Crystal structure of Na4[Cu4O2(SO4)4]·MeCl (Me: Na, Cu, ☐) – the synthetic Na-analogue of piypite (caratiite) | Mineralogical Magazine | Cambridge Core (original) (raw)
Abstract
The crystal structure of Na4[Cu4O2(SO4)4] MeCl (Me: Na,Cu,☐) has been solved by direct methods and refined to R1 = 0.028 using 2466 independent reflections. The compound is tetragonal with space group P4/n, a = 18.451(1)Å, c = 4.9520(2)Å, Z = 4. The two main building units running parallel to the c-axis comprise: (1) slabs containing SO4 and OCu4 tetrahedra; and (2) chains of corner sharing Me(Cl,O)6 octahedra. The displacement ellipsoids of the atoms occupying the Me sites and of the Cl ions indicate a short-range static disorder. Copper in the slabs exhibits a (4+1) coordination. Both elements form a tunnel structure in which additional Na atoms are incorporated for charge compensation. The structure is closely related to the mineral piypite (K4Cu4O2(SO4)4 MeCl (Me: Na,Cu,☐)): 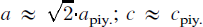 . Furthermore, the structure has a pronounced pseudo-translational symmetry: apart from some of the oxygen sites, all of the nineteen crystallographically distinct positions are related by a pseudo F centring.
. Furthermore, the structure has a pronounced pseudo-translational symmetry: apart from some of the oxygen sites, all of the nineteen crystallographically distinct positions are related by a pseudo F centring.
Type
Research Article
Copyright
Copyright © The Mineralogical Society of Great Britain and Ireland 2000
References
Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M.C., Polidori, G. and Camalli, M. (1992) SIR92 – a program for automatic solution of structures by direct methods. J. Appl. Crystallogr., 27, 435.Google Scholar
Effenberger, H. and Zemann, J. (1984) The crystal structure of caratiite. Mineral. Mag., 48, 541–6.CrossRefGoogle Scholar
Effenberger, H. (1985 a) Zur chemischen Zusammensetzung von Caratiit. Mitt. Österr. Miner. Ges., 130, 29-31.Google Scholar
Effenberger, H. (1985 b) Cu2O(SO4) Dolerophanite: refinement of the crystal structure, with a comparison of [OCu(II)4] tetrahedra in inorganic compounds. Monatshefte für Chemie, 116, 927–31.CrossRefGoogle Scholar
Giacovazzo, C., Scandale, E. and Scordari, F. (1976) The crystal structure of chlorothionite, CuK2Cl2SO4 . Zeits. Kristallogr., 144, 226–37.CrossRefGoogle Scholar
Hovestreydt, E. (1983) On the atomic scattering factor of O2– . Acta Crystallogr., A39, 268–9.CrossRefGoogle Scholar
Ibers, J.A. and Hamilton, W.C. (editors) (1974) International Tables for X-ray crystallography, Vol. IV. The Kynoch Press, Birmingham, UK.Google Scholar
Jambor, J.L. and Puziewicz., J. (1990) New Mineral Names. Amer. Mineral., 75, 1209–16.Google Scholar
Krivovichev, S.V., Filatov, S.K. and Semenova, T.F. (1998) Types of cationic complexes based on oxocentred tetrahedra [OM4] in the crystal structures of inorganic compounds. Russ. Chem. Rev., 67, 137–55.CrossRefGoogle Scholar
Sheldrick, G.M. (1993) SHELXL-93. A program for the refinement of crystal structures. Universität Göttingen, Germany.Google Scholar
Spek, A.L. (1999) PLATON, A multipurpose crystallographic tool. Utrecht University, Utrecht, The Netherlands.Google Scholar
Varaksina, T.V., Fundamensky, V.S., Filatov, S.K. and Vergasova, L.P. (1990) The crystal structure of kamchatkite, a new naturally occuring oxychloride sulphate of potassium and copper. Mineral. Mag., 54, 613–6.CrossRefGoogle Scholar
Wilson, A.J.C. (editor) (1995) International Tables for Crystallography, Vol. C. Kluwer Academic Publishers, London.Google Scholar