Chemotherapy-induced nausea and vomiting (CINV) and adherence to antiemetic guidelines: results of a survey of oncology nurses (original) (raw)
Introduction
Advances in understanding the physiology of chemotherapy-induced nausea and vomiting (CINV) combined with the development of new antiemetics, particularly over the past 25 years, have dramatically improved the prevention of CINV for patients undergoing emetogenic chemotherapy [1]. In fact, the American Society of Clinical Oncology (ASCO) recently recognized the development of effective antiemetic therapies among the top five advances in oncology over the last 50 years (since ASCO’s founding in 1964) [2].
Chemotherapy-induced nausea and vomiting, and in particular vomiting, can now be prevented in 65% and ~ 85% of patients, respectively [1, 3], with the use of current evidence-based guideline-recommended antiemetic regimens [4,5,[6](/article/10.1007/s00520-017-3866-6#ref-CR6 "National Comprehensive Cancer Network (NCCN Guidelines®), Antiemesis version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
. Accessed August 2017")\]. National and international evidenced-based guidelines are regularly updated and offer health care providers a guide for selecting the most appropriate antiemetics. Combination antiemetic regimens targeting multiple molecular pathways associated with emesis have become the standard of care for prevention of CINV. The most relevant, up-to-date guidelines include those of the Multinational Association of Supportive Care in Cancer/European Society of Medical Oncology (MASCC/ESMO) \[[5](/article/10.1007/s00520-017-3866-6#ref-CR5 "Hesketh PJ, Bohlke K, Lyman G et al (2016) Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 34(4):381–386")\], ASCO \[[4](/article/10.1007/s00520-017-3866-6#ref-CR4 "Roila F, Molassiotis A, Herrstedt J, et al. (2016) MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27:119–133")\], and the National Comprehensive Cancer Network (NCCN) \[[6](/article/10.1007/s00520-017-3866-6#ref-CR6 "National Comprehensive Cancer Network (NCCN Guidelines®), Antiemesis version 2.2017.
https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
. Accessed August 2017")\]. Despite evidence suggesting that administration of guideline-recommended antiemetic prophylaxis is correlated with improved CINV control, studies have suggested that adherence to antiemetic guidelines is suboptimal, with patients frequently not receiving recommended antiemetic combinations \[[7](#ref-CR7 "Aapro M, Molassiotis A, Dicato M et al (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 23(8):1986–1992"),[8](#ref-CR8 "Gilmore JW, Peacock NW, Gu A et al (2014) Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract 10(1):68–74"),[9](#ref-CR9 "Affronti ML, Schneider SM, Schlundt S et al (2014) Adherence to antiemetic guidelines in patients with malignant glioma: a quality improvement project to translate evidence into practice. Support Care Cancer 22(7):1897–1905"),[10](/article/10.1007/s00520-017-3866-6#ref-CR10 "Roeland E, Aapro M, Schwartzberg L (2015) Advances in the management of chemotherapy-Induced nausea and vomiting: new data from recent and ongoing trials. Clinical Roundtable Monograph. Clinical Advances in Hematology & Oncology")\].CINV not only is distressing for the patient [11], but it also can lead to dehydration, electrolyte imbalances, weight loss, and malnourishment, sometimes resulting in additional office visits, emergency department (ED) visits, or hospitalizations, thus requiring additional supportive care therapies. This increase in resource utilization increases the overall cost of cancer care [12]. Furthermore, dose reductions or delays in chemotherapy may be necessary as a result of CINV, which may negatively impact patient outcomes and quality of life [13, 14].
Oncology nurses, as part of a multidisciplinary team, are in a unique position to promote and reinforce guideline-recommended antiemetic prophylaxis and to improve health care providers’ adherence to evidence-based guideline recommendations. They also serve as a primary liaison with patients, providing education and instructions pertaining to antiemetics used in the clinic and at home.
Objectives
This survey (Supplementary File 1) was designed to (1) assess oncology nurses’ awareness of antiemetic guideline recommendations for the prevention of CINV, (2) evaluate nurses’ perception of CINV control within oncology nursing practices by querying estimated control rates and proportions of patients having chemotherapy postponed/stopped/changed or requiring ED/hospital visits due to CINV, (3) explore practice patterns of antiemetic use by asking nurses to report on antiemetic agents being used, (4) determine whether practice patterns were consistent with antiemetic guideline recommendations, and (5) query their perceptions of barriers to adherence.
Methods
Survey conduct and details
ONS:Edge, a for-profit subsidiary of the Oncology Nursing Society (ONS) until 2016, was centered on the transfer of cancer knowledge to oncology nurses to improve patient outcomes. As a part of ONS, ONS:Edge had access to practicing nurse members of ONS in the ONS database. As a result, they were selected to coordinate, manage, and analyze this survey.
In September 2015, approximately 8000 practicing US-based oncology nurses were invited by email to participate in a web-based, online exploratory de novo survey. Potential de-identified participants were ONS members selected based on information provided on their ONS membership form. All those invited were nurses working in a “patient care” functional area within an oncology specialty area who regularly facilitate administration of chemotherapy to patients in either outpatient or inpatient settings. The survey consisted of 21 questions, was distributed via email providing the survey objective and a link to the online survey on September 1, 2015, and remained open for 2 weeks. A 1-week reminder email was sent out to increase the sample size (Supplementary File 1). Only responders that opted in by opening the email and followed the steps to complete this web-based survey were included.
Assessments and statistical analyses
Results are reported for all nurses who opted in by completing the survey. The proportion of the total respondents was calculated for each of the survey responses.
Participants were asked to rate their confidence in their knowledge of the emetogenicity of chemotherapy agents/regimens with response options as “very confident,” “confident,” “somewhat confident,” and “not confident at all.” Chisquare analyses were utilized to compare responses and/or evaluate trends in response based on demographical characteristics (e.g., confidence in knowledge of the emetogenicity of chemotherapy was compared across various practice settings, for different nursing roles/positions, and based on years of oncology experience).
A weighted rank score was calculated for the response regarding the greatest challenges/unmet needs in preventing and managing CINV in their practices. Respondents were asked to rank the top three responses from a list of nine; the top ranked response got three points, the second received two points, and the third received one point. These points were added with the final ranking based on total points accumulated for each response.
Guideline adherence was calculated based on actual reported use of guideline-recommended agents using the up-to-date guidelines available at the time the survey was conducted [4,5,[6](/article/10.1007/s00520-017-3866-6#ref-CR6 "National Comprehensive Cancer Network (NCCN Guidelines®), Antiemesis version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
. Accessed August 2017")\]. The agents considered adherent were consistent with the general recommendations of ASCO, MASCC, and NCCN guidelines \[[4](#ref-CR4 "Roila F, Molassiotis A, Herrstedt J, et al. (2016) MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27:119–133"),[5](#ref-CR5 "Hesketh PJ, Bohlke K, Lyman G et al (2016) Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 34(4):381–386"),[6](/article/10.1007/s00520-017-3866-6#ref-CR6 "National Comprehensive Cancer Network (NCCN Guidelines®), Antiemesis version 2.2017.
https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
. Accessed August 2017")\]. If nurses reported using the guideline-recommended agents, they were considered “adherent,” regardless of whether they were also using additional antiemetic agents.Calculation of guideline adherence was based on the following definitions of adherence.
- Highly emetogenic chemotherapy (HEC): acute phase (0–24 h, day 1) after chemotherapy initiation: neurokinin-1 receptor antagonist (NK1 RA) + serotonin receptor antagonist (5HT3 RA) + dexamethasone (DEX); delayed phase (25–120 h, days 2–5): DEX + NK1 RA or DEX + NK1 RA + 5HT3 RA (while a 5-HT3 RA is not a recommended agent in the HEC delayed phase, respondents who reported using a NK1 RA + DEX + a 5-HT3 RA were considered adherent because the minimum guideline-recommended agents (i.e., DEX + NK1 RA) were being used). This adherence calculation requiring the use of an NK1RA in the delayed phase assumes the use of an oral NK1 RA on day 1 and discounts the possible use of intravenous fosaprepitant on day 1.
- Moderately emetogenic chemotherapy (MEC): acute phase (0–24 h): 5-HT3 RA + DEX or NK1 RA + 5-HT3 RA + DEX; delayed phase (25–120 h): 5-HT3 RA or DEX.
For the open-ended question “How could adherence to antiemetic guidelines be improved in your practice,” content analysis was used to identify and group concepts expressed and calculate the frequency of those concepts. Analyses were done using IBM SPSS version 22/IBM Text Analytics for Surveys version 4.0.1.
Results
Of the 7974 respondents invited to participate, 531 (6.7%) completed the survey. The majority of nurse respondents were oncology-certified (66.7%) staff nurses (73.4%) that had > 15 years (31.1%) of oncology experience and worked full time (82.7%) in the outpatient setting (64.4%) (Table 1).
Table 1 Respondent demographics
Confidence in knowledge of chemotherapy emetogenicity
Ninety-seven percent of all respondents responded that they were at least “somewhat confident” in their knowledge of the emetogenic potential of various chemotherapeutic agents/regimens.
Nurses in the outpatient setting stated significantly greater confidence in their knowledge; 75% of those in outpatient settings said they were confident or very confident, compared to 57% of those in inpatient and 47% in the other settings (p = .002). The combined group of managers, nurse practitioners, and clinical nurse specialists (advanced nurses) were more confident in their knowledge than the combined group of staff nurses, case managers, and those in “other roles” (p = .046); 65% of staff nurses were confident or very confident, compared with 73% of managers, 70% of clinical nurse specialists, and 86% of nurse practitioners. More years in oncology (p = 0.012) and years as ONS members (p = .011) also were associated with greater confidence.
Awareness and use of antiemetic guidelines
Respondents were most familiar with (73%) and most often utilized (66%) the NCCN antiemetic guidelines in their practice settings. Approximately half of respondents were familiar with the ASCO guidelines with approximately a third using them in practice. Similarly, a third of respondents used guidelines developed within their own institutions, while familiarity and use with other guidelines such as from MASCC/ESMO was low (6%).
CINV control and impact of CINV on chemotherapy regimen/schedule, ED visits and hospitalizations
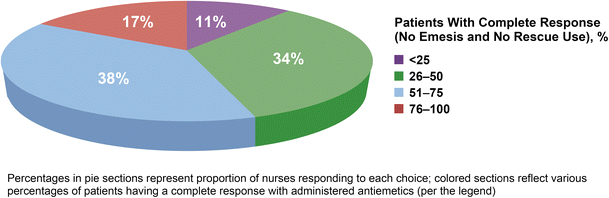
Forty-five percent of respondents reported that < 50% of their patients had CINV optimally controlled (i.e., experienced complete response [no emesis/no rescue]) with current selected antiemetics (Fig. 1). Forty-nine percent of respondents reported > 5% of their patients had chemotherapy delayed, discontinued, or dose-reduced due to CINV; 39% indicated that this occurred in 6–20% of their patients and 10% indicated this occurred in > 20% of their patients. Sixty-one percent reported having patients requiring ED visits or hospitalizations due to poorly controlled CINV during the course of treatment; this was similar in the inpatient and outpatient settings.
Fig. 1
Nurses’ perceptions of CINV control rates of their patients with currently administered antiemetics
Challenges managing CINV
The greatest perceived challenges or unmet needs in preventing and managing CINV within the respondents’ practices were reported as controlling CINV in the delayed phase and the impact of CINV on patients’ quality of life (QOL) (Fig. 2). The least significant challenges were institutional policies and access to more effective antiemetics.
Fig. 2
Greatest perceived challenges or unmet needs in preventing or managing CINV
Utilization of antiemetic agents in practice and adherence to antiemetic guidelines
HEC setting
In the HEC setting, most commonly used antiemetic combinations during the acute (0–24 h, day 1) and delayed phases (25–120 h, day 2 and beyond) were the guideline-recommended triplet combination of an NK1 RA + 5-HT3 RA + DEX (acute 73%, delayed 19%) or a 5-HT3 RA + DEX (acute 15%, delayed 36%). Key discrepancies between antiemetic use compared with guideline recommendations were underutilization of NK1 RAs on day 1 (19% not using) and high use of 5-HT3 RAs (78%) on day 2 and beyond (Fig. 3a), where the guideline recommendation is DEX and a follow-up NK1 RA if oral aprepitant was used on day 1. Despite these discrepancies, 77% of respondents felt that the antiemetics being used in their practice in the HEC setting were consistent with guideline recommendations. Calculated adherence to antiemetic guidelines was low (acute 73%, delayed 25%), particularly during the delayed phase.
Fig. 3
Classes of antiemetics being used to prevent CINV in the HEC setting (a) and the MEC setting (b)
MEC setting
In the MEC setting on day 1, a 5-HT3 RA + DEX was the most common antiemetic combination (66%) with the NK1 RA + 5-HT3 RA + DEX triplet regimen being used by 20% of respondents. During the delayed phase, the prominent combination was the 5-HT3 RA + DEX (41%). The guideline suggested agents of a 5-HT3 RA (NCCN) or DEX (NCCN/ASCO) during the delayed phase were underutilized regardless of whether used alone (30% and 10%, respectively) or in combination with each other (41% of respondents). An NK1RA was reported to be administered infrequently during the delayed phase [alone (0.5%), with a 5-HT3 RA (1%), with DEX (2%), or with both 5-HT3 RA and DEX (6%)]. The substantial use of phenothiazines (47%) and benzodiazepines (30%) on day 2 and beyond is inconsistent with guideline recommendations (Fig. 3b). Despite these inconsistencies with guideline recommendations, 74% of respondents felt that the antiemetics being used in their practice for patients receiving MEC were aligned with guideline recommendations and, in fact, the calculated adherence rates were high at 85% in the acute phase and 91% in the delayed phase.
Barriers interfering with utilizing recommended antiemetic regimens and approaches for improving adherence to antiemetic guidelines
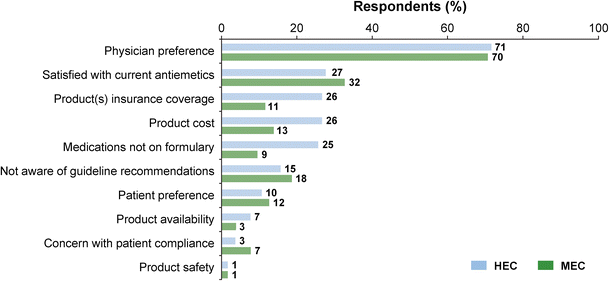
“Physician preference” was perceived by respondents (~ 3 times more) as the predominant barrier interfering with use of guideline-recommended antiemetic prophylaxis (Fig. 4). Responses to the open-ended question “How could adherence to antiemetic guidelines be improved in your practice” were given by 59 individuals (11.1% of respondents).
Fig. 4
Reported barriers/reasons interfering with using guideline-recommended antiemetics
Approaches that nurses suggested for improving adherence to guidelines included education, use of standardized protocols and orders, improving patient teaching, and follow-up. Education of providers (physicians, pharmacists, and nurses) was the most frequent suggestion, with physician education the most frequently noted. The next most common statement made was having practitioners follow current guidelines, although no additional statement pertaining to how to accomplish this was included. A few respondents made statements identifying specific medications that should be added to current practices; the addition of DEX and an NK1 RA were specifically mentioned in this regard.
Discussion
This survey revealed an opportunity to increase awareness of international antiemetic guidelines. Considering that these were US-based nurses, it was not surprising that nurses were most familiar with and used the US-based NCCN guidelines within their practices. About half (48%) were aware of ASCO guidelines, and despite the fact that MASCC comprised a panel of international antiemetic experts, the awareness and utilization of this guideline within these US practices were low.
While patient-specific risk factors certainly play a role in determining a patient’s emetic risk [15,16,17,18], emetogenicity of the chemotherapy remains the predominant factor used by guideline committees in determining emetic risk and appropriate antiemetic prophylaxis. The knowledge gap between nurses in the outpatient setting (where 75% indicated confidence in their understanding of chemotherapy emetogenicity) versus inpatient (57%) and other (47%) settings suggests a need for education in these areas.
Approximately 75% of nurses felt that the antiemetics being used in their practice were consistent with guideline recommendations. However, CINV is not optimally controlled as reflected in nurses’ reports of low control rates, high proportions of patients with ED visits/hospitalizations, and the fact that some patients are postponing or stopping their chemotherapy as a result of CINV. Similar findings were seen in a recent Internet-based survey of nurses and physicians where 32% of health care providers delayed or discontinued a patient’s chemotherapy regimen in the previous year because of CINV [14]. The discrepancy between the reports of inadequate CINV control and the nurses’ belief that guidelines are being followed highlights a critical need to increase awareness of the specifics within the guideline recommendations and address barriers for administering appropriate antiemetics.
Practice patterns of antiemetic use revealed inconsistencies with guideline recommendations in both HEC and MEC settings and particularly during the delayed phase. Benzodiazepines and phenothiazines, neither of which are guideline-recommended agents, are being overutilized in the delayed phase in both settings. With the caveat that adherence calculations did not account for using additional antiemetics beyond those which are recommended, the calculated guideline adherence suggests that a reasonable proportion of respondents’ practices are following the recommendations in the MEC setting, where 85% and 91% were using antiemetics consistent with guidelines in the acute and delayed phases, respectively. However, in the HEC setting, NK1 RAs are seemingly underutilized, with a guideline adherence rate of 73% in the acute phase and 25% in the delayed phase. As minimal changes have occurred to the guidelines since this survey was conducted, the adherence rates and conclusions from this survey remain applicable. Guideline adherence revealed in this survey was consistent with prior studies indicating that adherence to antiemetic guidelines was unacceptably low [7,8,9]; these studies also revealed that guideline-consistent antiemetic prophylaxis correlated with improved CINV prevention. In the referenced US-based study, similar results to this survey were observed with adherence at its lowest in the delayed phase of the HEC setting (29% adherence).
Unfortunately, few studies have evaluated barriers interfering with administration of guideline-recommended antiemetic regimens and approaches to improving adherence to guidelines [3]. A common theme from the limited research is that communicating CINV outcomes to physicians is key and that multifaceted strategies are necessary, as a single approach has little, if any impact [3]. This survey queried nurses’ perceptions of the barriers to adherence. “Physician preference” was by far the predominant barrier interfering with administration of guideline-recommended prophylactic treatment; a better understanding of this is important in order for this to be addressed. Suggestions to improve adherence focused on education of health care providers, use of standardized protocols, electronic orders incorporating evidence-based guideline recommended antiemetics, and improving patient teaching and follow-up. Improved communication between patients, doctors, and nurses may also be helpful as would the utilization of a tool such as the MASCC Antiemesis tool (MAT) to increase awareness of CINV control (or lack thereof).
The nurses’ rankings of “preventing CINV in the delayed phase” and “managing its impact on patients’ quality of life” as the greatest challenges for them validates the findings seen in practice patterns, where antiemetics given during the delayed phases are most inconsistent with guideline recommendations. Other challenges included preventing CINV in the acute phase and patient adherence with antiemetics. Interestingly, lack of access to more effective antiemetics ranked low, suggesting that nurses believe that the agents available today should effectively prevent CINV in the majority of patients.
While the results of this survey of oncology nurses are enlightening, it is important to address its limitations. The proportion of invitees completing the survey was low (only 7%); the short duration of recruitment of survey participants (i.e., 2 weeks) likely contributed to this. Also, it is unclear whether the survey sample reflects a similar profile as the general membership of ONS. While the demographics of the respondents appear to reflect a reasonably heterogeneous sample of the US oncology nursing population, it is impossible to know for certain if the responses are biased or if they reflect a broad range of clinical practices within the USA. In addition, the question asking whether respondents had patients requiring ED visits/hospitalizations due to uncontrolled CINV was too general and did not incorporate a time frame relative to the administration of chemotherapy nor did it explore the proportion of their patients with ED visits/hospitalizations. Given the likely severity of CINV leading to these events, further information on this topic would have been very interesting. As with any survey, the answers prompt readers to have follow-up questions not available within the survey such as why older agents such as benzodiazepines and phenothiazines continue to be frequently used when more effective antiemetics are available or why physician preference ranked as the number one barrier to administration of guideline-recommended agents. In addition, understanding physician perspectives within these same practices compared to nurses could provide compelling insights to be examined further.
Conclusions
With the broad antiemetic armamentarium of effective agents available, it is unfortunate that CINV control for some patients continues to be suboptimal, that practice patterns of antiemetic use suggest that guideline-recommended antiemetic combinations are not always being administered, and that challenges exist for managing CINV and its impact on patients in clinical practice. This survey highlights opportunities to increase awareness and education of evidence-based antiemetic guidelines, develop multidisciplinary efforts to increase adherence to guidelines, and identify practical multifaceted approaches for overcoming barriers that interfere with the use of guideline-recommended antiemetics. The ultimate goal is to provide appropriate prophylaxis of CINV and improve quality of life for all patients receiving emetogenic chemotherapy, thereby decreasing costs, ED visits, and re-hospitalization rates due to inadequate treatment, and allow patients to complete their chemotherapy as planned.
Oncology nurses, as part of a multidisciplinary team, are in a unique position to promote and reinforce guideline-recommended antiemetic prophylaxis and to improve health care providers’ adherence to evidence-based guideline recommendations. In addition, given their role in educating patients and caregivers on the administration of follow-up antiemetics at home, they can offer guidance and education to patients and caregivers on the importance of taking antiemetics as prescribed during the delayed phase in order to optimize outcomes. Nurses are an integral part of efforts to improve adherence to antiemetic guideline recommendations and prevention of CINV in the USA and globally. In addition, this survey focused on the role of oncology nurses and physicians; however, other health care providers such as pharmacists also play important roles in the treatment decisions of patients, especially in the oncology setting.
References
- Navari R, Aapro M (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. NEJM 374:1356–1367
Article CAS PubMed Google Scholar - ASCO 50th anniversary poll names top 5 advances past 50 years. The ASCO Post, October 15, 2014
- Jordan K, Jahn F, Aapro M (2015) Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol 26(6):1081–1090
Article CAS PubMed Google Scholar - Roila F, Molassiotis A, Herrstedt J, et al. (2016) MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27:119–133
- Hesketh PJ, Bohlke K, Lyman G et al (2016) Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 34(4):381–386
Article PubMed Google Scholar - National Comprehensive Cancer Network (NCCN Guidelines®), Antiemesis version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed August 2017
- Aapro M, Molassiotis A, Dicato M et al (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 23(8):1986–1992
Article CAS PubMed Google Scholar - Gilmore JW, Peacock NW, Gu A et al (2014) Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract 10(1):68–74
Article PubMed Google Scholar - Affronti ML, Schneider SM, Schlundt S et al (2014) Adherence to antiemetic guidelines in patients with malignant glioma: a quality improvement project to translate evidence into practice. Support Care Cancer 22(7):1897–1905
Article PubMed PubMed Central Google Scholar - Roeland E, Aapro M, Schwartzberg L (2015) Advances in the management of chemotherapy-Induced nausea and vomiting: new data from recent and ongoing trials. Clinical Roundtable Monograph. Clinical Advances in Hematology & Oncology
- de Boer-Dennert M, de Wit R, Schmitz PIM et al (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Article PubMed PubMed Central Google Scholar - Schwartzberg L, Harrow B, Lal L et al (2015) Resource utilization for chemotherapy-induced nausea and vomiting events for patients with solid tumors treated with antiemetic regimens. Am Health Drug Benefits 8(5):273–282
PubMed PubMed Central Google Scholar - Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358:2482–2494
Article CAS PubMed Google Scholar - Van Laar ES, Desai JM, Jatoi A (2015) Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providers. Support Care Cancer 23:151–157
Article PubMed PubMed Central Google Scholar - Dranitsaris G et al (2009) Identifying patients at high risk for nausea and vomiting after chemotherapy: development of a practical prediction tool I Acute nausea and vomiting. J Support Oncol 7:W1–W8
Google Scholar - Petrella T et al (2009) Identifying patients at high risk for nausea and vomiting after chemotherapy: development of a practical prediction tool II Delayed nausea and vomiting. J Support Oncol 7:W9–W16
Google Scholar - Dranitsaris G, Bouganim N, Milano C et al (2013) Prospective validation of a prediction tool for identifying patients at high risk for chemotherapy-induced nausea and vomiting. J Support Oncol 11(1):14–21
PubMed Google Scholar - Molassiotis A, Stamataki Z, Kontopantelis E et al (2013) Development and preliminary validation of risk prediction model for chemotherapy-induced nausea and vomiting. Support Care Cancer 21(10):2759–2767
Article CAS PubMed Google Scholar