Heart Transplantation: Practice Essentials, Background, Disease Processes Necessitating Heart Transplantation (original) (raw)
Practice Essentials
Heart transplantation is the replacement of a failing heart with a heart from a suitable donor. [1] After a decline between 1993 and 2004, heart transplant volumes reported to the International Society of Heart and Lung Transplantion (ISHLT) Transplant Registry have been steadily increasing, especially in recent years, with more than 6,000 heart transplants performed annually worldwide. [2]
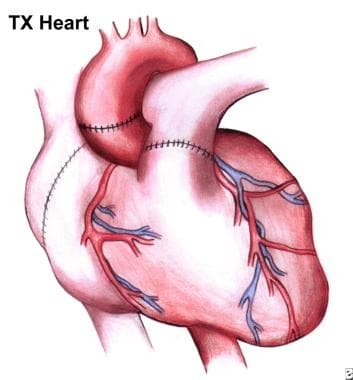
Heart transplantation (HTx) has become the preferred therapy for select patients, with a 1-year survival of almost 90% and a conditional half-life (the time at which 50% of patients who survived the first year are still alive) of 13 years. [3] See the image below.
Completed operation. Note suture lines on now-implanted heart.
Indications for heart transplantation
Heart transplantation is generally reserved for patients with end-stage chronic heart failure (CHF) who are estimated to have less than 1 year to live without the transplant and who are not candidates for or have not been helped by conventional medical therapy. Because of the poor condition of their heart, most heart transplantation candidates are excluded from other surgical options. Specific indications for a transplant include the following:
- Dilated cardiomyopathy
- Ischemic cardiomyopathy
- Congenital heart disease for which no conventional therapy exists or for which conventional therapy has failed
- Ejection fraction of less than 20%
- Intractable angina or malignant cardiac arrhythmias for which conventional therapy has been exhausted
- Pulmonary vascular resistance of less than 2 Wood units
- Age younger than 65 years
- Ability to comply with medical follow-up care
Workup
Evaluation of the heart transplant candidate includes laboratory tests, imaging studies, and other tests as appropriate.
Laboratory studies
- Virus: Including hepatitis viruses, human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV); used to determine past exposure and currently active disease
- Fungus and tuberculosis (TB): Used to determine past exposure and to predict reactivation
- Prostate-specific antigen (PSA): If elevated, initiate appropriate evaluation and therapy before completing the evaluation for transplantation
- Papanicolaou test: Results should be negative before listing for transplantation
- Complete blood count (CBC): With differential, platelet count, prothrombin time (PT), activated partial thromboplastin time (aPTT), and a complete chemistry profile (including liver panel, lipid profile, and urinalysis)
- Blood typing and screening, panel-reactive antibody (PRA) testing, and tissue typing: Used to determine the immunologic suitability of the patient for transplantation and donor matching
Imaging studies
- Coronary arteriography: Performed in cases of cardiomyopathy to determine if the cause of the cardiac dysfunction may be amenable to conventional therapies
- Echocardiography: Used to determine the cardiac ejection fraction and to monitor the cardiac function of patients on the transplantation waiting list
- Posteroanterior and lateral chest radiographs: Used to screen for other thoracic pathologies that may preclude transplantation.
- Bilateral mammograms: Should reveal no abnormalities before listing for transplantation
Cardiac and pulmonary evaluation
- Maximal venous oxygen consumption (MVO2): Used to assess overall cardiac function and as a predictor of the severity of congestive heart failure and survival
- Right- and left-heart catheterization: Used to determine if the disease process is reversible or treatable by more conventional therapy
- Pulmonary vascular resistance: Patients with fixed resistances above 4 Wood units are not candidates for heart transplantation
Biopsy
Endomyocardial biopsy of the potential candidate is not routinely performed. The procedure may be considered if a systemic process involving the heart is thought to be the cause of the cardiomyopathy.
Perform biopsies of appropriate areas if the patient exhibits symptoms of systemic disease. Biopsies are used to determine the extent and activity of the disease process. Systemic disease processes are a contraindication to cardiac transplantation.
Transplantation procedures
A cardiac allograft can be sewn in either a heterotopic or an orthotopic position. In heterotopic transplantation, the surgeon connects the allograft to the native heart in a parallel fashion. In orthotopic transplantation, the donor heart replaces the recipient heart.
Heterotopic heart transplantation
Heterotopic transplantation is an excellent technique for patients with severe pulmonary hypertension. Inherent problems with the technique, however, include pulmonary compression of the recipient, difficulty obtaining an endomyocardial bi;opsy, and the need for anticoagulation.
Orthotopic heart transplantation
In the orthotopic cardiac transplantation procedure, the recipient's ventricles are excised, leaving the great vessels; atrial excision varies with the surgical technique being employed. The donor heart is then sewn to these areas. Either of the following techniques may be used:
- Shumway-Lower (biatrial) technique (anastomosis of the donor's anterior atria to the posterior wall of the recipient’s atria): This older method is simpler and saves perhaps 10-15 minutes of ischemic time.
- Bicaval anastomosis (the recipient's left atrium is prepared as a single cuff incorporating all four pulmonary venous orifices, while the right atrium is preserved): One advantage of the bicaval method is that, by avoiding a large right atrium, the surgeon can maintain better atrial transport; another claimed advantage of this technique is a lower reported incidence of tricuspid regurgitation
Immunosuppressive therapy
Immunosuppression is started soon after surgery. Several regimens can be used, including pretransplantation induction therapy and simple postoperative maintenance therapy; the choice of regimen depends on the training and experience of the transplant center. [4, 5]
Most maintenance immunosuppressive protocols after HTx use a 3-drug regimen consisting of a calcineurin inhibitor (CNI) (cyclosporine or tacrolimus), an antimetabolite agent (mycophenolate mofetil or azathioprine), and tapering doses of corticosteroids over the first year post-transplantation. [3]
Complications
Posttransplant complications can include the following:
- Bleeding from suture lines
- Hyperacute rejection
- Infection
- Psychiatric disturbances from steroid therapy
- Cardiac rejection
- Allograft vascular diseaseatient education
For patient education resources, see the Heart Transplant Directory.

Background
Heart transplantation is generally reserved for patients with end-stage chronic heart failure (CHF) who are estimated to have less than 1 year to live without the transplant and who are not candidates for, or have not been helped by, conventional medical therapy. In addition, most candidates are excluded from other surgical options because of the poor condition of the heart.
Candidacy determination and evaluation are key components of the process, as are postoperative follow-up care and immunosuppression management. Proper execution of these steps can culminate in an extremely satisfying outcome for both the physician and patient. [6]
Candidates for cardiac transplantation generally present with New York Heart Association (NYHA) class III (moderate) symptoms or class IV (severe) symptoms. [7] Evaluation demonstrates ejection fractions of less than 25%. Attempts are made to stabilize the cardiac condition while the evaluation process is undertaken.
Interim therapy can include oral agents as well as inotropic support. Mechanical support with the intra-aortic balloon pump (IABP) or implantable assist devices may be appropriate in some patients as a bridge to transplantation. [8, 9, 10] However, mechanical support does not improve waiting list survival in adult patients with congenital heart disease. [11]
The annual frequency of heart transplantation is about 1% of the general population with heart failure (both candidates and noncandidates). Improved medical management of CHF has decreased the candidate population; however, organ availability remains an issue. [12, 13] Further information on organ availability and waiting lists is available from the United Network for Organ Sharing.

Disease Processes Necessitating Heart Transplantation
The disease processes that necessitate cardiac transplantation can be divided into the following categories:
- Dilated cardiomyopathy (54%) - This often has an unclear origin
- Ischemic cardiomyopathy (45%) - This percentage is rising because of the increase in coronary artery disease (CAD) in younger age groups
- Congenital heart disease and other diseases not amenable to surgical correction (1%)
The pathophysiology of cardiomyopathy that may necessitate cardiac replacement depends on the primary disease process. Chronic ischemic conditions precipitate myocardial cell damage, with progressive enlargement of the myocyte followed by cell death and scarring. The condition can be treated with angioplasty or bypass; however, the small-vessel disease is progressive and thus causes progressive loss of myocardial tissue. This eventually results in significant functional loss and progressive cardiac dilatation.
The pathologic process involved in the functional deterioration of a dilated cardiomyopathy is still unclear. Mechanical dilatation and disruption of energy stores appear to play roles.
The pathophysiology of the transplanted heart is unique. The denervation of the organ makes it dependent on its intrinsic rate. As a result of the lack of neuronal input, some left ventricular hypertrophy results. The right-side function is directly dependent on the ischemic time before reimplantation and the adequacy of preservation. The right ventricle is easily damaged and may initially function as a passive conduit until recovery occurs.
The rejection process that can occur in the allograft has 2 primary forms, cellular and humoral. Cellular rejection is the classic form of rejection and is characterized by perivascular infiltration of lymphocytes with subsequent myocyte damage and necrosis if left untreated.
Humoral rejection is much more difficult to characterize and diagnose. It is thought to be a generalized antibody response initiated by several unknown factors. The antibody deposition into the myocardium results in global cardiac dysfunction. This diagnosis is generally made on the basis of clinical suspicion and exclusion; endomyocardial biopsy is of little value in this context.
CAD is a late pathologic process common to all cardiac allografts, characterized by myointimal hyperplasia of small and medium-sized vessels. The lesions are diffuse and may appear any time from 3 months to several years after implantation. The inciting causes are unclear, though cytomegalovirus (CMV) infection and chronic rejection have been implicated. The mechanism of the process is thought to depend on growth-factor production in the allograft initiated by circulating lymphocytes. Currently, there is no treatment other than retransplantation.

Indications
The general indications for cardiac transplantation include deteriorating cardiac function and a prognosis of less than 1 year to live. Specific indications include the following:
- Dilated cardiomyopathy
- Ischemic cardiomyopathy
- Congenital heart disease for which no conventional therapy exists or for which conventional therapy has failed
- Ejection fraction less than 20%
- Intractable angina or malignant cardiac arrhythmias for which conventional therapy has been exhausted
- Pulmonary vascular resistance of less than 2 Wood units
- Age younger than 65 years
- Ability to comply with medical follow-up care
The 2016 International Society for Heart Lung Transplantation updated criteria for heart transplantation are as follows [14, 15] :
- Heart failure prognosis scores should be performed along with cardiopulmonary exercise testing to determine prognosis and guide listing for transplantation for ambulatory patients. An estimated 1-yr survival of < 80%, as calculated by the Seattle Heart Failure Model (SHFM), or a Heart Failure Survival Score (HFSS) in the high/medium risk range should be considered as reasonable cut points for listing
- Patients should not be listed solely on the criteria of heart failure survival prognostic scores
- Right heart catheterization (RHC) should be performed on all adult candidates in preparation for listing for cardiac transplantation and periodically until transplantation**.**
- After left ventricular assist device (LVAD), reevaluation of hemodynamics should be done after 3-6 mo to ascertain reversibility of pulmonary hypertension
- Pre-transplant body mass index (BMI) > 35 kg/m2 is associated with a worse outcome after cardiac transplantation; for such obese patients, it is reasonable to recommend weight loss to achieve a BMI of ≤35 kg/m2 before listing for cardiac transplantation.
- It is reasonable to consider the presence of irreversible renal dysfunction (estimated glomerular filtration rate [eGFR] < 30 ml/min/1.73 m2) as a relative contraindication for heart transplantation alone.
- Clinically severe symptomatic cerebrovascular disease may be considered a contraindication to transplantation
- Assessment of frailty (3 of 5 possible symptoms, including unintentional weight loss of ≥10 lb within the past year, muscle loss, fatigue, slow walking speed, and low levels of physical activity) could be considered when assessing candidacy
- Use of mechanical circulatory support should be considered for patients with potentially reversible or treatable comorbidities, such as cancer, obesity, kidney failure, tobacco use, and pharmacologically irreversible pulmonary hypertension, with subsequent reevaluation to establish candidacy
- Any patient for whom social supports are deemed insufficient to achieve compliant care in the outpatient setting may be regarded as having a relative contraindication to transplant. The benefit of heart transplantation in patients with severe cognitive-behavioral disabilities or dementia (eg, self-injurious behavior, inability to ever understand and cooperate with medical care) has not been established and has the potential for harm; therefore, heart transplantation cannot be recommended for this subgroup of patients
- Retransplantation is indicated for those patients who develop significant cardiac allograft vasculopathy (CAV) with refractory cardiac allograft dysfunction, without evidence of ongoing rejection
The Organ Procurement and Transplantation Network (OPTN) assigns all transplant candidates a status based on their severity of illness, geographic distance between the donor and recipient, length of time on the waitlist, and blood group compatibility. The current OPTN adult heart allocation policy, updated in 2018, are outlined in the table below. [16]
Table. Organ Procurement and Transplantation Network Adult Heart Allocation Criteria for Medical Urgency Status (Open Table in a new window)
| Status | Criteria |
|---|---|
| 1 | Venoarterial extracorporeal membrane oxygenation (VA ECMO)Non-dischargeable, surgically implanted, non-endovascular biventricular support deviceMechanical circulatory support device (MCSD) with life-threatening ventricular arrhythmias |
| 2 | Non-dischargeable, surgically implanted, non-endovascular left ventricular assist device (LVAD)Intra-aortic balloon pump (IABP)Ventricular tachycardia/fibrillation, mechanical support not requiredMCSD with device malfunction/mechanical failureTotal artificial heart, biventricular assist device, right ventricular assist device, or ventricular assist device for single-ventricle patientsPercutaneous endovascular MCSD |
| 3 | Dischargeable LVAD for discretionary 30 daysMultiple inotropes or single high-dose inotrope with continuous hemodynamic monitoringVA ECMO after 7 days; percutaneous endovascular circulatory support device or IABP after 14 daysNon-dischargeable, surgically implanted, non-endovascular LVAD after 14 daysMCSD with one of the following: device infection, hemolysis, pump thrombosis, right heart failure, mucosal bleeding, aortic insufficiency |
| 4 | Dischargeable LVAD without discretionary 30 daysInotropes without hemodynamic monitoringRetransplantDiagnosis of one of the following: congenital heart disease (CHD), ischemic heart disease with intractable angina, hypertrophic cardiomyopathy, restrictive cardiomyopathy, amyloidosis |
| 5 | On the waitlist for at least one other organ at the same hospital |
| 6 | All remaining active candidates |

Contraindications
Contraindications for heart transplantation include the following:
- Age older than 65 years - This is a relative contraindication; patients who are older than 65 years are evaluated on an individual basis
- Fixed pulmonary vascular resistance of greater than 4 Wood units
- Active systemic infection
- Active malignancy - Patients with prior malignancies who have demonstrated a 3- to 5-year disease-free interval may be considered, depending on the tumor type and the evaluating program
- An ongoing history of substance abuse (eg, alcohol, drugs, tobacco)
- Psychosocial instability
- Inability to comply with medical follow-up care [17]

Outcomes
The 1-year survival rate after cardiac transplantation is almost 90%, and the conditional half-life (the time at which 50% of patients who survived the first year are still alive) is 13 years. [3] After transplantation, adult patients with congenital heart disease have high 30-day mortality but better late survival. [11] The functional status of the recipient after the procedure is generally excellent, depending on the his or her level of motivation.
In patients with severe biventricular failure who received pneumatic biventricular assist devices as a bridge to transplant, the 1-year actuarial survival rate was 89%, compared with 92% in patients without a ventricular assist device. [18]
Hypertension, diabetes mellitus, and obesity are associated with exponential increases in postoperative mortality rates. Heart transplant recipients with all three of these metabolic risk factors were found to have a 63% increased mortality compared to patients without any of the risk factors. [19]
Arnaoutakis et al found that high-risk patients had better 1-year survival rates at high-volume centers (ie, centers that perform more than 15 procedures per year) than at lower-volume centers (79% vs 64%, respectively). These differences dissipated among lower-risk patients. Based on these findings, the authors recommended that all high-risk heart transplantation procedures be performed at higher-volume centers. [20]

Future and Controversies
The future of cardiac transplantation will be determined by the outcomes of several issues. One is the ongoing shortage of donor organs. In Europe, waiting time has tripled since 2000 and now exceeds one year. In order to address this shortage, the median age of donors has increased from 30 to almost 45 years. Even hearts from donors aged > 60 years are commonly used, with outcomes only slightly inferior to that of hearts from young donors. [21] The advent of effective antiviral treatment has allowed the use of hearts from donors with hepatitis C virus infection. [22, 23]
Novel technological solutions for organ preservation, such as warm machine perfusion that allows some degree of ex‐vivo evaluation of the organ and better control of the cold ischemia time, could potentially allow better utilization rates of marginal donor hearts. [21, 24] These new techniques also allow longer transport times, which widens the donor pool geographically. [22, 23]
Traditionally, the only source of organs for heart transplantation has been brain-dead donors. Techniques that allow the use of hearts donated after circulatory death [25] —a method long used for harvesting kidneys and other organs for transplantation—has the potential to markedly reduce the shortage of donor hearts. In 2022, the US Food and Drug Administration (FDA) approved the Organ Care System (OCS™) Heart System (Transmedics, Andover MA) for the preservation of donor hearts after circulatory death; the OCS Heart System is a portable device that mimics physiologic conditions, perfusing the heart and keeping it warm and beating. The device was previously approved for the preservation of donation-after-brain-death donor hearts that could not be preserved using standard cold storage. [26]
A study by Schroder et al, which used the OCS Heart System, demonstrated the noninferiority of transplantation with donor hearts that had been reanimated and assessed with the use of extracorporeal nonischemic perfusion after circulatory death, versus standard-care transplantation with hearts donated after brain death and preserved using cold storage. The risk-adjusted 6-month survival was 94% in the 80 recipients of a heart from a circulatory-death donor, as compared with 90% in the 86 recipients of a heart from a brain-death donor (P < 0.001 for noninferiority). [27]
Shortage of donor organs has also fueled a search for alternative therapies for the failing heart. Such therapies include left ventricular assist devices, dual-chamber pacing, total artificial hearts, new drug interventions, and genetic therapy. [23, 28] These efforts have proven to be successful in reducing the need for transplantation.
Research in the area of xenografts continues. [29, 30] Genetic engineering is being used to develop swine strains designed as organ donors for humans. This is done by eliminating molecular incompatibilities between the species—for example, knocking out porcine genes associated with antibody-mediated rejection and inserting human genes associated with immune acceptance of the organ. [31] In 2022, surgeons at the University of Maryland Medical Center, Baltimore, successfully transplanted a genetically engineered porcine heart into a 57-year-old man who had no other treatment options. The patient survived for 2 months. The cause of death remains uncertain; on autopsy, the allograft did not display any of the conventional signs of graft rejection. [31]
Another issue is the prevention of allograft vascular disease, which remains a paramount challenge. The pathology of allograft vascular disease is clearly multifactorial in origin, making the research and therapy equally complex. Resolution of this issue will prolong graft survival and lives.
A third issue is the question of recipient selection and listing status, which continues to pose medical and ethical dilemmas. If the donor situation were not an issue, then the listing of potential recipients would not be troublesome.
The final issue is financial. In this era of cost containment in health care, the escalating costs of heart transplantation raises the questions of who should pay for the therapy and whether the procedure should be available on demand.

- Griepp RB, Ergin MA. The history of experimental heart transplantation. J Heart Transplant. 1984. 3:145.
- Khush KK, Hsich E, Potena L, Cherikh WS, Chambers DC, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult heart transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant. 2021 Oct. 40 (10):1035-1049. [QxMD MEDLINE Link].
- Kittleson MM, Kobashigawa JA. Cardiac Transplantation: Current Outcomes and Contemporary Controversies. JACC Heart Fail. 2017 Dec. 5 (12):857-868. [QxMD MEDLINE Link]. [Full Text].
- Griffith BP, Hardesty RL, Deeb GM, et al. Cardiac transplantation with cyclosporin A and prednisone. Ann Surg. 1982 Sep. 196(3):324-9. [QxMD MEDLINE Link].
- Ye F, Ying-Bin X, Yu-Guo W, Hetzer R. Tacrolimus versus cyclosporine microemulsion for heart transplant recipients: a meta-analysis. J Heart Lung Transplant. 2009 Jan. 28(1):58-66. [QxMD MEDLINE Link].
- Ramakrishna H, Jaroszewski DE, Arabia FA. Adult cardiac transplantation: A review of perioperative management Part - I. Ann Card Anaesth. 2009 Jan-Jun. 12(1):71-8. [QxMD MEDLINE Link].
- Heart Failure Society of America. The Stages of Heart Failure - New York Heart Association (NYHA) Classification. Heart Failure Society of America. Available at https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure#.WbL0RU2ovyN. Accessed: June 23, 2022.
- Hill JD. Bridging to cardiac transplantation. Ann Thorac Surg. 1989 Jan. 47(1):167-71. [QxMD MEDLINE Link].
- Portner PM, Oyer PE, Pennington DG, et al. Implantable electrical left ventricular assist system: bridge to transplantation and the future. Ann Thorac Surg. 1989 Jan. 47(1):142-50. [QxMD MEDLINE Link].
- Holman WL, Kormos RL, Naftel DC, Miller MA, Pagani FD, Blume E, et al. Predictors of death and transplant in patients with a mechanical circulatory support device: a multi-institutional study. J Heart Lung Transplant. 2009 Jan. 28(1):44-50. [QxMD MEDLINE Link].
- Davies RR, Russo MJ, Yang J, et al. Listing and transplanting adults with congenital heart disease. Circulation. 2011 Feb 22. 123(7):759-67. [QxMD MEDLINE Link].
- Kramer BL, Massie BM, Topic N. Controlled trial of captopril in chronic heart failure: a rest and exercise hemodynamic study. Circulation. 1983 Apr. 67(4):807-16. [QxMD MEDLINE Link].
- Overcast TD, Evans RW, Bowen LE, et al. Problems in the identification of potential organ donors. Misconceptions and fallacies associated with donor cards. JAMA. 1984 Mar 23-30. 251(12):1559-62. [QxMD MEDLINE Link].
- Busks M. Wider Eligibility for Heart Transplantation in New Guidelines. Medscape Medical News. Available at https://www.medscape.com/viewarticle/857149. January 13, 2016; Accessed: February 26, 2016.
- [Guideline] Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016 Jan. 35 (1):1-23. [QxMD MEDLINE Link]. [Full Text].
- Adult heart allocation. Organ Procurement & Transplantation Network. Available at https://optn.transplant.hrsa.gov/professionals/by-organ/heart-lung/adult-heart-allocation. October 18, 2018; Accessed: June 29, 2022.
- Copeland JG, Emery RW, Levinson MM, et al. Selection of patients for cardiac transplantation. Circulation. 1987 Jan. 75(1):2-9. [QxMD MEDLINE Link].
- Moriguchi J, Davis S, Jocson R, Esmailian F, Ardehali A, Laks H, et al. Successful use of a pneumatic biventricular assist device as a bridge to transplantation in cardiogenic shock. J Heart Lung Transplant. 2011 Oct. 30(10):1143-7. [QxMD MEDLINE Link].
- Kilic A, Conte JV, Shah AS, Yuh DD. Orthotopic Heart Transplantation in Patients With Metabolic Risk Factors. Ann Thorac Surg. 2012 Feb 2. [QxMD MEDLINE Link].
- Arnaoutakis GJ, George TJ, Allen JG, Russell SD, Shah AS, Conte JV, et al. Institutional volume and the effect of recipient risk on short-term mortality after orthotopic heart transplant. J Thorac Cardiovasc Surg. 2012 Jan. 143(1):157-67, 167.e1. [QxMD MEDLINE Link].
- Crespo-Leiro MG, Gustafsson F. Heart transplantation turns 50 and is going strong. Eur J Heart Fail. 2017 Dec. 19 (12):1564-1565. [QxMD MEDLINE Link]. [Full Text].
- Coniglio AC, Patel CB, Kittleson M, Schlendorf K, Schroder JN, DeVore AD. Innovations in Heart Transplantation: A Review. J Card Fail. 2022 Mar. 28 (3):467-476. [QxMD MEDLINE Link].
- Crespo-Leiro MG, Costanzo MR, Gustafsson F, Khush KK, Macdonald PS, Potena L, et al. Heart transplantation: focus on donor recovery strategies, left ventricular assist devices, and novel therapies. Eur Heart J. 2022 Jun 14. 43 (23):2237-2246. [QxMD MEDLINE Link].
- Pinnelas R, Kobashigawa JA. Ex vivo normothermic perfusion in heart transplantation: a review of the TransMedics(®) Organ Care System. Future Cardiol. 2022 Jan. 18 (1):5-15. [QxMD MEDLINE Link].
- Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017 Dec. 36 (12):1311-1318. [QxMD MEDLINE Link].
- Organ Care System (OCS) Heart System – P180051/S001. U.S. Food & Drug Administration. Available at https://www.fda.gov/medical-devices/recently-approved-devices/organ-care-system-ocs-heart-system-p180051s001. May 18, 2022; Accessed: June 8, 2023.
- Schroder JN, Patel CB, DeVore AD, et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N Engl J Med. 2023 Jun 8. 388 (23):2121-2131. [QxMD MEDLINE Link].
- Henn MC, Mokadam NA. Total artificial heart as a bridge to transplantation. Curr Opin Organ Transplant. 2022 Jun 1. 27 (3):222-228. [QxMD MEDLINE Link].
- Pierson RN 3rd, Fishman JA, Lewis GD, D'Alessandro DA, Connolly MR, Burdorf L, et al. Progress Toward Cardiac Xenotransplantation. Circulation. 2020 Oct 6. 142 (14):1389-1398. [QxMD MEDLINE Link]. [Full Text].
- Farr M, Stehlik J. Heart Xenotransplant: A Door That Is Finally Opening. Circulation. 2022 Mar 22. 145 (12):871-873. [QxMD MEDLINE Link]. [Full Text].
- Boulet J, Cunningham JW, Mehra MR. Cardiac Xenotransplantation: Challenges, Evolution, and Advances. J Am Coll Cardiol Basic Trans Science. June 15, 2022. [Full Text].
- [Guideline] Te H, Doucette K. Viral hepatitis: Guidelines by the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant. 2019 Sep. 33 (9):e13514. [QxMD MEDLINE Link].
- Caves PK, Stinson EB, Billingham M, Shumway NE. Percutaneous transvenous endomyocardial biopsy in human heart recipients. Experience with a new technique. Ann Thorac Surg. 1973 Oct. 16(4):325-36. [QxMD MEDLINE Link].
- Hunt J, Lerman M, Magee MJ, et al. Improvement of renal dysfunction by conversion from calcineurin inhibitors to sirolimus after heart transplantation. J Heart Lung Transplant. 2005 Nov. 24(11):1863-7. [QxMD MEDLINE Link].
- Kaczmarek I, Sadoni S, Schmoeckel M, et al. The need for a tailored immunosuppression in older heart transplant recipients. J Heart Lung Transplant. 2005 Nov. 24(11):1965-8. [QxMD MEDLINE Link].
- Pedotti P, Mattucci DA, Gabbrielli F, Venettoni S, Costa AN, Taioli E. Analysis of the complex effect of donor's age on survival of subjects who underwent heart transplantation. Transplantation. 2005 Oct 27. 80(8):1026-32. [QxMD MEDLINE Link].
- McGee E, McCarthy PM, Hoercher KJ, et al. Donor Tricuspid Annuloplasty Reduces Post-Transplant Tricuspid Regurgitation (Abstract 22). The Kaufman Center for Heart Failure, The Cleveland Clinic. International Society for Heart and Lung Transplantation Meeting, San Francisco,. April 21-24, 2004.
- Chan MC, Giannetti N, Kato T, et al. Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant. 2001 Jul. 20(7):709-17. [QxMD MEDLINE Link].
- Khan MS, Mery CM, Zafar F, Adachi I, Heinle JS, Cabrera AG, et al. Is mechanically bridging patients with a failing cardiac graft to retransplantation an effective therapy? Analysis of the United Network of Organ Sharing database. J Heart Lung Transplant. 2012 Aug 17. [QxMD MEDLINE Link].
- Hofflin JM, Potasman I, Baldwin JC, et al. Infectious complications in heart transplant recipients receiving cyclosporine and corticosteroids. Ann Intern Med. 1987 Feb. 106(2):209-16. [QxMD MEDLINE Link].
- Tambur AR, Pamboukian SV, Costanzo MR, et al. The presence of HLA-directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005 Oct 27. 80(8):1019-25. [QxMD MEDLINE Link].
- Kfoury AG, Renlund DG, Snow GL, Stehlik J, Folsom JW, Fisher PW, et al. A clinical correlation study of severity of antibody-mediated rejection and cardiovascular mortality in heart transplantation. J Heart Lung Transplant. 2009 Jan. 28(1):51-7. [QxMD MEDLINE Link].
- Sweeney MS, Macris MP, Frazier OH, et al. The treatment of advanced cardiac allograft rejection. Ann Thorac Surg. 1988 Oct. 46(4):378-81. [QxMD MEDLINE Link].
- Miller CA, Sarma J, Naish J, Yonan N, Williams SG, Shaw SM, et al. Multiparametric Cardiovascular Magnetic Resonance Assessment of Cardiac Allograft Vasculopathy. J Am Coll Cardiol. 2013 Dec 9. [QxMD MEDLINE Link].
- Tremmel JA, Ng MK, Ikeno F, Hunt SA, Lee DP, Yeung AC, et al. Comparison of drug-eluting versus bare metal stents in cardiac allograft vasculopathy. Am J Cardiol. 2011 Sep 1. 108(5):665-8. [QxMD MEDLINE Link].
- Ford MA, Almond CS, Gauvreau K, Piercey G, Blume ED, Smoot LB, et al. Association of graft ischemic time with survival after heart transplant among children in the United States. J Heart Lung Transplant. 2011 Nov. 30(11):1244-9. [QxMD MEDLINE Link].
Author
Donald M Botta, Jr, MD Assistant Professor, Department of Surgery, Section of Cardiac Surgery, Surgical Director, Cardiac Transplantation, Director of Mechanical Circulatory Support, Yale University School of Medicine
Disclosure: Nothing to disclose.
Coauthor(s)
Mary C Mancini, MD, PhD, MMM
Mary C Mancini, MD, PhD, MMM is a member of the following medical societies: American Association for Thoracic Surgery, American College of Surgeons, American Surgical Association, Phi Beta Kappa, Society of Thoracic Surgeons
Disclosure: Nothing to disclose.
Chief Editor
John Geibel, MD, MSc, DSc, AGAF Vice Chair and Professor, Department of Surgery, Section of Gastrointestinal Medicine, Professor, Department of Cellular and Molecular Physiology, Yale University School of Medicine; Director of Surgical Research, Department of Surgery, Yale-New Haven Hospital; American Gastroenterological Association Fellow; Fellow of the Royal Society of Medicine
John Geibel, MD, MSc, DSc, AGAF is a member of the following medical societies: American Gastroenterological Association, American Physiological Society, American Society of Nephrology, Association for Academic Surgery, International Society of Nephrology, New York Academy of Sciences, Society for Surgery of the Alimentary Tract
Disclosure: Nothing to disclose.
Acknowledgements
Deepak M Gangahar, MBBS, MD Professor, Department of Surgery, Chief, Section of Cardiovascular and Thoracic Surgery, Surgical Director, Heart Transplant and VAD Services, University of Nebraska Medical Center
Deepak M Gangahar, MBBS, MD is a member of the following medical societies: American College of Cardiology, American College of Chest Physicians, American College of Surgeons, American Medical Association, International Society for Heart and Lung Transplantation, International Society for Minimally Invasive Cardiothoracic Surgery, Nebraska Medical Association, and Society of Thoracic Surgeons
Disclosure: Nothing to disclose.
Shreekanth V Karwande, MBBS Chair, Professor, Department of Surgery, Division of Cardiothoracic Surgery, University of Utah School of Medicine and Medical Center
Shreekanth V Karwande, MBBS is a member of the following medical societies: American Association for Thoracic Surgery, American College of Chest Physicians, American College of Surgeons, American Heart Association, Society of Critical Care Medicine, Society of Thoracic Surgeons, and Western Thoracic Surgical Association
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Richard Thurer, MD B and Donald Carlin Professor of Thoracic Surgical Oncology, University of Miami, Leonard M Miller School of Medicine
Richard Thurer, MD is a member of the following medical societies: American Association for Thoracic Surgery, American College of Chest Physicians, American College of Surgeons, American Medical Association, American Thoracic Society, Florida Medical Association, Society of Surgical Oncology, and Society of Thoracic Surgeons
Disclosure: Nothing to disclose.