Pacemaker-Mediated Tachycardia: Practice Essentials, Background, Pathophysiology (original) (raw)
Practice Essentials
Consider the following when evaluating a diagnosis of pacemaker-mediated tachycardia (PMT):
- What is the mechanism of PMT? Typical PMT occurs when the atrial channel of a dual-chamber cardiac implantable electronic device (CIED) begins to sense retrograde atrial activation (atrial electrogram) resulting from ventricular pacing or ventricular ectopy and causes tracking of these signals to result in ventricular pacing and an endless loop tachycardia.
- Which patient may experience PMT?
- A patient with a dual- or triple-chamber CIED (pacemaker or defibrillator) who has retrograde (V → A) conduction from the paced ventricles that results in sensed retrograde P waves
- Which type of CIED may cause PMT?
- A CIED programmed to an atrial "tracking" mode
- Programming examples: DDD (dual pacing, dual sensing, dual mode), DDDR (DDD with rate modulation), VDD (ventricle pacing, dual sensing, dual mode), VDDR (ventricle pacing, dual sensing, dual mode, rate modulation), or VAT (ventricle pacing, atrial sensing, triggering mode)
- These modes depend on the postventricular atrial refractory period (PVARP) parameter to prevent fast tracked rates (generally at or near the upper rate limit).
- How does PVARP affect PMT?
- For PMT to occur, the retrograde conduction (atrioventricular [AV]) time interval must exceed the value of the PVARP during retrograde conduction for the atrial electrogram to be tracked.
- Retrograde atrial electrograms (A waves) sensed after the end of PVARP can trigger a V paced event, resulting in the repetition of the same cycle and eventually an endless loop tachycardia.
- Retrograde conduction time, which is shorter than PVARP, will not initiate PMT but may initiate another form of endless loop tachycardia called repetitive nonreentrant ventriculoatrial synchrony (RNRVAS); see the section below.
- PVARP may be programmed to a fixed value or may be dynamic based on an algorithm. A typical step to eliminate PMT is to extend the PVARP; however, this may convert one form of endless loop tachycardia (PMT) into another form (RNRVAS).
- How is PMT initiated?
- An event that disturbs normal AV synchrony but allows for retrograde atrial activation to occur can initiate PMT.
- Examples: Premature atrial contraction (PAC), premature ventricular contraction (PVC), atrial oversensing, atrial undersensing, loss of atrial capture, magnet removal from a pacemaker
- How is PMT prevented or terminated?
- Program (extend) the PVARP to prevent inappropriate tracking.
- Use a nontracking mode such as DDI.
- Use magnet application to stop the PMT.
- Use algorithms to dynamically increase PVARP after a PVC.
- Use algorithms that detect PMT and intervene.
- How do algorithms detect PMT?
- A specific number of AS-VP (atrial sense–ventricular pace) intervals at the maximum tracking rate or a separate PMT detection rate
- Stability measurement of the A-V and/or V-A intervals during these AS-VP intervals
- Some algorithms modify the AV interval to rule out sinus tachycardia as a cause for AV synchrony rather than true PMT.
- If PMT is detected, how can it be terminated?
- Some algorithms extend PVARP automatically.
- Most algorithms withhold the VP output once endless loop tachycardia is identified to prevent the retrograde P wave appearance and thus terminate the PMT cycle.

Background
PMT is any condition in which a pacemaker or implantable defibrillator paces the ventricles at rates that are inappropriately fast. [1] This can be due to (1) a rate response setting that is too sensitive, (2) tracking of atrial noise (such as what may occur with electromagnetic interference or a make-break lead fracture), (3) inappropriate pacemaker manipulation with rate response turned on, or (4) tracking of an atrial tachyarrhythmia related to upper rate settings.
Traditionally, however, the term pacemaker-mediated tachycardia, also called endless loop tachycardia, refers to a form of a reentrant tachycardia that can occur in patients who have a dual-chamber or triple-chamber pacemaker or implantable defibrillator (cardiac implantable electronic device [CIED]) pacemakers. [2] The pacemaker forms the anterograde (atrium to ventricle [A → V]) limb of the circuit and the atrioventricular (AV) node (or a retrograde accessory pathway) is the retrograde limb (ventricle to atrium [V → A]) of the circuit. [3] This classic form is referred to and discussed as PMT for the rest of this article.
Typically, PMT occurs at the upper rate limit of the pacemaker, but this is not always the case depending on the pacemaker setting and the retrograde (VA) conduction time. If an endless loop tachycardia occurs at rates slower than the upper rate limit of the dual-chamber pacemaker programming, it is often referred to as a balanced endless loop tachycardia ("BELT").
See the electrocardiograms (ECGs) below.
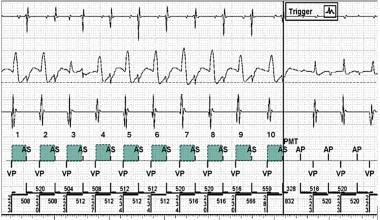
Pacemaker-Mediated Tachycardia. Example of a pacemaker-mediated tachycardia (PMT) algorithm detecting the number and stability of ventricular-paced–atrial-sensed (VP-AS) intervals at the PMT detection rate and terminating the PMT by withholding the VP output (arrow) and delivering atrial-paced (AP) output instead. From top to bottom: Atrial channel, far-field ventricular channel, bipolar ventricular channel, marker channel. The green squares depict the stability of the ventriculo-atrial (VA) interval. Retrograde AS outside of the postventricular atrial refractory period (PVARP) is the basis for the PMT. Note that an AS interval within the PVARP can form the basis for repetitive nonreentrant ventriculoatrial synchrony (RNRVAS). Please see a separate section. Image from Michael Orlov, MD.
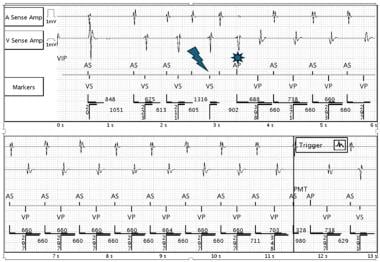
Pacemaker-Mediated Tachycardia. Example of a PMT initiated by a premature ventricular contraction (lightning bolt), followed by atrial paced (AP) with loss of capture (sun symbol) and loss of atrioventricular (AV) synchrony. The subsequent ventricular paced (VP) rhythm initiates the retrograde conduction. Labels for the channels are shown at the upper part of the tracing. Image from Michael Orlov, MD.
RNRVAS is a VA synchrony pacemaker-mediated arrhythmia that only occurs in the presence of retrograde VA conduction and dual-chamber or cardiac resynchronization devices with tracking (eg, DDD, DDDR) or nontracking pacing modes that allow AV-sequential pacing (eg, DDI, DDIR). [4] This form of endless loop tachycardia is similar to PMT but differs fundamentally as the retrograde A falls within the PVARP and is therefore "ignored" for timing purposes. The subsequent A pace event is determined by the sensor indicated rate. It will most likely fall into the refractory period and will not capture the atrium but will trigger the AV interval. Subsequent V pace will result in retrograde conduction again falling into the PVARP, and the cycle will become repetitive. This may result in mode switching, as there will be twice as many atrial events as there are V's (false mode switch for perceived atrial tachyarrhythmia/atrial fibrillation [AT-AF]). RNRVAS may be also proarrhythmic, causing real AF. [5] Unlike PMT, there are no specific algorithms to detect RNRVAS; several algorithms attempt to prevent RNRVAS by extending a waiting period after the retrograde atrial event in an attempt to avoid atrial noncapture and the subsequent sequence of events described above leading to endless loop tachycardia.
In 2021, Gjermeni et al reported a novel pacemaker-mediated arrhythmia that closely mimics RNRVAS but exhibits a different mechanism and was independent of VA conduction (pseudo-RNRVAS). [6] The investigators interrogated 840 dual-chamber or biventricular devices and identified 9 patients with this arrhythmia. The authors concluded that pseudo-RNRVAS is underrecognized because there are no specific device algorithms to detect and store them. [6]

Pathophysiology
The following is the most common scenario causing PMT.
A dual-chamber pacemaker that is programmed DDD or VAT, but not DDI, is implanted. The patient must have retrograde (V→A) conduction with an atrial activation time that is longer than the programmed PVARP. A ventricular-paced beat or a properly timed PVC conducts retrograde via the AV node (or an accessory pathway, if present) to the atrium. If the atrial depolarization occurs after the PVARP but before the next timed atrial-paced beat, ventricular pacing will be triggered by the sensed atrial event at the programmed AV interval for that rate.
PMT tends to occur at or near the programmed upper rate limit and depends upon the programmed AV delay and the PVARP. This generates an incessant reentrant arrhythmia circuit that persists as long as there is continuous VA conduction with atrial activation outside the PVARP. The pacemaker forms the antegrade limb of the circuit and VA conduction forms the retrograde limb as the essential critical components. [3] In many instances, the PVARP varies so that it shortens with rate, which can make PMT more likely. The AV interval can be programmed to change (shorten) with the rate, which tends to reduce the risk of PMT. Depending on the pacemaker programming and the VA conduction, the rate of the PMT may vary, but it is bounded by (and generally occurs near or at) the programmed upper rate limit.
Although PMT is commonly initiated by an isolated ventricular ectopic beat, it can also be initiated by failure to capture the atrium with a paced beat followed by a ventricular paced beat. If this occurs, the atrium is then amenable to depolarization by the impulse conducted retrograde from the ventricle. The tachycardia continues until retrograde conduction ends or the atrium becomes refractory. Specific CIED algorithms may stop PMT if tracked rates persist at the upper rate limit. The pacemaker can be programmed to lengthen the PVARP after a PVC or with an incessant tachycardia at the upper rate limit or prevent one atrial sensed event from being tracked. Up to one third of patients with antegrade complete AV block have intact (or intermittent) retrograde (V→A) conduction. It has been recommended that testing for retrograde conduction be performed in all patients with AV block to optimize device programming and prevent PMTs. [7]
Other situations that can result in PMT include intermittent atrial undersensing. An atrial premature beat with a long A sense–V pace interval (due to a long programmed AV interval) in a patient with antegrade (AV) heart block can also initiate PMT, as any antegrade concealed depolarization otherwise present cannot prevent VA conduction in this instance. [8] A short AV interval is likely to mitigate against PMT but, in some patients, an effort is made to reduce right ventricular pacing and, therefore, the AV interval is left long. This can exacerbate PMT. Furthermore, specific programming algorithms that temporarily lengthen the AV interval to search for intrinsic conduction (the "VIP algorithm," "AV search hysteresis," for example) may increase the risk of PMT in select patients. The 2024 PVC response Atrial-Pace algorithm designed to prevent PMT may trigger atrial high rate episodes. [9]
RNRVAS has similarities to PMT; patients with RNRVAS can have symptoms similar to PMT. In this instance, retrograde atrial activation occurs in the PVARP and thus the atrial activation in not acted upon, but this affects subsequent atrial pacing with functional loss of capture. RNRVAS is generally not at the upper rate limit of the pacemaker, and it can be exacerbated by long programmed AV intervals, as well as faster pacing rates in a DDDR pacemaker. It can cause atrial pacing in a vulnerable period to initiate atrial arrhythmias. [5]

Prognosis
Prognosis is not directly altered by an episode of PMT, but it is defined by the patient's underlying cardiac or medical condition.
Indirectly, in a rare event such as PMT-induced syncope, a patient could sustain injury as a result of the syncope.
Persistent PMT can cause hypotension, ischemia, and heart failure symptoms.
Morbidity/mortality
Patients may experience palpitations, rapid heart rates, lightheadedness, syncope, or chest discomfort.
Complications
PMT is rarely associated with any serious complications such as presyncope or syncope, but endless loop tachycardia can cause excessive ventricular pacing and at a fast rate that exacerbates myocardial ischemia and/or heart failure. It can also initiate hypotension, especially with the abrupt increases in heart rate. PMT may be difficult to detect if it is BELT, as it may not be stored in the pacemaker device and is only picked up on long-term monitoring.
In many patients, the condition may be asymptomatic and is noted only with ECG or Holter monitoring.
With the appropriate programming interventions described above, the problem usually is resolved and, in most modern pacemakers, it can be detected and treated by the device itself.
In patients who develop chest pain (angina pectoris) associated with the rapid pacing rate, consider a stress test to evaluate for coronary artery disease.

- Monteil B, Ploux S, Eschalier R, et al. Pacemaker-mediated tachycardia: manufacturer specifics and spectrum of cases. Pacing Clin Electrophysiol. 2015 Dec. 38(12):1489-98. [QxMD MEDLINE Link].
- Abu-haniyeh A, Hajouli S. Pacemaker mediated tachycardia. StatPearls [Internet]. 2023 Jul 25. [QxMD MEDLINE Link]. [Full Text].
- Love CJ. Pacemaker troubleshooting and follow-up. In: Ellenbogen KA, Kay GN, Lau CP, Wilkoff BL, eds. Clinical Cardiac Pacing Defibrillation and Resynchronization Therapy. 3rd ed. Philadelphia, PA: Elsevier; 2007. 1005-62.
- Sharma PS, Kaszala K, Tan AY, et al. Repetitive nonreentrant ventriculoatrial synchrony: an underrecognized cause of pacemaker-related arrhythmia. Heart Rhythm. 2016 Aug. 13(8):1739-47. [QxMD MEDLINE Link].
- Orlov MV, Olshansky B, Benditt DG, et al. Is competitive atrial pacing a possible trigger for atrial fibrillation? Observations from the RATE registry. Heart Rhythm. 2021 Jan. 18(1):3-9. [QxMD MEDLINE Link].
- Gjermeni E, Doering M, Richter S, Hindricks G, Bode K. Novel pacemaker-mediated arrhythmia without ventriculoatrial conduction can induce atrial fibrillation. JACC Clin Electrophysiol. 2021 Jan. 7(1):1-5. [QxMD MEDLINE Link]. [Full Text].
- Richter S, Muessigbrodt A, Salmas J, et al. Ventriculoatrial conduction and related pacemaker-mediated arrhythmias in patients implanted for atrioventricular block: an old problem revisited. Int J Cardiol. 2013 Oct 9. 168(4):3300-8. [QxMD MEDLINE Link].
- Frumin H, Furman S. Endless loop tachycardia started by an atrial premature complex in a patient with a dual chamber pacemaker. J Am Coll Cardiol. 1985 Mar. 5(3):707-10. [QxMD MEDLINE Link].
- Sane M, Marjamaa A, Kuusisto J, Raatikainen P, Karvonen J. "PVC response Atrial-Pace," an algorithm designed for preventing pacemaker-induced tachycardia after premature ventricular contractions, triggers atrial high rate episodes. Heart Rhythm. 2024 Apr. 21(4):495-6. [QxMD MEDLINE Link].
- Abu-haniyeh A, Hajouli S. Pacemaker mediated tachycardia. StatPearls [Internet]. 2022 Jan. [QxMD MEDLINE Link]. [Full Text].
- Greenspon AJ, Greenberg RM, Frankl WS. Tracking of atrial flutter during DDD pacing: another form of pacemaker-mediated tachycardia. Pacing Clin Electrophysiol. 1984 Nov. 7(6 pt 1):955-60. [QxMD MEDLINE Link].
- Rozanski JJ, Blankstein RL, Lister JW. Pacer arrhythmias: myopotential triggering of pacemaker mediated tachycardia. Pacing Clin Electrophysiol. 1983 Jul. 6(4):795-7. [QxMD MEDLINE Link].
- Griffin J, Smithline H, Cook J. Runaway pacemaker: a case report and review. J Emerg Med. 2000 Aug. 19(2):177-81. [QxMD MEDLINE Link].
- Klementowicz PT, Furman S. Selective atrial sensing in dual chamber pacemakers eliminates endless loop tachycardia. J Am Coll Cardiol. 1986 Mar. 7(3):590-4. [QxMD MEDLINE Link]. [Full Text].
- Frohlig G, Schwerdt H, Schieffer H, Bette L. Atrial signal variations and pacemaker malsensing during exercise: a study in the time and frequency domain. J Am Coll Cardiol. 1988 Apr. 11(4):806-13. [QxMD MEDLINE Link]. [Full Text].
- Horie K, Otomo K, Mori S, Kikuchi Y, Meguro T. Uncommon presentation of drug-refractory pacemaker-mediated common atrioventricular nodal reentrant tachycardia and a simple solution by reprogramming. Intern Med. 2015. 54(9):1063-6. [QxMD MEDLINE Link].
- Ip JE, Lerman BB. Validation of device algorithm to differentiate pacemaker-mediated tachycardia from tachycardia due to atrial tracking. Heart Rhythm. 2016 Aug. 13(8):1612-7. [QxMD MEDLINE Link].
- Strik M, Frontera A, Eschalier R, et al. Accuracy of the pacemaker-mediated tachycardia algorithm in Boston Scientific devices. J Electrocardiol. 2016 Jul-Aug. 49(4):522-9. [QxMD MEDLINE Link]. [Full Text].
Author
Brian Olshansky, MD, FESC, FAHA, FACC, FHRS Professor Emeritus of Medicine, Department of Internal Medicine, University of Iowa College of Medicine
Brian Olshansky, MD, FESC, FAHA, FACC, FHRS is a member of the following medical societies: American College of Cardiology, American Heart Association, Cardiac Electrophysiology Society, European Society of Cardiology, Heart Rhythm Society
Disclosure: Nothing to disclose.
Coauthor(s)
Michael V Orlov, MD, PhD, FACC Professor of Medicine, Fellowship Program Director, Department of Cardiac Electrophysiology, Steward St. Elizabeth’s Medical Center of Boston
Michael V Orlov, MD, PhD, FACC is a member of the following medical societies: American College of Cardiology, Heart Rhythm Society
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: Eagle Point Medical LB.
Timothy Gerald McIntyre, MSBME Patient Care Specialist, Cardiac RMS, CRM Consultant, Boston Scientific
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: CardiacRMS by DOCGO
Received income in an amount equal to or greater than $250 from: CardiacRMS by DOCGO.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
Jose M Dizon, MD Professor of Clinical Medicine, Clinical Electrophysiology Laboratory, Division of Cardiology, Columbia University College of Physicians and Surgeons; Attending Physician, Department of Medicine, New York-Presbyterian/Columbia University Medical Center
Jose M Dizon, MD is a member of the following medical societies: American College of Cardiology, Heart Rhythm Society
Disclosure: Nothing to disclose.
Additional Contributors
Justin D Pearlman, MD, ME, PhD, FACC, MA Chief, Division of Cardiology, Director of Cardiology Consultative Service, Director of Cardiology Clinic Service, Director of Cardiology Non-Invasive Laboratory, Chair of Institutional Review Board, University of California, Los Angeles, David Geffen School of Medicine
Justin D Pearlman, MD, ME, PhD, FACC, MA is a member of the following medical societies: American College of Cardiology, International Society for Magnetic Resonance in Medicine, American College of Physicians, American Federation for Medical Research, Radiological Society of North America
Disclosure: Nothing to disclose.
Noel G Boyle, MB, BCh, MD, PhD Professor of Medicine, UCLA Cardiac Arrhythmia Center, Ronald Reagan UCLA Medical Center
Noel G Boyle, MB, BCh, MD, PhD is a member of the following medical societies: American College of Cardiology, European Society of Cardiology, Heart Rhythm Society, American College of Physicians
Disclosure: Nothing to disclose.
Rakesh Gopinathannair, MD, MA Director of Cardiac Electrophysiology, University of Louisville; Assistant Professor of Medicine, Division of Cardiovascular Medicine, University of Louisville School of Medicine
Rakesh Gopinathannair, MD, MA is a member of the following medical societies: American College of Cardiology, Heart Rhythm Society
Disclosure: Received consulting fee from St. Jude Medical for consulting; Received honoraria from Boston Scientific for speaking and teaching.