Crohn Disease Treatment & Management: Approach Considerations, Pharmacologic Therapy for Diarrhea, Anti-inflammatory and Immunosuppressant Therapy for Active Crohn Disease (original) (raw)
Approach Considerations
Treatment of Crohn disease is made on the basis of the disease site, pattern, activity, and severity. [12] The general goals of treatment for Crohn disease are as follows:
- To achieve the best possible clinical, laboratory, and histologic control of the inflammatory disease with the least adverse effects from medication
- To permit the patient to function as normally as possible
- In children, to promote growth with adequate nutrition
Treatment of Crohn disease has changed over the past few years, reflecting new therapies that can target specific locations in the gastrointestinal (GI) tract and specific cytokines. The development of biologic anti–tumor necrosis factor (anti-TNF) agents (eg, infliximab, adalimumab, certolizumab pegol, and natalizumab) has significantly advanced the treatment of Crohn disease and improved the induction and maintenance of clinical remission in patients with moderate to severe disease, especially in those who are corticosteroid dependent. [84] Anti-TNF agents are effective for induction and maintenance of remission in patients with Crohn disease and active inflammation. [12]
Antibiotics can decrease anal fistula discharge and may induce remission in Crohn disease. [12]
If medical therapy for active Crohn disease fails, surgical resection of the inflamed bowel, with restoration of continuity, is indicated. Urgent surgery may be required in rare cases of sustained or recurrent hemorrhage, perforation, abscess, and toxic megacolon. Partial small bowel obstruction or intra-abdominal abscess may sometimes be treated conservatively with intravenous (IV) hydration, nasogastric suction, and parenteral nutrition if there is no evidence of ischemia. [2, 85]
If pharmacotherapy or nutritional therapy is ineffective or unadaptable for those with active colonic disease, the combination with granulocyte monocyte apheresis may be considered. [12]
The first randomized controlled trial of stem cell transplantation in treatment-resistant Crohn disease was performed in 2013, in 45 patients with moderately to severely active disease. All patients underwent stem cell mobilization with cyclophosphamide and filgrastim and were then randomly assigned to immediate stem cell transplantation (at 1 month) or delayed transplantation (at 13 months; control group). At 1-year follow-up, objective endoscopic findings were substantially better in the treatment group; on the Simple Endoscopic Score for Crohn's Disease (SES-CD), the mean lower gastrointestinal score fell from 13 to 4 in the treatment group but remained unchanged in the control group. The treatment group also showed a greater decrease than the control group in median Crohn's Disease Activity Index score (approximately 165 vs 50 points). Two thirds of the treatment group were able to discontinue immunosuppressive drugs and steroids at 1 year, compared with 15% of the control group. [86]
About 7% of large bowel strictures in patients with long-standing Crohn disease are malignant; these should be surveyed with multiple biopsies and cytologic brushing for neoplastic transformation. [7] Resection is generally performed when strictures cannot be appropriately surveyed, if neoplastic changes are observed, or obstruction is persistent. [7]
Outpatient vs inpatient management
Many patients with an exacerbation of Crohn disease can be treated on an outpatient basis. However, if a serious complication of Crohn disease (eg, obstruction, perforation, abscess, or hemorrhage) is a concern or if outpatient treatment fails, IV therapy (eg, corticosteroids, antibiotics, or total parenteral nutrition [TPN]) may be required, and hospitalization is warranted.
Patients should be examined on a regular basis, with the frequency of examination depending on the severity and activity of their disease. Follow-up laboratory workup and diagnostic testing should be performed regularly as needed to monitor the safety and success of therapy. Clinical and biochemical responses to treatment should be determined in the 12-week period after the initiation of therapy, and endoscopy or transmural responses to therapy should be evaluated in the 6 months after the start of therapy. [59]
Step-up vs top-down approach
Therapy for mild Crohn disease is typically administered in a sequential “step-up” approach, in which less aggressive and less toxic treatments are initiated first, followed by more potent medications or procedures if the initial therapy fails. Patients are treated with preparations of 5-aminosalicylic acid (5-ASA), antibiotics, and nutritional therapy. However, the use of 5-ASA for the treatment of Crohn disease is controversial; only a small subset of patients may benefit from this agent. Although 5-ASA is effective for reducing disease activity in active Crohn disease, it typically has a lower efficacy for Crohn disease than for ulcerative colitis, and its efficacy for maintenance of remission remains unproven. [12]
If no response occurs or if the disease is more severe than initially thought, corticosteroids and inhibitors of DNA synthesis (ie, immunomodulators) with 6-mercaptopurine (6-MP)/azathioprine or methotrexate are administered. Finally, biologic agents (infliximab, adalimumab, certolizumab pegol, and natalizumab) and surgical therapies can be useful.
For the treatment of moderate to severe Crohn disease, current recommendations include the “top-down” approach, which differs from the conventional step-up approach in that more potent agents are administered initially. Top-down therapies include biologic agents and steroids as needed versus combination therapy with both biologic drugs and immunomodulator agents.
Randomized trials have shown that the combination of azathioprine or 6-MP with a biologic agent is more effective in the induction of remission, steroid-free remission, and mucosal healing than either azathioprine/6-MP alone or a biologic agent alone. [87] However, it is unclear whether these findings hold true in clinical practice; thus, physicians must consider the risks and benefits of therapy to avoid overtreatment.
Azathioprine or 6-MP is effective for maintenance of remission in Crohn disease; each is effective for avoiding surgery and for preventing postoperative and endoscopic relapse. [12] Both agents can be used in combination with infliximab.
At present, it is clear that a subset of patients with Crohn disease is at a higher risk for complications of the disease; these individuals should be considered for top-down therapy. Poor prognostic indicators include young age at diagnosis, perianal disease, upper GI tract involvement, multiple extraintestinal manifestations (EIMs), active tobacco use, and perforating (ie, fistulizing) disease.

Pharmacologic Therapy for Diarrhea
Diarrhea may develop as a result of active disease. Other possible causes include acute infection (ie, C difficile), bacterial overgrowth, loss of ileocecal valve, short bowel syndrome, lactase deficiency, concomitant celiac disease, and functional bowel syndrome. Chronic diarrhea in Crohn disease responds well to antidiarrheal agents such as loperamide, bile acid binders (for bile acid diarrhea), diphenoxylate with atropine, and tincture of opium. Such agents should be considered with care in active colitis because of the risk of toxic megacolon.
Patients with terminal ileal disease or previous terminal ileal resection may not absorb bile acids normally, and this abnormality can lead to secretory diarrhea in the colon. These patients may benefit from bile acid sequestrants (eg, cholestyramine or colestipol). Those who have extensive ileal disease or have undergone resection of more than 100 cm of the ileum have defective bile salt absorption and develop steatorrhea; they benefit from a low-fat diet and medium-chain triglyceride preparations. Bile sequestrants exacerbate this type of diarrhea.
Abdominal cramps may be reduced with antispasm agents such as propantheline, dicyclomine, or hyoscyamine. However, these drugs should not be used if there is the possibility of bowel obstruction. [2, 85]

Anti-inflammatory and Immunosuppressant Therapy for Active Crohn Disease
For colon and small bowel inflammation in Crohn disease, anti-inflammatory drugs or antibiotics are helpful. Sulfasalazine is useful mainly in colonic disease; the active compound 5-ASA is released in the large bowel by bacterial degradation of the parent compound. Sulfasalazine does not alleviate small bowel disease and has no additive effect or steroid-sparing effect when used together with corticosteroids. In addition, in contrast to its action in ulcerative colitis, sulfasalazine seems not to maintain remission in Crohn disease. [88]
Products such as mesalamine, which release 5-ASA in the distal small bowel when triggered by pH changes, are more useful in patients with small bowel Crohn disease. Long-term maintenance with mesalamine may delay clinical relapse. Controlled release of mesalamine is thought to begin at the pylorus and to continue at a constant rate throughout the small bowel and colon; consequently, this drug is sometimes used when proximal intestinal and gastric Crohn disease is found.
5-ASA provides only modest benefit in preventing relapse of Crohn disease in remission after surgery. It can be considered for mild Crohn disease when immunosuppressive therapy is either not warranted or contraindicated.
A short course of corticosteroid therapy is indicated in patients with severe systemic symptoms (eg, fever, nausea, or weight loss) and in those whose condition does not respond to anti-inflammatory agents. Prednisone is generally helpful in acute inflammation without signs of obvious infection. In patients with a tender, palpable mass, the possibility of an underlying abscess should be excluded before steroids are started. Adding antibiotics such as ciprofloxacin or metronidazole is always beneficial if coexisting infection exists.
Steroids are not indicated for maintenance, because of serious complications (eg, aseptic necrosis of the hip, osteoporosis, cataract, diabetes, and hypertension). Accordingly, once remission is achieved, the agent is slowly tapered. It should be noted that steroids do not modify disease or induce sustained mucosal healing.
Enteric-coated ileal-release preparations with decreased systemic effects (eg, budesonide) have been developed to treat ileal and cecal Crohn disease. Budesonide induces remission in active Crohn disease but is less effective than other standard glucocorticosteroids and is of no benefit in preventing relapse. [89] For relapse after steroid withdrawal, other treatment options are required.
If steroid withdrawal proves difficult, immunosuppressants such as azathioprine or its active metabolite 6-MP may be considered. Treatment response is usually observed within 3-6 months. Because of the risk of bone marrow suppression, careful supervision is needed.
Before the initiation of therapy, thiopurine methyltransferase (TPMT) activity should be measured to identify patients predisposed to altered drug metabolism, which increases the risk of early leukopenia. Measurement of 6-thioguanine nucleotide (6-TG) metabolites is helpful in assessing compliance and adjusting dosing.
Methotrexate is effective in inducing and maintaining remission in Crohn disease in adults; it has also been shown to be effective and well tolerated for maintenance of remission in children. [90] The onset of action is shorter for methotrexate than for 6-MP, and the once-weekly dosing is sometimes preferred. Whether oral therapy is as effective as parenteral therapy is unclear.
A systemic review of the efficacy of biologic therapies in IBD (see below) confirmed that placebo is inferior to anti−TNF-α antibodies and natalizumab in inducing remission of active Crohn disease. [91]

Biologic Therapy
Tumor necrosis factor (TNF), a key inflammatory cytokine and mediator of intestinal inflammation, is expressed prominently in inflammatory bowel disease (IBD). Patients likely to benefit from anti-TNF therapy include the following [84] :
- Patients who have moderate to severe debilitating symptoms of Crohn disease, who have documented active inflammation, or who are dependent on corticosteroids and unable to taper these agents without return of symptoms
- Patients who do not show evidence of active bowel infection as a cause of GI symptoms
Before administering anti-TNF agents, clinicians should screen patients for Mycobacterium tuberculosis. In addition, caution is advised if a patient is a hepatitis B virus carrier. [84]
In September 2011, the US Food and Drug Administration (FDA) issued a notification regarding updates to the Black Box Warning for the entire class of TNF-α blockers. [92] The advisory addressed the risk of Legionella and Listeria infections, as well as the consistency of the information in the Boxed Warning and Warnings and Precautions sections regarding the risk of serious infections and the associated disease-causing organisms. [92]
Another area of concern with the use of these anti−TNF-α medications is that several patients have been reported to develop a rare hepatosplenic T-cell lymphoma when treated with dual therapy of 6-MP or azathioprine as well as a TNF-α inhibitor. Although this has been a rare complication, all reported cases have been in adolescents and young adults.
A meta-analysis that evaluated the efficacy of anti-TNF treatment with or without immunomodulator (IM) therapy in patients with Crohn disease following ileal pouch anal anastomosis (IPAA) for ulcerative colitis showed no difference in clinical outcomes (both complete and partial clinical response rates) with anti-TNF monotherapy relative to anti-TNF therapy administered concurrently with IM. [93] However, use of anti-TNF monotherapy showed a trend toward a higher risk of major and minor adverse events compared with anti-TNF with IM.
Infliximab
Infliximab is a chimeric mouse-human monoclonal antibody against TNF-α that has shown promise in Crohn disease treatment; it blocks TNF-α in the serum and at the cell surface, leading to the lysis of TNF-producing macrophages and T cells. Infliximab has also been approved for the treatment of pediatric Crohn disease.
According to the American Gastroenterological Association (AGA), infliximab is indicated for the following:
- Treatment of patients with Crohn disease who do not achieve adequate clinical response despite treatment with conventional therapy (ie, a corticosteroid or an immunosuppressive agent)
- Treatment of fistulizing Crohn disease that is refractory to conventional therapy (ie, antibiotics, surgical drainage with examination under anesthesia, immunosuppressive therapy, or combinations thereof) [94]
Patients who respond to induction therapy with infliximab should receive maintenance therapy. [94]
In one study, nearly 65% of refractory cases of Crohn disease responded well to treatment with infliximab (5 mg/kg), and one third went into complete remission. [95] Patients who relapsed after the initial response responded again to further infusions.
Infliximab is also effective in patients who have refractory perianal and enterocutaneous fistulae. Current clinical practice is to give 5 mg/kg IV at 0 weeks, 2 weeks, and 6 weeks, followed by maintenance IV infusions every 8 weeks. On average, the effect lasts for 12 weeks.
Important adverse effects include the development of a lupus like syndrome, multiple sclerosis, psoriasiform rash, and opportunistic or fungal infections (eg, Pneumocystis jiroveci pneumonia or histoplasmosis). Anti–double-stranded DNA is not always associated with clinical lupus. An added benefit of infliximab treatment is the potential ability to taper steroids, which will decrease further adverse effects. [96, 97]
In a study of 115 patients with Crohn disease who were treated for 1 year with infliximab and an antimetabolite, with at least 6 months of corticosteroid-free remission, and then followed up at 1 year (median, 28 months), nearly half (52/115; 45.2%) experienced a relapse. [98] The 1-year relapse rate was 43.9%.
In this study, risk factors for relapse included male sex, leukocyte count higher than 6.0 × 109/L, C-reactive protein (CRP) level of 5.0 mg/L or higher, and fecal calprotectin level of 300 µg/g or higher. [98] However, retreatment with infliximab was effective in 88% of patients with a relapse and was well tolerated.
Unfortunately, infliximab is immunogenic, and long holidays between infusions may result in the development of antibodies to infliximab that lead to infusion reactions, loss of efficacy, and delayed hypersensitivity reactions. [99]
Adalimumab and certolizumab pegol
Two other anti−TNF-α agents, adalimumab and certolizumab pegol, may be less immunogenic than infliximab and have shown efficacy in the treatment of Crohn disease that is refractory to the standard medical treatment of corticosteroids and inhibitors of DNA synthesis. [99]
Adalimumab is a recombinant human immunoglobulin (Ig) G1 monoclonal antibody that binds with a high affinity and specificity to human soluble TNF-α but not to lymphotoxin (TNF-β). Study results have shown that the immunogenicity of adalimumab is low compared with that of the chimeric agent infliximab. [99]
Two placebo-controlled trials, CLASSIC I and II (CLinical assessment of Adalimumab Safety and efficacy Studied as Induction therapy in Crohn’s disease), showed that adalimumab was effective for both induction and maintenance of remission in patients who were previously naive to anti-TNF therapy. [26, 100]
The CHARM (Crohn’s trial of the fully Human antibody Adalimumab for Remission Maintenance) trial demonstrated the same effect in a mixed population of patients who were either naive to infliximab therapy or who had previously been on infliximab therapy. [101] In patients who had lost response to or become intolerant of infliximab, the GAIN (Gauging Adalimumab efficacy in Infliximab Nonresponders) trial results showed a benefit from adalimumab therapy induction with remission at 4 weeks. [102]
Furthermore, an open-label study conducted in France that assessed the long-term efficacy and safety of adalimumab maintenance therapy in this population showed that it was well tolerated and effective in maintaining clinical remission in patients who had Crohn disease with a lost response to or intolerance of infliximab. [26, 103, 104]
A review of randomized clinical trials using adalimumab in the treatment of Crohn disease recommended initiating adalimumab as a loading dose of 160/80 mg subcutaneously at week 0/week 2, followed by 40 mg every other week as a maintenance dose in order to determine whether there is a response. [84]
In the PRECISE (Pegylated Antibody Fragment Evaluation in Crohn’s Disease: Safety and Efficacy) trials, certolizumab pegol, a humanized Fab’ antibody fragment conjugated to polyethylene glycol, demonstrated efficacy in maintaining remission in patients with moderately to severely active Crohn disease.
In PRECISE 1, certolizumab yielded greater clinical response (37%) in patients with high CRP levels (≥ 10 mg/L) at week 6 than placebo (26%), as well as greater persistence of response at 6 months (22% vs 12%, respectively). [105] Remission rates did not differ between treatment and placebo groups. In PRECISE 2, when week 6 responders were randomized to drug or placebo, certolizumab yielded clinical remission in 36% and clinical response in 63%. [106] In PRECISE 3, 41% of patients achieved remission at 12 months and 36% at 18 months. [107]
Natalizumab
Natalizumab is a humanized monoclonal antibody that prevents the accumulation of lymphocytes in the diseased bowel by binding α4β7 integrin (gut specific). It also binds to α4β1 integrin (CNS specific). Clinical data indicate that this drug is effective in inducing clinical response and remission of active moderate to severe Crohn disease. It is administered in a single 300-mg dose every 4 weeks up to 12 weeks, at which time it may be stopped if it is not effective.
This drug was initially taken off of the market in 2005 as a result of reported cases of progressive multifocal leukoencephalopathy (PML) in patients with multiple sclerosis [2] ; it was then reintroduced into the market with restrictions for the indication of refractory multiple sclerosis in 2006 and Crohn disease in 2008.
PML is an opportunistic infection caused by the JC virus that typically only occurs in patients who are immunocompromised. As of August 1, 2012, there were 271 confirmed cases of PML worldwide; in individuals treated with natalizumab, one was a patient with Crohn disease who was receiving 35 infusions of natalizumab. [108] Individuals at risk include those with the following [109] :
- JC virus antibody positivity
- Previous exposure to immunosuppressant therapy
- More than 2 years of treatment with natalizumab
The risk of developing PML is less than 1 in 1000 users, but it increases to 11 per 1000 users if all 3 risk factors are present. [109]
Natalizumab therapy is currently reserved for individuals with moderate to severe Crohn disease who are intolerant of or have lost response to other biologic or immunosuppressant therapies. The ENACT (Evaluation of Natalizumab As Continuous Therapy) [110] and ENCORE (Efficacy of Natalizumab in Crohn’s Disease Response and Remission) [111] trials evaluated the efficacy of natalizumab in the induction of response (ENACT 1 and ENCORE) and maintenance of response (ENACT 2) in patients with active Crohn disease.
In ENACT 1, natalizumab induced clinical response at week 10 in patients with an elevated C-RP level. [110] ENCORE found a 48% clinical response rate and 26% clinical remission rate at week 8. [111] In ENACT 2, of patients from ENACT 1 who had had a clinical response to natalizumab and who were rerandomized to maintenance therapy with natalizumab 300 mg or placebo every 4 weeks for 1 year, 61% of patients in the natalizumab group maintained response, and 44% achieved remission through weeks 36 and 60. [110]
Vedolizumab
Vedolizumab, another integrin antagonist, is approved for Crohn disease and ulcerative colitis. It is specific for α4β7 integrin. Approval was based on a large phase 3 clinical trial conducted to simultaneously evaluate vedolizumab for both UC and CD that included several clinical studies involving 2,700 patients in nearly 40 countries.
Among patients with CD who had a response to induction therapy with vedolizumab, 39.0% of those assigned to vedolizumab every 8 weeks were in clinical remission at week 52, compared with 21.6% assigned to placebo (P< 0.001). [112]
Ustekinumab
Ustekinumab inhibits interleukin (IL)-12 and IL-23 cytokines, which play a key role in inflammatory and immune responses. In September 2016, the FDA approved ustekinumab for adults with moderately to severely active Crohn disease who have [113, 114] :
- Failed or were intolerant to immunomodulators or corticosteroids, but never failed treatment with a TNF blocker, OR
- Failed or were intolerant to treatment with 1 or more TNF blockers
FDA approval was based on three phase 3 studies (UNITI-1, UNITI-2, IM UNITI) in more than 1300 patients. [113, 114] Of patients who were either new to, experienced with, or failed biologic therapy (TNF blockers), between 34% (UNITI-1 study) and 56% (UNITI-2 study) of patients experienced relief of symptoms within 6 weeks after receiving ustekinumab as a one-time IV infusion. Noticeable improvement was observed as early as 3 weeks. A majority of those who responded to induction dosing and continued treatment with subcutaneous (SC) maintenance doses every 8 weeks were in remission at the end of 44 weeks (52 weeks from the initiation of the induction dose). [115, 116, 117]
Results from the ENEIDA registry study that evaluated the the real-world, short-term effectiveness of ustekinumab in 305 patients with highly refractory Crohn disease found clinical remission rates of 47% at week 8 and 58% at week 14 of treatment, normalization of fecal calprotectin levels in 46% at week 8 and 54% at week 14, and normalization of C-reactive protein levels in 35% at week 8 and 41% at week 14. [118]
Other agents
Tacrolimus may be effective in treating Crohn disease. A systematic review of the role of tacrolimus found remission rates of 44.3% for patients with luminal Crohn disease and 28.6% for patients with perianal disease when this agent was used systemically. [119] The review noted that in studies of topical use of tacrolimus, 35.7% of patients achieved remission and 28.6% a partial response. [119]
Side effects included tremor, paresthesia, and headache; recurrent nephrotoxicity occurred in 16% of patients. [119] Although this review appears to support the use of tacrolimus, the investigators noted that randomized controlled trials are needed.
Mycophenolate mofetil has been used in the short- and long-term treatment of difficult IBD. This agent inhibits a de novo pathway of purine synthesis in lymphocytes, leading to intracellular depletion of guanosine monophosphate and resulting in the suppression of cytotoxic T cells and the formation of antibodies by activated B cells. A dose of 500 mg twice daily in 2 divided doses is well tolerated by patients and can be used to reduce the steroid dose. [2, 85]
Early studies have suggested the use of the helminth Trichuris suis for the treatment of Crohn disease. This suggestion is based on the observation that the disease is common in highly industrialized Western countries, where helminths are rare, but uncommon in less developed areas of the world, where most people carry the worms. It is believed that helminths diminish immune responsiveness in naturally colonized humans and reduce inflammation in experimental colitis. Studies evaluating the use of T suis eggs for this purpose are under way.
Evidence supporting the efficacy of low-dose oral naltrexone for the treatment of Crohn disease is limited; the 2 main studies had small patient cohorts and short duration of follow-up. Thus, at present, there is no clear indication for the use of low-dose oral naltrexone for Crohn disease. However, a small subpopulation may benefit from treatment. Further, larger studies may be warranted.

Management of Fistulae
Fistulae between bowel loops (ileoileal, ileocecal, ileosigmoid, enterovesicular, enterocutaneous, cologastric, and coloduodenal) can occur in patients with progressive Crohn disease. Surgical intervention may be required; left untreated, fistulae can cause complications such as unexplained diarrhea, abdominal pain, or abscess formation. Occasionally, medical management with oral metronidazole or ciprofloxacin can be used to treat underlying infections and symptoms until more definitive medical or surgical planning can be established.
In general, localized and systemic sepsis do not occur in fistulae that originate in diseased bowel and involve other intra-abdominal organs or the skin. [7] However, the presence of sepsis necessitates the initiation of broad-spectrum antibiotic agents. Radiologic studies should be performed to rule out concomitant abscesses, which should be drained when present. In cases of persistent sepsis, the diseased bowel is generally excised, whether an abscess is present or not (see Surgical Intervention).
Perianal fistulae can be a debilitating complication of Crohn disease. A multidisciplinary and top-down approach may be required to induce remission of more complex fistulae. A small study demonstrated that the combination of ciprofloxacin and metronidazole in 14 patients with perianal fistulae healed the fistulae in 3 patients and improved the condition of 85% of the patients. [120]
There is good evidence that the combination of antibiotics, current medical therapy (anti–TNF-α agents with or without azathioprine/6-MP) and surgical drainage of abscesses followed by seton placement is of greatest efficacy in improving the outcome of perianal fistulizing disease. [82]
In addition, the use of endoscopic ultrasonography (EUS), magnetic resonance imaging (MRI), or both to identify the anatomy and monitor fistula activity in conjunction with the above-mentioned management approach has been shown to help in the maintenance of fistula closure. [82]

Nutritional Therapy and Diet Modification
Nutritional therapy is another important modality for the treatment of disease, malnutrition, and growth failure in Crohn disease. Although ineffective as a primary therapy, nutritional manipulations that facilitate bowel rest can be effective adjuncts in the treatment of active Crohn disease. A dramatic reversal of malnutrition and a change in growth velocity can be expected in all children treated with adequate nutrition in conjunction with medical therapy to control symptoms of Crohn disease.
Both parenteral and enteral nutrition are effective. Additionally, exclusive enteral nutrition (EEN) has been shown to be as effective as corticosteroids for the induction of remission and might promote better gastrointestinal (GI) tract mucosal healing. [121] An elemental diet is effective for maintaining remission. [12] Consumption of at least 1200 kcal/day has been associated with lower rates of disease relapse, but patients frequently relapse after initiation of a normal diet. [122, 123, 124]
Because most patients have appetite suppression, overnight nasogastric feeds are often used in children. Nighttime supplemental enteral nutrition without daytime dietary restrictions has been shown to be beneficial in maintaining disease remission. Although the exact mechanism of action is unknown, beneficial effects could be due to an altered intestinal flora, a reduced antigen load, and decreased inflammatory cytokine levels.
Patients with Crohn disease require a balanced diet. Fiber supplementation is said to be beneficial for patients with colonic disease, in that dietary fiber can be converted to short-chain fatty acids, which provide fuel for colonic mucosal healing; a low-roughage diet is usually indicated for patients with obstructive symptoms.
Because patients with Crohn disease of the small intestine are often lactose-intolerant, avoidance of dairy products may be indicated. However, calcium supplementation may be required. Osteoporosis is a common nutritional complication, resulting not only from decreased calcium absorption in those with active small bowel disease but also from the release of cytokines from inflammatory cells, which stimulate osteoclast activity and lead to increased bone breakdown. Corticosteroid use is another risk factor for osteoporosis. [122, 123, 124]
An international survey of enteral nutrition formula protocols for children with Crohn disease found that the most common duration of EEN administration was 6-8 weeks and that 90% of centers used polymeric formulas with a variety of flavorings added. [125] The most common recommendation for the reintroduction of food after EEN was gradually introducing food as the use of formula was decreased (52%) or beginning a low-fiber diet (26%). [125]
Patients who undergo extensive resection of the terminal portion of the ileum may benefit from a low-fat diet with the addition of medium-chain triglyceride preparations.
Selected patients may require TPN. Short-term use of TPN (given preoperatively) is appropriate for patients with active inflammation, abscesses, fistulae, and severe malnutrition. Long-term TPN is suitable for patients who have undergone extensive intestinal resection, resulting in short bowel syndrome. [85]

Surgical Intervention
Indications
Surgery plays an integral role in controlling the symptoms and treating the complications of Crohn disease, but operative resection is not curative. Because of the high rate of disease recurrence after segmental bowel resection, the guiding principle of surgical management of Crohn disease is preservation of intestinal length and function. [1] Recommended indications for surgical intervention include the following [1] :
- Persistent symptoms despite high-dose corticosteroid therapy
- Treatment-related complications, including intra-abdominal abscesses
- Medically intractable fistulae
- Fibrotic strictures with obstructive symptoms
- Toxic megacolon
- Intractable hemorrhage
- Perforation
- Cancer
In 2007, the Standards Practice Task Force of the American Society of Colon and Rectal Surgeons (ASCRS) published recommendations for surgery in patients with Crohn disease (see Table 2, below). [7]
Table 2. ASCRS Indications for Surgical Management of Crohn Disease (Open Table in a new window)
| Operative Indication | Factors for Considering Surgery |
|---|---|
| Failed medical therapy | Presence of disease-related symptoms not responsive to medical management; condition demonstrates an inadequate response When first- and second-line therapies do not induce remission safely in severe disease Before escalating medical therapy in severe or steroid-dependent disease with limited extent (eg, disease with stricturing behavior, patients who have contraindications or risk factors for further medical therapy) |
| Perforation | Presence of symptoms or signs of free perforation Immediate resection of perforated segment (has a relatively high mortality) After small bowel resection or perforation, other procedures can be performed, as needed (eg, end stoma, diverted or nondiverted anastomosis) |
| When large anteroparietal, interloop, intramesenteric, or retroperitoneal abscesses cannot be or are unsuccessfully managed with antibiotics and percutaneous drainage Perform surgical drainage in such cases, with or without resection | |
| Persistent enteric fistulae and symptoms or signs of localized or systemic sepsis despite appropriate medical management Persistent sepsis warrants excision of the diseased bowel, whether or not an abscess is present For target or “innocent bystander” organs, diseased bowel is typically resected, noninflamed bowel primarily closed, and other internal organs primarily closed or allowed to heal by secondary intentionNote: Operative intervention may be avoided for asymptomatic internal fistulae | |
| Obstruction | Presence of symptomatic strictures in regions not amenable or responsive to medical therapy |
| Presence of asymptomatic colonic strictures that cannot be adequately surveyed by biopsy or cytology brushing | |
| Inflammation | Presence of acute colitis and symptoms or signs of impending or actual perforation (eg, transverse colon distention > 6 cm on abdominal x-ray or persistent gaseous colonic distention indicate toxic megacolon, pneumatosis coli, evolving local peritonitis, multiple organ failure) Presence of severe or fulminant colitis |
| Worsening acute colitis or failure to significantly improve despite 48-96 hours of appropriate medical therapy | |
| Hemorrhage | Presence of massive hemorrhaging of any origin that (1) cannot be or fails to be managed with interventional or endoscopic techniques and (2) occurs in hemodynamically unstable patients Mesenteric angiography with embolization may be attempted when adequate endoscopic visualization is not possible or when the bleeding source cannot be identified; if this technique is not successful or the patient is hemodynamically unstable, laparotomy with or without intraoperative endoscopy and resection of the responsible bowel segment may be required |
| Neoplasia | Presence of chronic Crohn disease of the ileocolon or colon (endoscopic surveillance) Presence of adenomatous-appearing polyps (excision) |
| Presence of carcinoma, DALM, high-grade dysplasia, multifocal colonic or rectal low-grade dysplasia (resection) | |
| Presence of chronic Crohn disease of the terminal ileum, ileocolon, or upper GI region | |
| Growth retardation and EIMs | Presence of significant growth retardation in prepubertal patients despite appropriate medical therapy |
| Presence of symptomatic dermatologic, oral, ophthalmologic, or joint disorders refractory to medical therapy (resection of diseased intestine) | |
| ASCRS = American Society of Colon and Rectal Surgeons; DALM = dysplasia-associated lesion or mass; EIM = extraintestinal manifestation; GI = gastrointestinal.Source: Strong SA, Koltun WA, Hyman NH, Buie WD, for the Standards Practice Task Force of The American Society of Colon and Rectal Surgeons. Practice parameters for the surgical management of Crohn’s disease. Dis Colon Rectum. 2007;50(11):1735-46. [7] |
Recommended procedures
Unlike ulcerative colitis, Crohn disease has no surgical cure. Most patients with Crohn disease require surgical intervention during their lifetime. Within 15 years of diagnosis, 70% of patients with Crohn disease have required 1 or more surgical procedures, and many require multiple procedures. [42]
Approximately 85-90% of patients develop disease recurrence within the first postoperative year. Therefore, every attempt at conserving the small bowel should be made in the surgical approach to Crohn disease. However, repeated intestinal resection for Crohn disease is a major cause of short bowel syndrome. Several agents have been shown to decrease the likelihood of disease recurrence in individuals who have had ileocolic resections, including antibiotics, azathioprine/6-MP, and biologic agents. [126, 127, 128, 129, 130]
The ASCRS has identified recommended surgical procedures for site-specific Crohn disease (see Table 3, below). [7]
Table 3. ASCRS Recommendations for Site-Specific Operative Management of Crohn Disease (Open Table in a new window)
| Site | Surgical Intervention |
|---|---|
| Terminal ileum, ileocolon, upper GI tract | Resection of the affected bowel for jejunal, proximal ileal, terminal ileal, or ileocolic disease in the absence of existing or impending short bowel syndrome Ileocolostomy or proximal loop ileostomy in cases where there is concern about damage to nondiseased bowel, superior mesenteric vessels, retroperitoneal structures Drainage of any septic foci with later definitive resection (after several months’ delay) |
| Strictureplasty for nonphlegmonous jejunal, ileal, or ileocolic strictures in the absence of existing or impending short bowel syndrome Strictureplasty when multiple jejunal or proximal/terminal ileum strictures are present | |
| Bypass or strictureplasty for symptomatic gastric or duodenal disease | |
| Endoscopic dilatation of symptomatic, accessible strictures of the intestinal tract Note: Surgical services should be available in case of perforation | |
| Colon | Subtotal or total colectomy with end ileostomy for colonic disease requiring emergency or urgent surgery (via laparoscopic or open approach) |
| Segmental or total colectomy with or without primary anastomosis for colonic disease requiring elective surgery | |
| Total proctocolectomy or proctectomy with stoma creation for rectal disease requiring surgery | |
| ASCRS = American Society of Colon and Rectal Surgeons; GI = gastrointestinal.Source: Strong SA, Koltun WA, Hyman NH, Buie WD, for the Standards Practice Task Force of The American Society of Colon and Rectal Surgeons. Practice parameters for the surgical management of Crohn’s disease. Dis Colon Rectum. 2007;50(11):1735-46. [7] |
The most common complication of Crohn disease, occurring in 30-50% of patients, is small bowel obstruction. Typically, it is due to intestinal strictures from repeated bouts of inflammation and subsequent fibrosis. For a partial or complete obstruction refractory to nonsurgical management, surgical intervention is required. Surgical options include resection of the strictured bowel and strictureplasty. For long (> 12 cm) strictures or multiple strictures in close proximity, surgical resection with primary anastomosis is often required.
Strictureplasty for multiple shorter strictures has the benefit of conserving the bowel. A Foley catheter (inflated to 25 mm) can be passed through the lumen to detect additional distal strictures. The strictured bowel is incised longitudinally to a point 1-2 cm beyond the narrowing and then closed transversely without resection.
For long or multiple confluent strictures, a stricturoplasty that resembles a Finney side-to-side pyloroplasty (“essentially a side-to-side gastroduodenostomy” [131] or a “side-to-side anastomosis of antrum and duodenum that … does not exclude the pyloric area” [132] ) can be used to conserve bowel length.
Hydrostatic balloon dilatation of ileocolic strictures has been performed, but its effects may not be long lasting. Bypass procedures are usually reserved for duodenal obstructions. [133, 134]
Other complications of Crohn disease that may require operative intervention include free perforation, abscesses, fistulae, toxic megacolon, and massive hemorrhage. More than 10% of patients with Crohn disease have an intra-abdominal or pelvic abscess during their lifetime. Unfortunately, many patients at risk for perforation or abscess will be on corticosteroids, which are known to suppress peritoneal signs and fever and mask the presenting signs of infection. Computed tomography (CT) helps confirm the diagnosis.
Abscesses must be drained, either surgically or percutaneously, and treated with broad-spectrum antibiotics. Although surgical drainage is more often successful, attempting percutaneous drainage first may spare some patients an operation. [135]
Enteroenteric, enterocutaneous, enterovesical, and rectovaginal fistulae are often treated initially according to the principles of fistula healing and medical therapy. If medical therapy is unsuccessful, resection of the involved bowel is required in symptomatic patients.
Toxic megacolon and massive hemorrhage are much less common complications but may require urgent bowel resection when present. Total abdominal colectomy with a Hartmann pouch has been advocated for fulminant toxic megacolon; this allows future restoration of bowel continuity with a sphincter-preserving ileorectal anastomosis. However, a permanent ileostomy may ultimately be required to treat recurrent rectal disease. [136]
In a study comprising a strictly defined cohort of patients, Kiran et al were not able to identify segmental bowel resection as an independent risk factor for recurrence or stoma formation; additionally, they found no reduction in quality of life scores to suggest an adverse effect of recurrence. [137] Nevertheless, segmental colectomy provides good function, and the data support practice of a conservative approach with anastomosis in anatomically linked Crohn disease.
Perianal Crohn disease presents a particularly difficult management challenge. Fissures, fistulae, and abscess may be multiple and recurrent, and repeat operations may lead to sphincter damage and incontinence.
True abscess requires drainage. When a fistula tract can be identified, a seton can be used to prevent premature skin closure and recurrent abscesses. These indwelling setons should be left in place for an extended period (up to 6-12 months) to allow complete epithelialization of the tract as visualized by MRI or EUS. This approach leads to a chronically draining fistula tract. In cases where severe perianal disease has destroyed the sphincter, proctectomy with permanent ileostomy may be necessary. [80, 138, 139, 140]
Laparoscopic versus open resection
The laparoscopic approach to Crohn disease has been shown to be feasible as well as safe. [141, 142] Complications of Crohn disease such as abscesses, phlegmons, and recurrent disease have been safely treated laparoscopically and are not contraindications to laparoscopy in these patients.
Although open resection is still performed by many surgeons and should be considered the criterion standard, the laparoscopic approach is being employed with increasing frequency. In children, laparoscopic intestinal resections have been used for proctectomy and pull-through procedures in Hirschsprung disease for more than a decade. [143] Segmental intestinal resections in Crohn disease can easily be accomplished as well.
No difference in recurrence rates has been found in adults undergoing laparoscopic as opposed to open ileocolic resection, and the laparoscopic approach has been found to shorten the duration of postoperative ileus significantly. [144, 145] Adult patients who undergo laparoscopic ileocecectomy tend to experience a better quality of life than those who undergo the equivalent open procedure. In addition, patients undergoing laparoscopic resection report that they are more satisfied with the physical appearance of their surgical scar. [146]
A study comparing laparoscopic ileocolic resection with infliximab in the treatment of distal ileitis is in progress in the Netherlands. The primary outcomes of the study are quality of life and costs, with recurrence being a secondary outcome. [147] To date, no data have been published on recurrence rates in children undergoing open versus laparoscopic resection.
Preparation for resection
Preoperatively, a recent evaluation of the extent of intestinal disease with appropriate radiologic and endoscopic studies is essential. Steroids are tapered as much as is tolerable, and the patient’s nutritional status is optimized.
In cases where stomas may be required, preoperative counseling better prepares the patients and their families for this possibility. A stomal therapist or nurse should be involved with patient care before the surgical procedure. Patients should also be counseled about the expectations of surgery, because future recurrences are likely.
Most patients will have received corticosteroids recently. Therefore, perioperative steroid dosing will likely be required.
Perianal, rectal, and sigmoidoscopic examinations are often performed while the patient is under anesthesia to determine the presence and extent of perianal disease.
The goal of surgical resection is to remove the grossly involved bowel; microscopic disease at resection margins is acceptable. Primary anastomosis of bowel can usually be achieved. Occasionally, a proximal functioning stoma or Brooke ileostomy is required in patients in whom an anastomosis would be unsafe.
Operative steps: laparoscopic resection
After the patient is placed under general endotracheal anesthesia and a urinary catheter is introduced, the abdomen is prepared and draped widely. A 12-mm incision is made in the umbilicus, through which a 12-mm cannula is introduced for future insertion of the endoscopic stapling device. Two 5-mm incisions are made, one in the left mid abdomen and the other in the left suprapubic region; through these, grasping forceps are inserted for retraction.
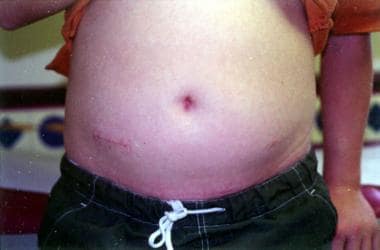
The final port (if necessary) is initially 5 or 10 mm in length and is placed in the right lower abdomen in a location similar to an open appendectomy incision. This incision is subsequently enlarged to approximately 2 cm, and the specimen is extracted from the abdominal cavity through this incision (see the image below). In addition, the 2 ends of the intestine to be anastomosed are exteriorized through this incision, and a 2-layer extracorporeal anastomosis is created.
Crohn disease. This postoperative photograph depicts incisions used for laparoscopic ileocolectomy in a 14-year-old male adolescent with obstruction of the terminal ileum. Note the 2-cm incision in the right lower abdomen, through which the specimen was extracted and extracorporeal anastomosis performed. A 12-mm umbilical incision is nicely hidden in the depths of the umbilicus. A 5-mm incision is visible in the left lower abdomen, and another is in the left suprapubic region just above the top of the pants.
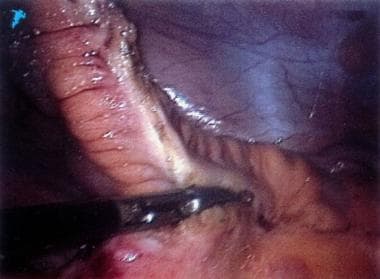
The first step in the operation is ligation and division of the proximal ileum with the endoscopic stapler. Next, with either an UltraCision Harmonic Scalpel (Ethicon Endosurgery, Cincinnati, OH) or a LigaSure device (Valley Lab, Boulder, CO), the mesentery of the proximal right colon is coagulated and transected (see the image below). Then, the right lower abdominal incision is enlarged to 2 cm and the specimen is exteriorized.
Crohn disease. On this laparoscopic photograph, the mesentery of the terminal ileum is being coagulated with a sealing device (LigaSure). Note that the ligation of the mesentery proceeds near the border of the ileum rather than at the base of the mesentery.
Alternatively, the umbilical incision may be enlarged to allow exteriorization of the specimen. With this technique, the distal margin of resection is more precisely determined, and the distal resection margin can be divided with the surgical stapler. This procedure may also be performed intracorporeally with an endoscopic stapler.
Once the resected specimen is removed, the proximal small intestine is delivered through the right lower abdominal incision (or the enlarged umbilical incision), and a 2-layer extracorporeal anastomosis is created between the proximal and distal margins. The bowel is then returned to the abdominal cavity, and all incisions are closed.
Postoperative management
Postoperatively, steroids are tapered appropriately. Patients who were receiving low-dose or short-term steroids preoperatively may be treated with a more rapid taper. Often, patients who have received long-term steroid therapy will be given so-called stress-dose steroids intraoperatively to prevent adrenal insufficiency during a time when the body is under high stress (as is the case in surgery). This stress dose is tapered rapidly. Parenteral nutrition is often continued until bowel function returns.
Complications of surgery
The most common complication of surgical treatment of Crohn disease is the development of intraperitoneal adhesions. Patients with Crohn disease undergoing abdominal surgery are also at increased risk for the development of enterocutaneous fistulae as a result of their surgery. Those who are being treated with steroids or immunosuppressive agents may be at increased risk of wound or intra-abdominal infections.

Consultations
Crohn disease is a chronic disease that requires treatment by a team of experts consisting of primary care providers, gastroenterologists, psychologists, nutritionists, social workers, and nurses. A multidisciplinary approach involving the participation of specialists such as surgeons, dermatologists, rheumatologists, endocrinologists, and obstetricians is often necessary to manage complications of the disease, as well as potential side effects of therapy, if these occur unexpectedly.
A critical factor in the successful management of Crohn disease is the willingness of the patient to participate and cooperate with the team. Adherence to therapy and the management plan is essential in improving outcomes. Patients and parents must be educated and receive support to treat this disorder effectively.

Preventive Care
The American College of Gastroenterology released their guideline on preventive care in inflammatory bowel disease (IBD) in 2017. [148] Their preventive health maintenance recommendations are outlined below.
Strong recommendations
These include the following:
- Patients with IBD (both ulcerative colitis and Crohn disease) should undergo screening for melanoma independent of the use of biologic therapy. IBD patients on immunomodulators (6-mercaptopurine or azathioprine) should undergo screening for nonmelanoma skin cancer (NMSC) while using these agents, particularly those older than 50 years.
- Patients with conventional risk factors for abnormal bone mineral density with ulcerative colitis and Crohn disease should undergo screening for osteoporosis with bone mineral density testing at the time of diagnosis and periodically after diagnosis.
- Adults with IBD older than 50 years should consider vaccination against herpes zoster, including certain subgroups of immunosuppressed patients.
Conditional recommendations
These include the following:
- Patients with conventional risk factors for abnormal bone mineral density with ulcerative colitis and CD should undergo screening for osteoporosis with bone mineral density testing at the time of diagnosis and periodically after diagnosis.
- Adults with IBD should receive age-appropriate vaccinations before initiation of immune suppression when possible.
- Household members of immunosuppressed patients can receive live vaccines with certain precautions.
- All adult patients with IBD should undergo annual vaccination against influenza. Those on immunosuppressive therapies and their household contacts should receive the non-live trivalent inactivated influenza vaccine, but not the live inhaled influenza vaccine.
- Adult patients with IBD receiving immunosuppressive therapy should receive pneumococcal vaccination with both the PCV13 and PPSV23 vaccines, in accordance with national guidelines.
- Adults with IBD should be assessed for prior exposure to varicella and vaccinated if naive before initiation of immunosuppressive therapy when possible.
- Patients with IBD who are immunosuppressed and traveling to areas that are endemic for yellow fever should consult with a travel medicine or infectious disease specialist prior to travel
- Adolescents with IBD should receive meningococcal vaccination in accordance with routine vaccination recommendations.
- Vaccination against tetanus, diphtheria, and pertussis (Tdap); hepatitis A and B (HAV, HBV, respectively); and human papillomavirus (HPV) should be administered as per Advisory Committee on Immunization Practice (ACIP) guidelines.
- Women with IBD on immunosuppressive therapy should undergo annual cervical cancer screening.
- Screening for depression and anxiety is recommended in patients with IBD.

- Kornbluth A, Sachar DB, Salomon P. Crohn's disease. Feldman M, Scharschmidt BF, Sleisenger MH, eds. Sleisenger & Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, and Management. 6th ed. Philadelphia, Pa: WB Saunders Co; 1998. Vol 2: 1708-34.
- Panes J, Gomollon F, Taxonera C, et al. Crohn's disease: a review of current treatment with a focus on biologics. Drugs. 2007. 67(17):2511-37.
- Tierney LM. Crohn's disease. Tierney LM, McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis and Treatment. 40th ed. New York, NY: McGraw-Hill Professional Publishing; 2001. 638-42.
- Mackner LM, Bickmeier RM, Crandall WV. Academic achievement, attendance, and school-related quality of life in pediatric inflammatory bowel disease. J Dev Behav Pediatr. 2012 Feb. 33(2):106-11. [QxMD MEDLINE Link].
- Rabbett H, Elbadri A, Thwaites R, et al. Quality of life in children with Crohn's disease. J Pediatr Gastroenterol Nutr. 1996 Dec. 23(5):528-33. [QxMD MEDLINE Link].
- Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. Nov 2007. 133(5):1670-89. [Full Text].
- Strong SA, Koltun WA, Hyman NH, Buie WD. Practice parameters for the surgical management of Crohn's disease. Dis Colon Rectum. 2007 Nov. 50(11):1735-46. [QxMD MEDLINE Link].
- Farmer RG, Hawk WA, Turnbull RB Jr. Clinical patterns in Crohn's disease: a statistical study of 615 cases. Gastroenterology. 1975 Apr. 68(4 Pt 1):627-35. [QxMD MEDLINE Link].
- D'Haens G, Baert F, van Assche G, et al, for the Belgian Inflammatory Bowel Disease Research Group., North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008 Feb 23. 371(9613):660-7. [QxMD MEDLINE Link].
- Tsianos EV, Katsanos KH, Tsianos VE. Role of genetics in the diagnosis and prognosis of Crohn's disease. World J Gastroenterol. 2012 Jan 14. 18(2):105-18. [QxMD MEDLINE Link]. [Full Text].
- Thoreson R, Cullen JJ. Pathophysiology of inflammatory bowel disease: an overview. Surg Clin North Am. 2007 Jun. 87(3):575-85. [QxMD MEDLINE Link].
- [Guideline] Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018 Mar. 53 (3):305-53. [QxMD MEDLINE Link]. [Full Text].
- Hampe J, Grebe J, Nikolaus S, et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn's disease: a cohort study. Lancet. 2002 May 11. 359(9318):1661-5. [QxMD MEDLINE Link].
- Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001 May 31. 411(6837):599-603. [QxMD MEDLINE Link].
- Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006 Dec 1. 314(5804):1461-3. [QxMD MEDLINE Link].
- Glas J, Seiderer J, Wetzke M, et al. rs1004819 is the main disease-associated IL23R variant in German Crohn's disease patients: combined analysis of IL23R, CARD15, and OCTN1/2 variants. PLoS One. 2007 Sep 5. 2(9):e819. [QxMD MEDLINE Link]. [Full Text].
- Van Limbergen J, Russell RK, Nimmo ER, et al. IL23R Arg381Gln is associated with childhood onset inflammatory bowel disease in Scotland. Gut. 2007 Aug. 56(8):1173-4. [QxMD MEDLINE Link]. [Full Text].
- Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008 Aug. 40(8):955-62. [QxMD MEDLINE Link]. [Full Text].
- Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007 Feb. 39(2):207-11. [QxMD MEDLINE Link].
- Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007 May. 39(5):596-604. [QxMD MEDLINE Link]. [Full Text].
- Parkes M, Barrett JC, Prescott NJ, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007 Jul. 39(7):830-2. [QxMD MEDLINE Link]. [Full Text].
- Libioulle C, Louis E, Hansoul S, et al. Novel Crohn disease locus identified by genome-wide association maps to a gene desert on 5p13.1 and modulates expression of PTGER4. PLoS Genet. 2007 Apr 20. 3(4):e58. [QxMD MEDLINE Link]. [Full Text].
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007 Jun 7. 447(7145):661-78. [QxMD MEDLINE Link]. [Full Text].
- Hedin C, Whelan K, Lindsay JO. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. Proc Nutr Soc. 2007 Aug. 66(3):307-15. [QxMD MEDLINE Link].
- Baumgart DC. Endoscopic surveillance in Crohn's disease and ulcerative colitis: who needs what and when?. Dig Dis. 2011. 29 Suppl 1:32-5. [QxMD MEDLINE Link].
- Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. Sep 2007. 56(9):1232-9. [Full Text].
- Lindberg E, Jarnerot G, Huitfeldt B. Smoking in Crohn's disease: effect on localisation and clinical course. Gut. 1992 Jun. 33(6):779-82. [QxMD MEDLINE Link].
- D'Souza S, Levy E, Mack D, et al. Dietary patterns and risk for Crohn's disease in children. Inflamm Bowel Dis. 2008 Mar. 14(3):367-73. [QxMD MEDLINE Link].
- Davis RL, Kramarz P, Bohlke K, et al, for the Vaccine Safety Datalink Team. Measles-mumps-rubella and other measles-containing vaccines do not increase the risk for inflammatory bowel disease: a case-control study from the Vaccine Safety Datalink project. Arch Pediatr Adolesc Med. 2001 Mar. 155(3):354-9. [QxMD MEDLINE Link].
- Reif S, Lavy A, Keter D, et al. Appendectomy is more frequent but not a risk factor in Crohn's disease while being protective in ulcerative colitis: a comparison of surgical procedures in inflammatory bowel disease. Am J Gastroenterol. 2001 Mar. 96(3):829-32. [QxMD MEDLINE Link].
- Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998 Jun. 114(6):1161-8. [QxMD MEDLINE Link].
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007 Dec. 5(12):1424-9. [QxMD MEDLINE Link].
- Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996 Nov. 39(5):690-7. [QxMD MEDLINE Link]. [Full Text].
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004 May. 126(6):1504-17. [QxMD MEDLINE Link].
- Lovasz BD, Golovics PA, Vegh Z, Lakatos PL. New trends in inflammatory bowel disease epidemiology and disease course in Eastern Europe. Dig Liver Dis. 2013 Apr. 45(4):269-76. [QxMD MEDLINE Link].
- Economou M, Zambeli E, Michopoulos S. Incidence and prevalence of Crohn’s disease and its etiological influences. Annals of Gastroenterology. Available at https://www.annalsgastro.gr/index.php/annalsgastro/article/view/743. 2009. 22(3):158-67; Accessed: December 11, 2012.
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan. 142(1):46-54.e42; quiz e30. [QxMD MEDLINE Link].
- Calkins BM, Lilienfeld AM, Garland CF, Mendeloff AI. Trends in incidence rates of ulcerative colitis and Crohn's disease. Dig Dis Sci. 1984 Oct. 29(10):913-20. [QxMD MEDLINE Link].
- Duerr RH. Update on the genetics of inflammatory bowel disease. J Clin Gastroenterol. 2003 Nov-Dec. 37(5):358-67. [QxMD MEDLINE Link].
- Jess T, Loftus EV Jr, Harmsen WS, et al. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940-2004. Gut. 2006 Sep. 55(9):1248-54. [QxMD MEDLINE Link]. [Full Text].
- Jess T, Frisch M, Simonsen J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin Gastroenterol Hepatol. 2013 Jan. 11(1):43-8. [QxMD MEDLINE Link].
- Munkholm P, Langholz E, Davidsen M, Binder V. Intestinal cancer risk and mortality in patients with Crohn's disease. Gastroenterology. 1993 Dec. 105(6):1716-23. [QxMD MEDLINE Link].
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998 Jul. 115(1):182-205. [QxMD MEDLINE Link].
- Friedman S, Blumberg RS. Inflammatory bowel disease. Braunwald E, Fauci AS, Kasper DS, et al, eds. Harrison's Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill Professional Publishing; 2001. Vol 2: 1679-91.
- Wilkins T, Jarvis K, Patel J. Diagnosis and management of Crohn's disease. Am Fam Physician. 2011 Dec 15. 84(12):1365-75. [QxMD MEDLINE Link].
- Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005 Dec 14. 11(46):7227-36. [QxMD MEDLINE Link]. [Full Text].
- Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006 Apr 15. 23(8):1097-104. [QxMD MEDLINE Link].
- Jess T, Simonsen J, Jorgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012 Aug. 143(2):375-81.e1; quiz e13-4. [QxMD MEDLINE Link].
- Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012 Aug. 143(2):382-9. [QxMD MEDLINE Link].
- Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001 Apr. 96(4):1116-22. [QxMD MEDLINE Link].
- Isaacs KL. How prevalent are extraintestinal manifestations at the initial diagnosis of IBD?. Inflamm Bowel Dis. 2008 Oct. 14 Suppl 2:S198-9. [QxMD MEDLINE Link].
- Aghazadeh R, Zali MR, Bahari A, Amin K, Ghahghaie F, Firouzi F. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol. 2005 Nov. 20(11):1691-5. [QxMD MEDLINE Link].
- Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000 Feb. 6(1):8-15. [QxMD MEDLINE Link].
- Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005 Sep. 19 Suppl A:5-36. [QxMD MEDLINE Link].
- Leach ST, Nahidi L, Tilakaratne S, Day AS, Lemberg DA. Development and assessment of a modified Pediatric Crohn Disease Activity Index. J Pediatr Gastroenterol Nutr. 2010 Aug. 51(2):232-6. [QxMD MEDLINE Link].
- Kappelman MD, Crandall WV, Colletti RB, et al. Short pediatric Crohn's disease activity index for quality improvement and observational research. Inflamm Bowel Dis. 2011 Jan. 17(1):112-7. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Bernstein CN, Eliakim A, Fedail S, et al. World Gastroenterology Organisation global guidelines inflammatory bowel disease: update August 2015. J Clin Gastroenterol. 2016 Nov/Dec. 50 (10):803-18. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] World Gastroenterology Organisation. World Gastroenterology Organisation global guideline: inflammatory bowel disease: a global perspective. Munich, Germany: World Gastroenterology Organisation; 2009. Available at https://guideline.gov/content.aspx?id=15231. Accessed: December 12, 2012.
- McNamara D. New IBD guidelines aim to simplify care. Medscape Medical News. Available at https://www.medscape.com/viewarticle/892853. February 20, 2018; Accessed: June 6, 2018.
- Govani SM, Guentner AS, Waljee AK, Higgins PD. Risk stratification of emergency department patients with Crohn's disease could reduce computed tomography use by nearly half. Clin Gastroenterol Hepatol. 2014 Oct. 12(10):1702-1707.e3. [QxMD MEDLINE Link]. [Full Text].
- Newnham E, Hawkes E, Surender A, James SL, Gearry R, Gibson PR. Quantifying exposure to diagnostic medical radiation in patients with inflammatory bowel disease: are we contributing to malignancy?. Aliment Pharmacol Ther. 2007 Oct 1. 26(7):1019-24. [QxMD MEDLINE Link].
- Desmond AN, O'Regan K, Curran C, et al. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008 Nov. 57(11):1524-9. [QxMD MEDLINE Link].
- Kambadakone AR, Prakash P, Hahn PF, Sahani DV. Low-dose CT examinations in Crohn's disease: Impact on image quality, diagnostic performance, and radiation dose. AJR Am J Roentgenol. 2010 Jul. 195(1):78-88. [QxMD MEDLINE Link].
- Craig O, O'Neill S, O'Neill F, et al. Diagnostic accuracy of computed tomography using lower doses of radiation for patients with Crohn's disease. Clin Gastroenterol Hepatol. 2012 Aug. 10(8):886-92. [QxMD MEDLINE Link].
- Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther. 2011 Jul. 34(2):125-45. [QxMD MEDLINE Link].
- Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009 Jan. 41(1):56-66. [QxMD MEDLINE Link].
- D'Inca R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007 Apr. 22(4):429-37. [QxMD MEDLINE Link].
- Mackalski BA, Bernstein CN. New diagnostic imaging tools for inflammatory bowel disease. Gut. May 2006. 55(5):733-41.
- Saibeni S, Rondonotti E, Iozzelli A, et al. Imaging of the small bowel in Crohn's disease: a review of old and new techniques. World J Gastroenterol. 2007 Jun 28. 13(24):3279-87. [QxMD MEDLINE Link].
- Schreyer AG, Seitz J, Feuerbach S, Rogler G, Herfarth H. Modern imaging using computer tomography and magnetic resonance imaging for inflammatory bowel disease (IBD) AU1. Inflamm Bowel Dis. 2004 Jan. 10(1):45-54. [QxMD MEDLINE Link].
- [Guideline] Kidd R, Mezwa DG, Ralls PW, et al. Imaging recommendations for patients with newly suspected Crohn's disease, and in patients with known Crohn's disease and acute exacerbation or suspected complications. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000 Jun. 215 Suppl:181-92. [QxMD MEDLINE Link].
- Fidler JL, Rosen MP, Blake MA, et al, for the Expert Panel on Gastrointestinal Imaging. ACR Appropriateness Criteria: Crohn disease. [online publication]. Reston, Va: American College of Radiology; 2011. Available at https://guideline.gov/content.aspx?id=35137. Accessed: April 5, 2011.
- Pilleul F, Godefroy C, Yzebe-Beziat D, Dugougeat-Pilleul F, Lachaux A, Valette PJ. Magnetic resonance imaging in Crohn's disease. Gastroenterol Clin Biol. 2005 Aug-Sep. 29(8-9):803-8. [QxMD MEDLINE Link].
- Florie J, Horsthuis K, Hommes DW, et al. Magnetic resonance imaging compared with ileocolonoscopy in evaluating disease severity in Crohn's disease. Clin Gastroenterol Hepatol. 2005 Dec. 3(12):1221-8. [QxMD MEDLINE Link].
- Rimola J, Ordas I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011 Aug. 17(8):1759-68. [QxMD MEDLINE Link].
- Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009 Jun. 251(3):751-61. [QxMD MEDLINE Link].
- Low RN, Francis IR, Politoske D, Bennett M. Crohn's disease evaluation: comparison of contrast-enhanced MR imaging and single-phase helical CT scanning. J Magn Reson Imaging. 2000 Feb. 11(2):127-35. [QxMD MEDLINE Link].
- Fiorino G, Bonifacio C, Peyrin-Biroulet L, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn's disease. Inflamm Bowel Dis. 2011 May. 17(5):1073-80. [QxMD MEDLINE Link].
- Hafeez R, Punwani S, Boulos P, et al. Diagnostic and therapeutic impact of MR enterography in Crohn's disease. Clin Radiol. 2011 Dec. 66(12):1148-58. [QxMD MEDLINE Link].
- Guidi L, Ratto C, Semeraro S, et al. Combined therapy with infliximab and seton drainage for perianal fistulizing Crohn's disease with anal endosonographic monitoring: a single-centre experience. Tech Coloproctol. 2008 Jun. 12(2):111-7. [QxMD MEDLINE Link].
- Schwartz DA, White CM, Wise PE, Herline AJ. Use of endoscopic ultrasound to guide combination medical and surgical therapy for patients with Crohn's perianal fistulas. Inflamm Bowel Dis. 2005 Aug. 11(8):727-32. [QxMD MEDLINE Link].
- Wise PE, Schwartz DA. The evaluation and treatment of Crohn perianal fistulae: EUA, EUS, MRI, and other imaging modalities. Gastroenterol Clin North Am. 2012 Jun. 41(2):379-91. [QxMD MEDLINE Link].
- Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006 Apr. 63(4):558-65. [QxMD MEDLINE Link].
- Rubin DT, Panaccione R, Chao J, Robinson AM. A practical, evidence-based guide to the use of adalimumab in Crohn's disease. Curr Med Res Opin. 2011 Sep. 27(9):1803-13. [QxMD MEDLINE Link].
- Robinson M. Optimizing therapy for inflammatory bowel disease. Am J Gastroenterol. 1997 Dec. 92(12 Suppl):12S-17S. [QxMD MEDLINE Link].
- Helwick C. Stem cell transplantation halts Crohn's disease. Medscape Medical News from WebMD. Available at https://www.medscape.com/viewarticle/804570. May 22, 2013; Accessed: June 4, 2013.
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010 Apr 15. 362(15):1383-95. [QxMD MEDLINE Link].
- Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn's disease. Cochrane Database Syst Rev. 2010 Dec 8. CD008870. [QxMD MEDLINE Link].
- Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011 Apr. 106(4):590-9. [QxMD MEDLINE Link].
- Turner D, Grossman AB, Rosh J, et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn's disease. Am J Gastroenterol. 2007 Dec. 102(12):2804-12; quiz 2803, 2813. [QxMD MEDLINE Link].
- Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011 Apr. 106(4):644-59. [QxMD MEDLINE Link].
- US Food and Drug Administration. Tumor necrosis factor-alpha (TNFα) blockers: label change - boxed warning updated for risk of infection from Legionella and Listeria. Posted September 7, 2011. Available at https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm270977.htm. Accessed: April 5, 2012.
- Yadav A, Kurada S, Foromera J, Falchuk KR, Feuerstein JD. Meta-analysis comparing the efficacy and adverse events of biologics and thiopurines for Crohn's Disease after surgery for ulcerative colitis. Dig Liver Dis. 2018 May 30. [QxMD MEDLINE Link].
- [Guideline] Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006 Mar. 130(3):935-9. [QxMD MEDLINE Link]. [Full Text].
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999 May 6. 340(18):1398-405. [QxMD MEDLINE Link].
- Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997 Oct 9. 337(15):1029-35. [QxMD MEDLINE Link]. [Full Text].
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999 May 6. 340(18):1398-405. [QxMD MEDLINE Link]. [Full Text].
- Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012 Jan. 142(1):63-70.e5; quiz e31. [QxMD MEDLINE Link].
- Peyrin-Biroulet L, Laclotte C, Bigard MA. Adalimumab maintenance therapy for Crohn's disease with intolerance or lost response to infliximab: an open-label study. Aliment Pharmacol Ther. 2007 Mar 15. 25(6):675-80. [QxMD MEDLINE Link]. [Full Text].
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006 Feb. 130(2):323-33; quiz 591. [QxMD MEDLINE Link].
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007 Jan. 132(1):52-65. [QxMD MEDLINE Link].
- Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004 Nov 11. 351(20):2069-79. [QxMD MEDLINE Link].
- Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007 Jun 19. 146(12):829-38. [QxMD MEDLINE Link]. [Full Text].
- Peppercorn MA. Clinical manifestations, diagnosis, and prognosis of ulcerative colitis in adults. UpToDate. September 15, 2008. [Full Text].
- Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007 Jul 19. 357(3):228-38. [QxMD MEDLINE Link].
- Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007 Jul 19. 357(3):239-50. [QxMD MEDLINE Link].
- Lichtenstein GR, Thomsen OO, Schreiber S, et al. Continuous therapy with certolizumab pegol maintains remission of patients with Crohn's disease for up to 18 months. Clin Gastroenterol Hepatol. 2010 Jul. 8(7):600-9. [QxMD MEDLINE Link].
- Biogen Idec Elan. TYSABRI (natalizumab) Safety Update: (17 August 2012). Available at https://www.tapp.com.au/members/Tysabri_Safety_Update_160812.pdf. Accessed: December 14, 2012.
- FDA. FDA Drug Safety Communication: New risk factor for progressive multifocal leukoencephalopathy (PML) associated with Tysabri (natalizumab) [safety announcement]. January 20, 2012. Available at https://www.fda.gov/Drugs/DrugSafety/ucm288186.htm. Accessed: December 14, 2012.
- Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005 Nov 3. 353(18):1912-25. [QxMD MEDLINE Link].
- Targan SR, Feagan BG, Fedorak RN, et al, for the International Efficacy of Natalizumab in Crohn's Disease Response and Remission (ENCORE) Trial Group. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology. 2007 May. 132(5):1672-83. [QxMD MEDLINE Link].
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013 Aug 22. 369(8):711-21. [QxMD MEDLINE Link].
- Brooks M. FDA clears ustekinumab (Stelara) for Crohn's disease. Medscape Medical News. Available at https://www.medscape.com/viewarticle/869259. September 26, 2016; Accessed: September 30, 2016.
- Johnson & Johnson. FDA approves STELARA (ustekinumab) for treatment of adults with moderately to severely active Crohn’s disease. Available at https://www.jnj.com/media-center/press-releases/fda-approves-stelara-ustekinumab-for-treatment-of-adults-with-moderately-to-severely-active-crohns-disease. September 26, 2016; Accessed: September 30, 2016.
- Sandborn W, Gasink C, Blank M, et al. O-001 A multicenter, double-blind, placebo-controlled phase 3 study of ustekinumab, a human IL-12/23P40 mAB, in moderate-severe Crohn's disease refractory to anti-TFNα: UNITI-1. Inflamm Bowel Dis. 2016 Mar. 22 suppl 1:S1. [QxMD MEDLINE Link].
- Feagan B, Gasink C, Lang Y, et al. A multicenter, randomized, double-blind, placebo-controlled phase 3 study of ustekinumab, a human monoclonal antibody to IL-12/23P40, in patients with moderately- to severely-active Crohn's disease who are naive or non-refractory to anti-TNF (UNITI-2). Presented at: American College of Gastroenterology 2015 Annual Scientific Meeting; Honolulu, Hawaii; October 16-21, 2015. [Full Text].
- Sanborn W, Feagan BG, Gasink C, et al. A phase 3 randomized, multicenter, double-blind, placebo-controlled study of ustekinumab maintenance therapy in moderate-severe Crohn's disease patients: results from IM-UNITI [abstract 768]. Presented at: Digestive Disease Week; San Diego, California; May 23, 2016. [Full Text].
- Iborra M, Beltran B, Fernandez-Clotet A, et al. Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn's disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2019 Aug. 50 (3):278-88. [QxMD MEDLINE Link]. [Full Text].
- McSharry K, Dalzell AM, Leiper K, El-Matary W. Systematic review: the role of tacrolimus in the management of Crohn's disease. Aliment Pharmacol Ther. 2011 Dec. 34(11-12):1282-94. [QxMD MEDLINE Link].
- Solomon MJ, McLeod RS, O’Connor BI, Steinhart H, Greenberg GR, Cohen Z. Combination of ciprofloxacin and metronidazole in severe perianal Crohn’s disease. Can J Gastroenterol. 1993. 7:571-3.
- Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn's disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006 Jun. 4(6):744-53. [QxMD MEDLINE Link].
- Harpavat M, Keljo DJ, Regueiro MD. Metabolic bone disease in inflammatory bowel disease. J Clin Gastroenterol. 2004 Mar. 38(3):218-24. [QxMD MEDLINE Link].
- Heuschkel R. Enteral nutrition in Crohn disease: more than just calories. J Pediatr Gastroenterol Nutr. 2004 Mar. 38(3):239-41. [QxMD MEDLINE Link].
- Razack R, Seidner DL. Nutrition in inflammatory bowel disease. Curr Opin Gastroenterol. 2007 Jul. 23(4):400-5. [QxMD MEDLINE Link].
- Whitten KE, Rogers P, Ooi CY, Day AS. International survey of enteral nutrition protocols used in children with Crohn's disease. J Dig Dis. 2012 Feb. 13(2):107-12. [QxMD MEDLINE Link].
- Markowitz J, Markowitz JE, Bousvaros A, et al. Workshop report: prevention of postoperative recurrence in Crohn's disease. J Pediatr Gastroenterol Nutr. 2005 Aug. 41(2):145-51. [QxMD MEDLINE Link].
- Ewe K, Herfarth C, Malchow H, Jesdinsky HJ. Postoperative recurrence of Crohn's disease in relation to radicality of operation and sulfasalazine prophylaxis: a multicenter trial. Digestion. 1989. 42(4):224-32. [QxMD MEDLINE Link].
- Alos R, Hinojosa J. Timing of surgery in Crohn's disease: a key issue in the management. World J Gastroenterol. 2008 Sep 28. 14(36):5532-9. [QxMD MEDLINE Link].
- Simillis C, Yamamoto T, Reese GE, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn's disease. Am J Gastroenterol. 2008 Jan. 103(1):196-205. [QxMD MEDLINE Link].
- Shen B. Managing medical complications and recurrence after surgery for Crohn's disease. Curr Gastroenterol Rep. 2008 Dec. 10(6):606-11. [QxMD MEDLINE Link].
- Cobb WS IV. Finney pyloroplasty. Ponsky JR, Rosen MJ, eds. Atlas of Surgical Techniques for the Upper Gastrointestinal Tract and Small Bowel. Philadelphia, Pa: Saunders Elsevier; 2010. 97-103.
- Angel CA. Finney pyloroplasty. Townsend CM Jr, Evers BM, eds. Atlas of General Surgical Techniques. Philadelphia, Pa: Saunders Elsevier; 2010. Chapter 24.
- Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn's disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007 Nov. 50(11):1968-86. [QxMD MEDLINE Link].
- Couckuyt H, Gevers AM, Coremans G, Hiele M, Rutgeerts P. Efficacy and safety of hydrostatic balloon dilatation of ileocolonic Crohn's strictures: a prospective longterm analysis. Gut. 1995 Apr. 36(4):577-80. [QxMD MEDLINE Link].
- Garcia JC, Persky SE, Bonis PA, Topazian M. Abscesses in Crohn's disease: outcome of medical versus surgical treatment. J Clin Gastroenterol. 2001 May-Jun. 32(5):409-12. [QxMD MEDLINE Link].
- Berg DF, Bahadursingh AM, Kaminski DL, Longo WE. Acute surgical emergencies in inflammatory bowel disease. Am J Surg. 2002 Jul. 184(1):45-51. [QxMD MEDLINE Link].
- Kiran RP, Nisar PJ, Church JM, Fazio VW. The role of primary surgical procedure in maintaining intestinal continuity for patients with Crohn's colitis. Ann Surg. 2011 Jun. 253(6):1130-5. [QxMD MEDLINE Link].
- Kamm MA, Ng SC. Perianal fistulizing Crohn's disease: a call to action. Clin Gastroenterol Hepatol. 2008 Jan. 6(1):7-10. [QxMD MEDLINE Link].
- Bode M, Eder S, Schurmann G. [Perianal fistulas in Crohn's disease--biologicals and surgery: is it worthwhile?]. Z Gastroenterol. 2008 Dec. 46(12):1376-83. [QxMD MEDLINE Link].
- Poritz LS, Rowe WA, Koltun WA. Remicade does not abolish the need for surgery in fistulizing Crohn's disease. Dis Colon Rectum. 2002 Jun. 45(6):771-5. [QxMD MEDLINE Link].
- Liu CD, Rolandelli R, Ashley SW, Evans B, Shin M, McFadden DW. Laparoscopic surgery for inflammatory bowel disease. Am Surg. Dec 1995. 61(12):1054-6.
- Sardinha TC, Wexner SD. Laparoscopy for inflammatory bowel disease: pros and cons. World J Surg. 1998 Apr. 22(4):370-4. [QxMD MEDLINE Link].
- Georgeson KE, Cohen RD, Hebra A, et al. Primary laparoscopic-assisted endorectal colon pull-through for Hirschsprung's disease: a new gold standard. Ann Surg. 1999 May. 229(5):678-82; discussion 682-3. [QxMD MEDLINE Link]. [Full Text].
- Lowney JK, Dietz DW, Birnbaum EH, Kodner IJ, Mutch MG, Fleshman JW. Is there any difference in recurrence rates in laparoscopic ileocolic resection for Crohn's disease compared with conventional surgery? A long-term, follow-up study. Dis Colon Rectum. 2006 Jan. 49(1):58-63. [QxMD MEDLINE Link].
- Chen HH, Wexner SD, Iroatulam AJ, et al. Laparoscopic colectomy compares favorably with colectomy by laparotomy for reduction of postoperative ileus. Dis Colon Rectum. 2000 Jan. 43(1):61-5. [QxMD MEDLINE Link].
- Eshuis EJ, Polle SW, Slors JF, et al. Long-term surgical recurrence, morbidity, quality of life, and body image of laparoscopic-assisted vs. open ileocolic resection for Crohn's disease: a comparative study. Dis Colon Rectum. 2008 Jun. 51(6):858-67. [QxMD MEDLINE Link].
- Eshuis EJ, Bemelman WA, van Bodegraven AA, et al. Laparoscopic ileocolic resection versus infliximab treatment of distal ileitis in Crohn's disease: a randomized multicenter trial (LIR!C-trial). BMC Surg. 2008 Aug 22. 8:15. [QxMD MEDLINE Link].
- [Guideline] Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017 Feb. 112 (2):241-58. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr. 113 (4):481-517. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Bruining DH, Zimmermann EM, Loftus EV Jr, et al. Consensus Recommendations for Evaluation, Interpretation, and Utilization of Computed Tomography and Magnetic Resonance Enterography in Patients With Small Bowel Crohn's Disease. Gastroenterology. 2018 Mar. 154 (4):1172-94. [QxMD MEDLINE Link].
- Experts offer guidance on cross-sectional enterography in Crohn’s disease. Reuters Health Information. Available at https://www.medscape.com/viewarticle/891631. January 23, 2018; Accessed: July 26, 2019.
- Willeman T, Jourdil JF, Gautier-Veyret E, Bonaz B, Stanke-Labesque F. A multiplex liquid chromatography tandem mass spectrometry method for the quantification of seven therapeutic monoclonal antibodies: Application for adalimumab therapeutic drug monitoring in patients with Crohn's disease. Anal Chim Acta. 2019 Aug 27. 1067:63-70. [QxMD MEDLINE Link].
- Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011 Apr. 106(4):661-73. [QxMD MEDLINE Link].
- Feagan BG, Rutgeerts PJ, Sands BE, et al. Induction therapy for ulcerative colitis: results of GEMINI I, a randomized, placebo-controlled, double-blind, multicenter phase 3 trial [abstract 943b]. Gastroenterology. 2012. 142(5):S160-61.
- Sakuraba A, Keyashian K, Correia C, et al. Natalizumab in Crohn's disease: results from a US tertiary inflammatory bowel disease center. Inflamm Bowel Dis. 2013 Mar. 19(3):621-6. [QxMD MEDLINE Link].
- Savarino E, Bodini G, Dulbecco P, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. Am J Gastroenterol. 2013 Nov. 108(11):1731-42. [QxMD MEDLINE Link]. [Full Text].
- Valentine JF, Fedorak RN, Feagan B, et al. Steroid-sparing properties of sargramostim in patients with corticosteroid-dependent Crohn's disease: a randomised, double-blind, placebo-controlled, phase 2 study. Gut. 2009 Oct. 58(10):1354-62. [QxMD MEDLINE Link].
- [Guideline] Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S, for the American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017 Sep. 153 (3):827-34. [QxMD MEDLINE Link]. [Full Text].
- Satta R, Pes GM, Rocchi C, Pes MC, Dore MP. Is probiotic use beneficial for skin lesions in patients with inflammatory bowel disease?. J Dermatolog Treat. 2019 Sep. 30 (6):612-6. [QxMD MEDLINE Link].
Author
Specialty Editor Board
Chief Editor
Acknowledgements
BS Anand, MD Professor, Department of Internal Medicine, Division of Gastroenterology, Baylor College of Medicine
BS Anand, MD is a member of the following medical societies: American Association for the Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association, and American Society for Gastrointestinal Endoscopy
Disclosure: Nothing to disclose.
Priyankha Balasundaram, MD Director, Kovai Heart Foundation, India; Resident Physician, Department of Surgery, Tulane University School of Medicine
Disclosure: Nothing to disclose.
Marcy L Coash, DO Staff Physician, Department of Internal Medicine, University of Connecticut
Marcy L Coash, DO is a member of the following medical societies: American Medical Student Association/Foundation and American Osteopathic Association
Disclosure: Nothing to disclose.
Senthil Nachimuthu , MD, FACP
Disclosure: Nothing to disclose.
Waqar A Qureshi, MD Associate Professor of Medicine, Chief of Endoscopy, Department of Internal Medicine, Division of Gastroenterology, Baylor College of Medicine and Veterans Affairs Medical Center
Waqar A Qureshi, MD is a member of the following medical societies: American College of Gastroenterology, American College of Physicians, American Gastroenterological Association, and American Society for Gastrointestinal Endoscopy
Disclosure: Nothing to disclose.
Priya Rangasamy, MD Fellow, Department of Gastroenterology/Hepatology, University of Connecticut Health Center
Priya Rangasamy, MD is a member of the following medical societies: American College of Gastroenterology, American Gastroenterological Association, and American Society for Gastrointestinal Endoscopy
Disclosure: Nothing to disclose.
Kathleen M Raynor, MD Staff Physician, Department of Internal Medicine, University of Connecticut School of Medicine
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
George Y Wu, MD, PhD Professor, Department of Medicine, Director, Hepatology Section, Herman Lopata Chair in Hepatitis Research, University of Connecticut School of Medicine
George Y Wu, MD, PhD is a member of the following medical societies: American Association for the Study of Liver Diseases, American Gastroenterological Association, American Medical Association, American Society for Clinical Investigation, and Association of American Physicians
Disclosure: Springer Consulting fee Consulting; Gilead Consulting fee Review panel membership; Gilead Honoraria Speaking and teaching; Bristol-Myers Squibb Honoraria Speaking and teaching; Springer Royalty Review panel membership