Comparison of the major flavonoid content of S. baicalensis, S. lateriflora, and their commercial products (original) (raw)
Abstract
According to the notification for definition of pharmaceuticals from the Director-General of the Pharmaceutical and Food Safety Bureau, Ministry of Health Labour and Welfare of Japan, the roots of Scutellaria baicalensis (Chinese skullcap) and S. lateriflora (skullcap) are classified as “the raw materials exclusively used as pharmaceuticals”, but their aerial parts are classified as “non-pharmaceuticals” so, in principle, there are no health claims for these materials and no descriptions of drug-like dosages or administration directions. Dried root of S. baicalensis is also registered in Japanese Pharmacopoeia XV as scutellaria root. Scutellaria root is considered to have the adverse drug reactions of interstitial pneumonia and drug-induced hepatopathy in kampo medicines (Japanese traditional herbal formulations), and baicalin, its major constituent, is considered to be the cause of the adverse reaction. This study was conducted to evaluate the validity of this borderline between pharmaceuticals and non-pharmaceuticals by analyzing the amounts of four flavonoids, including baicalin, in the roots, stems, and leaves of S. baicalensis and S. lateriflora, and in the commercial products herbal tea and dietary supplements prepared from S. lateriflora. These flavonoids were found in the root of S. baicalensis; its aerial parts, however, did not contain them. On the other hand, the amounts of those flavonoids in the aerial parts of S. lateriflora were larger than in the root. Herbal tea and dietary supplements of S. lateriflora obtained commercially also contained those flavonoids, and the dietary supplements contained amounts of them comparable with that in kampo medicine. These results suggest that classification that the aerial parts of S. lateriflora as non-pharmaceuticals in Japan needs reconsideration.
Access this article
Subscribe and save
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime Subscribe now
Buy Now
Price excludes VAT (USA)
Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
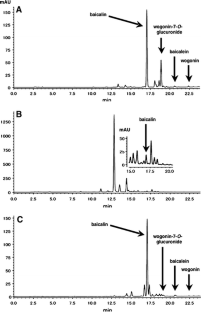
Fig. 1
Similar content being viewed by others
References
- The Society of Japanese Pharmacopoeia (2007) The Japanese pharmacopoeia (JP XV), 15th edn. Yakuji-Nippo, Tokyo
Google Scholar - The Japan Society for Oriental Medicine (2005) Introduction to KAMPO. Elsevier, Tokyo
Google Scholar - Tsao TF, Newman MG, Kwok YY, Horikoshi AK (1982) Effect of Chinese and western antimicrobial agents on selected oral bacteria. J Dent Res 61:1103–1106
PubMed CAS Google Scholar - Huang WH, Lee AR, Yang CH (2006) Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci Biotechnol Biochem 70:2371–2380
Article PubMed CAS Google Scholar - Park S, Hahm KB, Oh TY, Jin JH, Choue R (2004) Preventive effect of the flavonoid, wogonin, against ethanol-induced gastric mucosal damage in rats. Dig Dis Sci 49:384–394
Article PubMed CAS Google Scholar - Itoh Y, Sendo T, Oishi R (2006) Drug-induced hepatopathy. Folia Pharmacol Jpn 127:425–432
Article CAS Google Scholar - Yafune A, Tsutani K (1996) Hepatic injury induced by Scutellaria species and hepatic dysfunction caused by kampo formulations including ogon (Scutellariae Radix). Jpn J Clin Pharmacol Ther 27:635–345
Google Scholar - Terada M, Kitazawa H, Kawakami J, Adachi I (2002) Pharmacoepidemiology of interstitial pneumonia and liver dysfunction associated with kampo medicine. Jpn J Pharm Health Care 28:425–434
Google Scholar - Nakazawa Y, Suzuki S, Negichi E, Nakazaki M, Ueno K, Akiba T (2006) Investigation of changes in ALT levels after administration of kampo medicines at Akiba hospital. Jpn J Pharm Health Care 32:504–510
Google Scholar - Ministry of Health, Labour and Welfare of Japan (2001) Notification No. 243 (March 27, 2001) from Director-General of the Pharmaceutical and Food Safety Bureau, MHLW; partially revised by Notification No. 1115003 (April 17, 2007)
- Lininger SW, Gaby AR, Austin S, Brown DJ, Wright JV, Duncan A (2000) The natural pharmacy. Random House, NY
Google Scholar - Ishimaru K, Nishikawa K, Omoto T, Asai I, Yoshihira K, Shimomura K (1995) Two flavone 2′-glucosides from Scutellaria baicalensis. Phytochemistry 40:279–281
Article PubMed CAS Google Scholar - American Herbal Products Association (1997) Botanical safety handbook. CRC Press, Boca Raton
Google Scholar
Acknowledgments
We are grateful to Dr Nobuhiro Ohtake, Tsumura Co. Ltd. for kind supply of wogonin-7-_O_-glucuronide. This work was supported by a Grant-in Aid for Scientific Research of the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
- Graduate School of Pharmaceutical Sciences, Nagoya City University, 3-1 Tanabe-dori, Mizuho-ku, Nagoya, 467-8603, Japan
Toshiaki Makino & Hajime Mizukami - Research Center for Medicinal Plant Resources, Tsukuba Division, 1-2 Yawata-dai, Tsukuba, 305-0843, Japan
Atsuyuki Hishida - National Institute of Health Sciences (NIHS), 1-18-1 Kamiyoga, Setagaya-ku, Tokyo, 158-8501, Japan
Yukihiro Goda
Authors
- Toshiaki Makino
You can also search for this author inPubMed Google Scholar - Atsuyuki Hishida
You can also search for this author inPubMed Google Scholar - Yukihiro Goda
You can also search for this author inPubMed Google Scholar - Hajime Mizukami
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toToshiaki Makino.
Rights and permissions
About this article
Cite this article
Makino, T., Hishida, A., Goda, Y. et al. Comparison of the major flavonoid content of S. baicalensis, S. lateriflora, and their commercial products.J Nat Med 62, 294–299 (2008). https://doi.org/10.1007/s11418-008-0230-7
- Received: 19 October 2007
- Accepted: 09 January 2008
- Published: 15 February 2008
- Issue Date: July 2008
- DOI: https://doi.org/10.1007/s11418-008-0230-7