DELETE - Dermatologic Manifestations of Renal Disease: Overview, Dermatologic Manifestations of Diseases Associated With ESRD, Dermatologic Manifestations of Uremia (original) (raw)
Although many lives are saved and maintained by dialytic intervention, most individuals endure a great deal of morbidity as a result of the inadequacy of renal replacement therapy. The best therapeutic option for many patients with end-stage renal disease (ESRD) is renal allograft transplantation. Successful transplantation results in regression of many of the metabolic and cutaneous changes of uremia. The 2007 US Renal Data System (USRDS) report revealed that over 18,000 individuals were transplanted in 2006, raising the number of renal transplant recipients in the United States to over 140,000. Unfortunately, renal transplantation has its own set of complications, primarily resulting from the immunosuppressive medications that are essential for allograft survival.
Studies have shown that 50-100% of renal transplant recipients (RTRs) have a transplant-related cutaneous complaint. Dermatologic disorders associated with renal transplantation are a function of the immunosuppressive medications prescribed, as well as the immunosuppressed condition produced. Factors such as time after transplantation, geographic location, climate, and skin type greatly modify the clinical disorders associated with renal transplantation.
Medication-related dermatologic disorders
Medication-related disorders include the following:
- Cushingoid changes
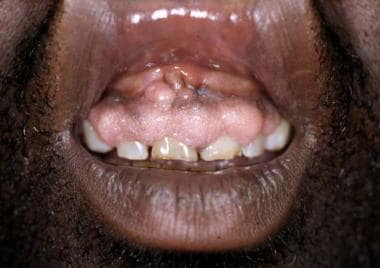
- Gingival hyperplasia (see the following image)
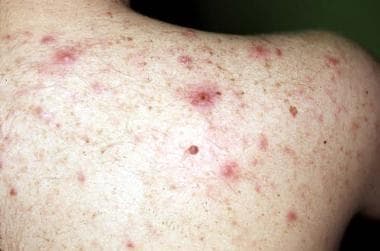
- Disorders of the pilosebaceous unit, including acne, folliculitis, hypertrichosis, keratosis pilaris, sebaceous gland hyperplasia, epidermal cysts (see the image below)
Many cutaneous changes seen in the renal transplant recipient (RTR) are related directly to medications used to suppress rejection of renal allograft. A full-blown Cushingoid appearance develops in 55-90% of patients and is associated with the high doses of corticosteroids used early after transplantation. Cutaneous findings include moon facies, development of a cervical fat pad (buffalo hump), striae distensae, cutaneous atrophy, and telangiectasias. Changes may resolve or improve when the corticosteroid dose is reduced, although these cutaneous changes may continue, as steroids are used long term. Gingival hyperplasia, which occurs in approximately one third of patients receiving cyclosporin A (CyA), tends to occur early and improve over time. See the image below.
Cyclosporin may induce gingival hyperplasia in approximately one third of renal transplant recipients.
Other cutaneous changes involve poorly understood alterations in the pilosebaceous unit and may result from either CyA or corticosteroid use. Acne develops in 15% of patients and primarily affects the chest and back (see the image below). Most severe in the first year, acne later improves with reduction of the corticosteroid dose. Sebaceous gland hyperplasia and epidermal cysts are found with increased frequency and have been associated with the use of both corticosteroids and CyA. Hypertrichosis develops in 60% of patients and may be associated with the development of keratosis pilaris.
Lesions of steroid-induced acne (evident on the back of a renal transplant patient) may be severe.
Immunosuppression-related disorders
Immunosuppression-related disorders include the following:
- Viral infections, including herpes simplex virus (HSV), varicella-zoster virus (VZV), Epstein-Barr virus (EBV)
- Bacterial infections, including Staphylococcus aureus, Bartonella henselae, mycobacteria, Mycobacterium tuberculosis, and atypical mycobacteria
- Fungal infections, including superficial mycoses (eg, dermatophytes, Pityrosporum species, candidiasis) and deep fungal infections (eg, Aspergillus, Cryptococcus, Nocardia, Rhizopus species)
- Parasitic infection, including scabies
- Actinic keratoses
- Malignancies, including squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, Kaposi sarcoma, melanoma
- Miscellaneous malignancies, including lymphoma, Merkel cell carcinoma (MCC), and dermatofibrosarcoma protuberans
- Miscellaneous disorders, including transfusion-associated graft-versus-host disease and porokeratosis
Infections
The iatrogenically induced decrease in cell-mediated immunity predisposes the renal transplant recipient (RTR) to infection by a variety of organisms. Timing and relative risk of the infections are determined by the degree of immunosuppression. Patients are at heightened risk for developing opportunistic infections during the first 6 months after transplantation because of the use of higher doses of immunosuppressive agents. Cutaneous examination is crucial in the surveillance for opportunistic infections, because cutaneous lesions frequently are the first sign of disseminated disease.
Mycobacterial infections
Later in the posttransplant period, patients may develop infections from a variety of acid-fast bacilli (AFB), specifically typical or atypical mycobacteria. [34, 35, 36] Although these infections are relatively unusual, they may cause significant morbidity. Multifocal disease is not uncommon. Organisms of the Mycobacterium fortuitum/chelonae complex are more common causes of AFB cutaneous infections, although others, such as M kansasii and M marinum, have also been reported. Histopathologic examination and tissue culture are necessary to make the correct diagnosis. Therapeutic options for these infections include antimicrobials, surgical debridement, and/or a reduction in immunosuppression.
Fungal infections
Fungal organisms are the most common cause of infection in the renal transplant recipient, occurring in 7-75%. [37] The wide variability in prevalence likely results from heterogeneity in diagnostic criteria, environmental exposures, geographic locations, and economic and hygienic factors.
Pityriasis versicolor has been shown to be the most common fungal infection and occurs in 18-48% of renal transplant recipients, which is a higher rate than found in the general population. Colonization of the upper back with Pityrosporum yeasts has been shown to occur 2-3 times more often in the renal transplant recipient relative to the general population. Pityrosporum organisms may predispose patients to increased incidence of folliculitis. Dermatophytosis, although common after renal transplantation, is no more common than in the general population.
Viral infections
Severe viral infections usually occur during the first year after transplantation and predominately result from herpes viruses. Cutaneous lesions resulting from infection with human papillomavirus (HPV) tend to develop later. Surveys suggest that the prevalence of HPV is 15-50% after the first year and increases to 77-95% by 5 years after transplantation.
Common and plane warts, which predominate, occur most frequently in sun-exposed areas and usually are multiple in number. HPV types 1, 2, 3, and 4 are found most commonly; however, many other HPV types have been reported in association with warty lesions in renal transplant recipients, including oncogenic types 16 and 18 and types 5 and 8, which usually are associated with epidermodysplasia verruciformis.
Eradication of these HPV infections is difficult, because the lesions respond poorly to therapy and recur frequently. Treating warts early and aggressively is best in the renal transplant recipient using routine modalities, such as cryotherapy, electrocoagulation, and carbon dioxide laser. Surgery and radiotherapy are not more effective and may result in scarring. Treatment with oral or topical retinoids may be an option for some patients.
Interferon alfa, which has been effective against warts in immunocompetent individuals, should not be used in the renal transplant recipient, because it may trigger allograft rejection. Imiquimod, an agent that can heighten the host's immune system by upregulating interferon, was initially thought to be similarly contraindicated in the transplant population. However, more recent studies have demonstrated it to be both effective and safe when used in a limited fashion.
Malignancy
Malignancies are more common after organ transplantation, and most are primary cutaneous malignancies. Incidence of nonmelanoma skin cancer is 20-40 times greater in renal transplant recipients (RTRs) than in the general population. This incidence increases as the time elapsed after transplantation increases because of the duration of immunosuppressive therapy. The cumulative risk of cutaneous malignancy is 10-30% at 5 years, 10-44% at 10 years, and 30-40% at 20 years. The prevalence of skin cancer varies according to geographic location, amount of ultraviolet (UV) light exposure, and predominant skin type. Most malignancies occur on sun-exposed areas and usually are found on the head, neck, and upper extremities. Multiple malignancies are a common occurrence.
Nonmelanoma skin cancer
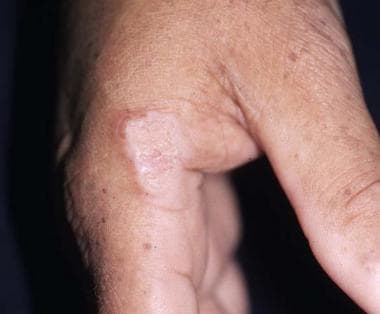
Squamous cell carcinoma (SCC) is the most common cutaneous malignancy in renal transplant recipients and occurs 50-250 times more frequently in these individuals than in the general population. In contrast, basal cell carcinoma (BCC) occurs 6-10 times more frequently in the renal transplant recipient. As a result of the markedly increased incidence of SCC, an inversion is seen in the ratio of basal cell carcinoma to SCC, from 4:1 in the general population to 1:3-4 in the renal transplant population. The image below illustrates SCC.
A hyperkeratotic plaque on this renal transplant recipient was proven to be squamous cell carcinoma. Similar lesions are frequently mistaken for warts.
Nonmelanoma malignancies occur at a younger age in renal transplant recipients and are characterized by a more rapid and aggressive course, a higher recurrence rate, and a greater metastatic potential. Actinic keratoses also occur at a younger age and develop at a faster rate in renal transplant recipients. Actinic keratoses frequently have more severe cytologic atypia and may have more rapid progression to SCC. Clinically, it may be difficult to distinguish actinic keratoses, SCCs, and warts.
Factors that predispose individuals to development of nonmelanoma skin cancer include exposure to ultraviolet (UV) light, skin phototypes I and II, immunosuppression and, possibly, human papillomavirus (HPV) infection. Most cancers occur in sun-exposed areas in lightly pigmented individuals.
Immunosuppressive medications reduce cell-mediated immunity and, thereby, greatly augment the potential for malignant transformation. Whether cyclosporin A (CyA) is more or less oncogenic than azathioprine remains controversial, because available data are conflicting. Azathioprine and its metabolites are mutagenic and toxic to Langerhans cells, but CyA is more immunosuppressive.
Mounting evidence suggests that sirolimus-based immune suppression is associated with a much lower rate of skin cancer development in the solid organ transplant recipient. Many patients who are at high risk for skin cancer are being switched to this regimen; however. the risks and benefits of each agent must be considered and therapy customized to each patient. [38, 39, 40]
The role HPV may play remains obscure, because data are not conclusive; however, multiple HPV strains have been documented in many cutaneous malignancies, and malignant transformation of warty lesions has been observed.
Management of nonmelanoma skin cancers includes sun avoidance, use of broad-spectrum sunscreens, early detection of malignant and precancerous lesions, and aggressive therapy. Complete surgical excision with margin control is necessary. Adjuvant radiotherapy or chemotherapy may have a role in association with surgery in certain patients.
Some studies have shown imiquimod to be safe and effective for superficial BCCs and actinic keratoses when used on small surface areas according to directions [41, 42, 43, 44] ; however, the safety and efficacy of this agent in immunosuppressed patients have not been established. Some authors advocate switching from cyclosporin or tacrolimus to sirolimus in hope of minimizing cancer risk. Be aware that minimizing ultraviolet B (UVB) light exposure adds further risk for vitamin D deficiency in this population. [45, 46]
Chemoprevention using systemic retinoids should be reserved for those at highest risk for multiple malignancies. Partial and complete remissions have been reported with retinoid use, but long-term therapy is necessary, because the beneficial effect is lost 2-3 months after stopping treatment. [47, 48] Adverse effects may preclude retinoid use. Potential adverse effects include birth defects, hyperlipidemia, and osteoporosis. In addition, CS and CyA use frequently are associated with hyperlipidemia and osteoporosis; concern exists that these effects may be amplified by addition of a retinoid. Reduction or discontinuation of immunosuppressive therapy should be considered but may not be acceptable in some patients because it may result in allograft rejection and loss.
Kaposi sarcoma
The incidence of Kaposi sarcoma is 400-500 times higher in renal transplant recipients than in the healthy population. [17] Kaposi sarcoma accounts for approximately 3-5% of transplant-related malignancies in renal transplant recipients. The incidence varies according to geographic region and depends on ethnic composition of the population. Generally, Kaposi sarcoma incidence is higher in those with Jewish, Mediterranean, or Arabic ancestry and in blacks.
The highest incidence is in the first year after transplantation. Kaposi sarcoma usually appears 2-24 months after transplantation, in contrast to the late development of most other transplant-related malignancies. [49, 50, 51] As in other populations studied, the development of Kaposi sarcoma in renal transplant recipients is associated with human herpes virus 8 (HHV-8). Mucocutaneous lesions occur in 60% of renal transplant recipients with Kaposi sarcoma and may be isolated in these areas; however, visceral disease is not uncommon.
Therapeutic options for the renal transplant recipient with Kaposi sarcoma include cessation of immunosuppressive medications, radiotherapy, chemotherapy, excision, and cryotherapy. Although complete regression has been described after discontinuation of immunosuppressive therapy, adjuvant therapy often is needed. Occasionally, Kaposi sarcoma may be fatal as a result of visceral involvement and despite therapy.
Melanoma
Melanoma occurs 2-9 times more frequently in the transplant population than in the general population, according to population registry data and published studies. [52] Interestingly, the risk of melanoma in renal transplant recipient may include transmission by the donor. Studies are conflicting regarding the prognosis of melanoma in the transplant patient, including the risk of recurrence of melanoma following transplantation. [53, 54]
Other malignancies
Although Kaposi sarcoma is the most commonly reported sarcoma in renal transplant recipient, other cutaneous sarcomas have been reported. Malignant fibrous histiocytomas, atypical fibroxanthomas, and dermatofibrosarcoma protuberans have been reported in renal transplant recipients, although the incidence of these malignancies is unknown. Several cases of Merkel cell carcinoma (MCC) occurring in renal transplant recipients have been reported. [55] Most lesions develop on the head and neck or the extremities. This is an aggressive tumor with a high propensity to recur or metastasize. Overall mortality associated with Merkel cell carcinoma is 35%. Interestingly, reports exist of SCC and MCC occurring simultaneously.
Although lymphomas are the second most common malignancy in transplant recipients, cutaneous lymphomas are relatively rare. Whereas cutaneous T-cell lymphomas have been described, cutaneous B-cell lymphomas are more common in the transplant population. [56]
Miscellaneous disorders
Transfusion-associated graft-versus-host disease has been reported in several renal transplant patients who received nonradiated packed red blood cells. [57] Patients are at increased risk during times of marked immunosuppression, including therapy for acute organ rejection. Transfusion-associated graft-versus-host disease usually has a poor prognosis.
Porokeratosis, a disorder of epidermal keratinization, has been reported as an unusual manifestation of immunosuppression in solid organ recipients. [58, 59, 60] Several of the clinical variants have been reported, including disseminated superficial porokeratosis, single lesions of porokeratosis of Mibelli, and rare cases of disseminated porokeratosis of Mibelli. Malignant transformation of lesions of porokeratosis has been described but not in the transplant population.