phosphorylation in L6 myotubes (n = 3). Data are mean ± SE. *p < 0.05 vs respective basal; † p < 0.05 vs control + insulin. (b) The dose-response effect of RSV on ACCβ phosphorylation in L6 myotubes (n = 3). Data are mean ± SE. *p < 0.05 vs respective control. (PDF 832 kb)
ESM Figure 5
ER stress markers in L6 myotubes. The dose-response effect of RSV on the phosphorylation of eIF2α (a) and the expression of CHOP (b) and BiP (c) in L6 myotubes (n = 3). Results are mean ± SE. *p < 0.05 vs respective control. (PDF 1291 kb)
ESM Figure 6
The time-course of the effect of resveratrol on protein phosphorylation. Human myotubes were exposed to 100 μmol/l resveratrol for 2, 4, 8, 12 or 24 h and then stimulated with or without 100 nmol/l insulin (I) for 10 min. The phosphorylation of AKT at Ser473 (a), AMPK at Thr172 (b), ACCβ at Ser79 (c) and eIF2α at Ser52 (d) was analysed. Results are mean ± SE from 3 male volunteers. a: *p < 0.05 vs respective basal, † p < 0.05 vs control insulin at 0 h, c and d: *p < 0.05 vs respective 0 h conditions. (PDF 1403 kb)
Rights and permissions
About this article
Cite this article
Skrobuk, P., von Kraemer, S., Semenova, M.M. et al. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells.Diabetologia 55, 3051–3060 (2012). https://doi.org/10.1007/s00125-012-2691-1
- Received: 23 April 2012
- Accepted: 25 July 2012
- Published: 17 August 2012
- Issue Date: November 2012
- DOI: https://doi.org/10.1007/s00125-012-2691-1