Psoriasis: Practice Essentials, Background, Pathophysiology (original) (raw)
Practice Essentials
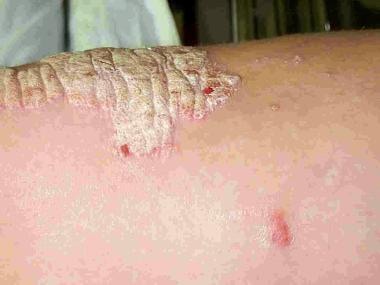
Psoriasis is a complex, chronic, multifactorial, inflammatory disease that involves hyperproliferation of the keratinocytes in the epidermis, with an increase in the epidermal cell turnover rate (see the image below). Environmental, genetic, and immunologic factors appear to play a role. The disease most commonly manifests on the skin of the elbows, knees, scalp, lumbosacral areas, intergluteal clefts, and glans penis. In up to 30% of patients, the joints are also affected.
Treatment is based on surface areas of involvement, body site(s) affected, the presence or absence of arthritis, and the thickness of the plaques and scale.
Plaque psoriasis is raised, roughened, and covered with white or silver scale with underlying erythema. Contributed by Randy Park, MD.
See Psoriasis: Manifestations, Management Options, and Mimics, a Critical Images slideshow, to help recognize the major psoriasis subtypes and distinguish them from other skin lesions.
Signs and symptoms
Signs and symptoms of psoriasis may include the following:
- Worsening of a long-term erythematous scaly area
- Sudden onset of many small areas of scaly redness
- Recent streptococcal throat infection, viral infection, immunization, use of antimalarial drug, or trauma
- Pain (especially in erythrodermic psoriasis and in some cases of traumatized plaques or in the joints affected by psoriatic arthritis)
- Pruritus (especially in eruptive, guttate psoriasis)
- Afebrile (except in pustular or erythrodermic psoriasis, in which the patient may have high fever)
- Dystrophic nails, which may resemble onychomycosis
- Long-term, steroid-responsive rash with recent presentation of joint pain
- Joint pain (psoriatic arthritis) without any visible skin findings
- Conjunctivitis or blepharitis
See Clinical Presentation for more detail.
Diagnosis
The diagnosis of psoriasis is clinical, and the type of psoriasis present affects the physical examination findings.
Types of psoriasis
Common types of psoriasis include the following:
- Chronic stationary psoriasis (psoriasis vulgaris): Most common type of psoriasis; involves the scalp, extensor surfaces, genitals, umbilicus, and lumbosacral and retroauricular regions
- Plaque psoriasis: Most commonly affects the extensor surfaces of the knees, elbows, scalp, and trunk
- Guttate psoriasis: Presents predominantly on the trunk; frequently appears suddenly, 2-3 weeks after an upper respiratory tract infection with group A beta-hemolytic streptococci; this variant is more likely to itch, sometimes severely
- Inverse psoriasis: Occurs on the flexural surfaces, armpit, and groin; under the breast; and in the skin folds; this is often misdiagnosed as a fungal infection
- Pustular psoriasis: Presents on the palms and soles or diffusely over the body
- Erythrodermic psoriasis: Typically encompasses nearly the entire body surface area with red skin and a diffuse, fine, peeling scale
- Scalp psoriasis: Affects approximately 50% of patients
- Nail psoriasis: May be indistinguishable from, and more prone to developing, onychomycosis
- Psoriatic arthritis: Affects approximately 10-30% of those with skin symptoms; usually in the hands and feet and, occasionally, the large joints
- Oral psoriasis: May present as severe cheilosis, with extension onto the surrounding skin, crossing the vermillion border
- Eruptive psoriasis: Involves the upper trunk and upper extremities; most often seen in younger patients
- Napkin psoriasis: Presence of psoriasis in children's diaper region
- Linear psoriasis: Psoriasis that occurs within a dermatome
Examination in patients with psoriasis includes the following:
- Dermatologic: Most commonly, scaling erythematous macules, papules, and plaques; area of skin involvement varies with the form of psoriasis
- Ocular: Ectropion and trichiasis, conjunctivitis and conjunctival hyperemia, and corneal dryness with punctate keratitis and corneal melt [1] ; blepharitis
- Musculoskeletal: Stiffness, pain, throbbing, swelling, or tenderness of the joints; distal joints most often affected (eg, fingers, toes, wrists, knees, ankles); may progress to a severe and mutilating arthritis of the hands, especially if treatment has been suboptimal
Testing
There is no specific or diagnostic blood test for psoriasis. Laboratory studies and findings for patients with psoriasis may include the following:
- Rheumatoid factor level: Negative
- Erythrocyte sedimentation rate: Usually normal, except in pustular and erythrodermic psoriasis, where it may be elevated along with the white blood cell count
- Uric acid level: May be elevated in psoriasis (especially in pustular psoriasis)
- Examination of fluid from pustules: Sterile bacterial culture with neutrophilic infiltrate
- Fungal studies: Especially important in cases of hand and foot psoriasis that seem to be worsening with the use of topical steroids or to determine if psoriatic nails are also infected with fungus
- Conjunctival impression cytology: Increased incidence of squamous metaplasia, neutrophil clumping, and snakelike chromatin
The differentiation of psoriatic arthritis from rheumatoid arthritis and gout can be facilitated by the absence of the typical laboratory findings of those conditions.
Consider obtaining the following baseline laboratory studies in patients being initiated on systemic therapies (eg, immunologic inhibitors):
- Complete blood cell (CBC) count
- Blood urea nitrogen (BUN) and creatinine levels
- Liver function tests
- Hepatitis panel
- Tuberculosis (TB) screening
- Human immunodeficiency virus (HIV) testing
- Pregnancy test
Other studies
- Radiographs of affected joints: Can be helpful in differentiating types of arthritis
- Joint radiographs: Can facilitate the diagnosis of psoriatic arthritis
- Bone scans: Can identify joint involvement early
- Dermatologic biopsy: Can be used to make the diagnosis when some cases of psoriasis are difficult to recognize (eg, pustular forms)
See Workup for more detail.
Management
Pharmacotherapy
Medications used in the management of psoriasis include the following:
- Topical corticosteroids (eg, triamcinolone acetonide 0.025-0.1% cream, betamethasone 0.025-0.1% cream)
- Topical aryl hydrocarbon receptor (AhR) agonists (eg, tapinarof)
- Ophthalmic corticosteroids (eg, prednisolone acetate 1% ophthalmic, dexamethasone ophthalmic)
- Intramuscular corticosteroids (eg, triamcinolone): Requires caution because the patient may have a significant flare as the medication wears off; 3 months should elapse between injections
- Intralesional corticosteroids: May be useful for resistant plaques and for the treatment of psoriatic nails
- Coal tar 0.5-33%
- Keratolytic agents (eg, anthralin, urea): Use of these medications may facilitate more direct steroid contact with the skin
- Vitamin D analogs (eg, calcitriol ointment, calcipotriene, calcipotriene and betamethasone topical ointment)
- Topical retinoids (eg, tazarotene aqueous gel and cream 0.05% and 0.1%)
- Antimetabolites (eg, methotrexate)
- Immunomodulators (eg, tacrolimus topical 0.1%, cyclosporine, alefacept, ustekinumab)
- Tumor necrosis factor (TNF) inhibitors (eg, infliximab, etanercept, adalimumab)
- Phosphodiesterase-4 inhibitors (eg, apremilast, roflumilast topical)
- Interleukin inhibitors (eg, ustekinumab, secukinumab, tildrakizumab, guselkumab, risankizumab, ixekizumab, brodalumab) [2, 3, 4, 5]
- Artificial tears
The American Academy of Dermatology (AAD) guidelines recommend treatment with methotrexate, cyclosporine, and acitretin with consideration of contraindications and drug interactions. [6]
A 2013 international consensus report on treatment optimization and transitioning for moderate to severe plaque psoriasis include the following recommendations [7] :
- Methotrexate, for as long as it remains effective and well tolerated
- Cyclosporine, generally used intermittently for inducing a clinical response with one or several courses over 3-6 months
- Transition from conventional systemic therapy to a biologic agent, either directly or with an overlap if transitioning is needed due to lack of efficacy, or with a treatment-free interval if transitioning is needed for safety reasons
- Combination therapy
- Continuous therapy for patients receiving biologic agents
- Switching biologic agents: If due to lack of efficacy, perform without a washout period; if for safety reasons, a treatment-free interval may be required
- Combinations of multiple agents (eg, methotrexate and a biologic) are necessary in some patients, but the long-term safety and optimal laboratory monitoring have yet to be defined
Other therapies
Management of psoriasis may also involve the following nondrug therapies:
- Light therapy with solar or ultraviolet radiation
- Stress reduction
- Biofeedback
- Climatotherapy
- Adjuncts, such as sunshine, sea bathing, moisturizers, oatmeal baths
- Punctal occlusion (and ocular lubricants): For keratoconjunctivitis sicca
- Bandage contact lens: To retard corneal melting
Surgical option
Ocular manifestations such as trichiasis and cicatricial ectropion usually require surgical treatment. Progression of corneal melting, inflammation, and vascularization may require lamellar or penetrating keratoplasty.
See Treatment and Medication for more detail.

Background
Psoriasis is a chronic, noncontagious, multisystem, inflammatory disorder. Patients with psoriasis have a genetic predisposition for the illness, which most commonly manifests itself on the skin of the elbows, knees, scalp, lumbosacral areas, intergluteal clefts, and glans penis. The joints are also affected by psoriasis in up to 30% of patients with the disease. (See Pathophysiology and Etiology.)
Psoriasis has a tendency to wax and wane with flares related to systemic or environmental factors, including life stress events and infection. It impacts quality of life and potentially long-term survival. There should be a higher clinical suspicion for depression in the patient with psoriasis. (See Prognosis.)
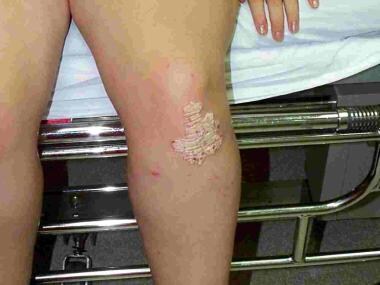
Multiple types of psoriasis are identified, with plaque-type psoriasis, also known as discoid psoriasis, being the most common type. Plaque psoriasis usually presents with plaques on the scalp, trunk, and limbs (see the image below). These plaques appear as focal, raised, inflamed, edematous lesions covered with silvery-white “micaceous” scales. (See Clinical Presentation.)
Plaque psoriasis is most common on the extensor surfaces of the knees and elbows. Contributed by Randy Park, MD.
Ocular signs occur in approximately 10% of psoriasis patients, and they are more common in men than in women. Patients with ocular findings almost always have psoriatic skin disease; however, it is rare for the eye to become involved before the skin. [8]
The diagnosis of psoriasis is clinical. (See Workup.) Management of psoriasis may involve topical or systemic medications, light therapy, stress reduction, climatotherapy, and various adjuncts such as sunshine, moisturizers, and salicylic acid. (See Treatment and Management.)
For more information, see the following:

Pathophysiology
Psoriasis is a complex, multifactorial disease that appears to be influenced by genetic and immune-mediated components. This is supported by the successful treatment of psoriasis with immune-mediating, biologic medications.
The pathogenesis of this disease is not completely understood. Multiple theories exist regarding triggers of the disease process including an infectious episode, traumatic insult, and stressful life event. In many patients, no obvious trigger exists at all. However, once triggered, there appears to be substantial leukocyte recruitment to the dermis and epidermis resulting in the characteristic psoriatic plaques.
Specifically, the epidermis is infiltrated by a large number of activated T cells, which appear to be capable of inducing keratinocyte proliferation. This is supported by histologic examination and immunohistochemical staining of psoriatic plaques revealing large populations of T cells within the psoriasis lesions. One report calculated that a patient with 20% body surface area affected with psoriasis lesions has around 8 billion blood circulating T cells compared with approximately 20 billion T cells located in the dermis and epidermis of psoriasis plaques. [9]
Ultimately, a ramped-up, deregulated inflammatory process ensues with a large production of various cytokines (eg, tumor necrosis factor-α [TNF-α], interferon-gamma, interleukin-12). Many of the clinical features of psoriasis are explained by the large production of such mediators. Interestingly, elevated levels of TNF-α specifically are found to correlate with flares of psoriasis.
One study adds further support that T-cell hyperactivity and the resulting proinflammatory mediators (in this case IL-17/23) play a major role in the pathogenesis of psoriasis. [10]
Key findings in the affected skin of patients with psoriasis include vascular engorgement due to superficial blood vessel dilation and altered epidermal cell cycle. Epidermal hyperplasia leads to an accelerated cell turnover rate (from 23 d to 3-5 d), leading to improper cell maturation.
Cells that normally lose their nuclei in the stratum granulosum retain their nuclei, a condition known as parakeratosis. In addition to parakeratosis, affected epidermal cells fail to release adequate levels of lipids, which normally cement adhesions of corneocytes. Subsequently, poorly adherent stratum corneum is formed leading to the flaking, scaly presentation of psoriasis lesions, the surface of which often resembles silver scales.
Conjunctival impression cytology demonstrated a higher incidence of squamous metaplasia, neutrophil clumping, and nuclear chromatin changes in patients with psoriasis. [11]

Etiology
Psoriasis involves hyperproliferation of the keratinocytes in the epidermis, with an increase in the epidermal cell turnover rate. (See Pathophysiology.) The cause of the loss of control of keratinocyte turnover is unknown. However, environmental, genetic, and immunologic factors appear to play a role.
Environmental factors
Many factors besides stress have also been observed to trigger exacerbations, including cold, trauma, infections (eg, streptococcal, staphylococcal, human immunodeficiency virus), alcohol, and drugs (eg, iodides, steroid withdrawal, aspirin, lithium, beta-blockers, botulinum A, antimalarials). One study showed an increased incidence of psoriasis in patients with chronic gingivitis. Satisfactory treatment of the gingivitis led to improved control of the psoriasis but did not influence longterm incidence, highlighting the multifactorial and genetic influences of this disease. [12]
Hot weather, sunlight, and pregnancy may be beneficial, although the latter is not universal. Perceived stress can exacerbate psoriasis. Some authors suggest that psoriasis is a stress-related disease and offer findings of increased concentrations of neurotransmitters in psoriatic plaques.
Genetic factors
Patients with psoriasis have a genetic predisposition for the disease. The gene locus is determined. The triggering event may be unknown in most cases, but it is likely immunologic. The first lesion commonly appears after an upper respiratory tract infection.
Psoriasis is associated with certain human leukocyte antigen (HLA) alleles, the strongest being human leukocyte antigen Cw6 (HLA-Cw6). In some families, psoriasis is an autosomal dominant trait. Additional HLA antigens that have shown associations with psoriasis and psoriatic subtypes include HLA-B27, HLA-B13, HLA-B17, and HLA-DR7. [13]
A multicenter meta-analysis confirmed that deletion of 2 late cornified envelope (LCE) genes, LCE3C and LCE3B, is a common genetic factor for susceptibility to psoriasis in different populations. [14]
Obesity is another factor associated with psoriasis. Whether it is related to weight alone, genetic predisposition to obesity, or a combination of the 2 is not certain. Onset or worsening of psoriasis with weight gain and/or improvement with weight loss is observed. A meta-analysis showed that a higher body mass index (≥30) is associated with a poorer response to biologics in patients with psoriasis. [15]
Immunologic factors
Evidence suggests that psoriasis is an autoimmune disease. Studies show high levels of dermal and circulating TNF-α. Treatment with TNF-α inhibitors is often successful. Psoriatic lesions are associated with increased activity of T cells in the underlying skin.
Psoriasis is related to excess T-cell activity. Experimental models can be induced by stimulation with streptococcal superantigen, which cross-reacts with dermal collagen. This small peptide has been shown to cause increased activity among T cells in patients with psoriasis but not in control groups. Some of the newer drugs used to treat severe psoriasis directly modify the function of lymphocytes.
Also of significance is that 2.5% of those with HIV develop worsening psoriasis with decreasing CD4 counts. This is paradoxical, in that the leading hypothesis on the pathogenesis of psoriasis supports T-cell hyperactivity and treatments geared to reduce T-cell counts help reduce psoriasis severity. This finding is possibly explained by a decrease in CD4 T cells, which leads to overactivity of CD8 T cells, which drives the worsening psoriasis. The HIV genome may drive keratinocyte proliferation directly.
HIV associated with opportunistic infections may see increased frequency of superantigen exposure leading to similar cascades as above mentioned.
Guttate psoriasis often appears following certain immunologically active events, such as streptococcal pharyngitis, cessation of steroid therapy, and use of antimalarial drugs.

Epidemiology
According to the National Institutes of Health (NIH), approximately 2.2% of the United States population has psoriasis. Internationally, the incidence of psoriasis varies dramatically. A study of 26,000 South American Indians did not reveal a single case of psoriasis, whereas in the Faeroe Islands, an incidence of 2.8% was observed. Overall, approximately 2-3% of people are affected by psoriasis worldwide. Psoriasis can begin at any age, yet there is a bimodal peak between age 20-30 years and 50-60 years. Approximately 10-15% of new cases begin in children younger than 10 years. The median age at onset is 28 years.
Psoriasis appears to be slightly more prevalent among women than among men; however, men are thought to be more likely to experience the ocular disease.
The incidence of psoriasis is dependent on the climate and genetic heritage of the population. It is less common in the tropics and in persons with dark skin. Psoriasis prevalence in African Americans is 1.3% compared with 2.5% in Whites. [16]
Psoriasis, even severe psoriasis, may occur in the pediatric age group, with a prevalence of 0.5-2% of children. A retrospective review found that Black children are significantly more likely than White children to have palmoplantar psoriasis, which is also more common among male children. [17] Both biologic and immunomodulating therapies may be used safely and effectively in pediatric patients. [18]

Prognosis
Although psoriasis is usually benign, it is a lifelong illness with remissions and exacerbations and is sometimes refractory to treatment. It progresses to arthritis in about 10% of cases. About 17-55% of patients experience remissions of varying lengths.
Mild psoriasis does not appear to increase risk of death. [19] However, men with severe psoriasis died 3.5 years earlier compared with men without the disease. Women with severe psoriasis died 4.4 years earlier compared with women without the disease. [19]
Psoriasis is associated with smoking, alcohol, metabolic syndrome, lymphoma, depression, suicide, potentially harmful drug and light therapies, and possibly melanoma and nonmelanoma skin cancers.
In a population-based cross-sectional study of 9035 psoriasis patients and 90,350 matched controls without psoriasis, those with more extensive psoriatic skin disease were at greater risk for major medical comorbidities, including heart and blood vessel disease, chronic lung disease, diabetes, kidney disease, joint problems, and other health conditions. [20, 21] Overall, the risk for any other type of serious illness was 11% higher for people with mild psoriasis, 15% higher for patients with moderate psoriasis, and 35% higher for those with severe psoriasis. [20, 21]
Findings from a large retrospective study in Canada show that the risk of mortality in patients with psoriasis is strongly associated with comorbidities such as liver injury, cardiovascular disease, and affective disorders; however, the risk seems to be reduced among those who receive biologic therapy. [22]
Heart disease
A systematic review of 90 studies confirmed that patients with psoriasis had a higher risk of ischemic heart disease, stroke, and peripheral arterial disease but also a greater prevalence of risk factors for cardiovascular disease, compared with controls. The authors concluded that large prospective studies with long-term followup are required to determine whether psoriasis is an independent risk factor for vascular disease or is merely associated with known risk factors. [23]
Another study identified psoriasis as an independent risk factor for cardiovascular disease in women, especially if they had psoriatic arthritis and suffered from psoriasis for a longer period (>9 y). [24] A large cross-sectional study found that almost one third of patients with severe psoriasis met criteria for coronary microvascular dysfunction. [25]
Hypertension
In a population-based cross-sectional study of 1322 hypertensive patients with psoriasis and 11,977 controls without psoriasis, Takeshita et al found that patients with psoriasis were more likely to suffer from uncontrolled hypertension than those without psoriasis. [26, 27] Patients with moderate-to-severe psoriasis affecting more than 3% of their body surface area were at the greatest risk.
The dose-response relation between uncontrolled hypertension and psoriasis severity remained significant after adjustment for age, sex, body mass index, smoking status, alcohol use, comorbid conditions, and current use of antihypertensive medications and nonsteroidal anti-inflammatory drugs, with odds ratios of 1.20 (95% confidence interval [CI], 0.99-1.45) for moderate psoriasis and 1.48 (95% CI, 1.08-2.04) for severe psoriasis. [27]
Kidney disease
Severe psoriasis was associated with a greatly increased risk of chronic kidney disease (CKD) in a recent study of more than 800,000 patients, including 142,883 with psoriasis, 7354 with severe psoriasis, and 689,702 without psoriasis. After adjustment for age, sex, cardiovascular disease, diabetes mellitus, hyperlipidemia, hypertension, use of nonsteroidal anti-inflammatory drugs, and body mass index, the adjusted hazard ratio for CKD among patients with severe psoriasis was 1.93. [28, 29]
In a nested analysis of 8731 psoriasis patients and 87,310 controls, the odds ratio of CKD after adjustment for age, sex, cardiovascular disease, diabetes, hypertension, hyperlipidemia, body mass index, use of nonsteroidal anti-inflammatory drugs, and duration of observation was 1.36 in patients with moderate psoriasis and 1.58 in those with severe psoriasis. The relative risk for CKD was highest in younger patients. [28, 29]
Quality of life
Psoriasis can significantly influence a person’s quality of life. The physical and mental disability experienced with this disease can be comparable or in excess of that found in patients with other chronic illnesses such as cancer, arthritis, hypertension, heart disease, diabetes, and depression. A study by Kurd et al further supports the notion that psoriasis impacts quality of life and potentially long-term survival. [30] There should be a higher clinical suspicion for depression in the patient with psoriasis.
While the clinical presentation of psoriasis, and whatever improvements are made during therapy, is usually measured using the PASI (Psoriasis Area and Severity Index) score, measurement of the effect on the quality of life of the psoriasis patient may be better assessed by the DLQI (Dermatology Life Quality Index) or the CDLQI (Children’s Dermatology Life Quality Index). Measurements using these tools generally show improved quality of life with more aggressive treatment such as systemic agents. [31, 32]
Studies show that psoriasis of the palms and soles tends to have greater impact on the patient’s quality of life compared with those who have more extensive psoriatic involvement not involving the palms and soles. [33, 34]

Patient Education
Dry eye and its manifestations may be present. Avoiding drying conditions and using lubricants can be effective. Patient recognition of these symptoms is vital for effective early treatment of this disease. Most cases of psoriasis can be controlled at a tolerable level with the regular application of care measures.

- Huynh N, Cervantes-Castaneda RA, Bhat P, Gallagher MJ, Foster CS. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008 May-Jun. 16(3):89-93. [QxMD MEDLINE Link].
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013 Apr. 168 (4):844-54. [QxMD MEDLINE Link].
- Kimball AB, Gordon KB, Fakharzadeh S, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol. 2012 Apr. 166 (4):861-72. [QxMD MEDLINE Link].
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015 Oct. 373 (14):1318-28. [QxMD MEDLINE Link]. [Full Text].
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018 Aug 25. 392 (10148):650-661. [QxMD MEDLINE Link].
- [Guideline] Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009 Sep. 61(3):451-85. [QxMD MEDLINE Link].
- Mrowietz U, de Jong EM, Kragballe K, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014 Apr. 28 (4):438-53. [QxMD MEDLINE Link].
- Gulliver W. Long-term prognosis in patients with psoriasis. Br J Dermatol. 2008 Aug. 159 Suppl 2:2-9. [QxMD MEDLINE Link].
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005 Mar. 64 Suppl 2:ii30-6. [QxMD MEDLINE Link]. [Full Text].
- Keaney TC, Kirsner RS. New insights into the mechanism of narrow-band UVB therapy for psoriasis. J Invest Dermatol. 2010 Nov. 130(11):2534. [QxMD MEDLINE Link].
- Pietrzak AT, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, et al. Cytokines and anticytokines in psoriasis. Clin Chim Acta. 2008 Aug. 394(1-2):7-21. [QxMD MEDLINE Link].
- Keller JJ, Lin HC. The effects of chronic periodontitis and its treatment on the subsequent risk of psoriasis. Br J Dermatol. 2012 Dec. 167 (6):1338-44. [QxMD MEDLINE Link].
- Woodrow JC, Ilchysyn A. HLA antigens in psoriasis and psoriatic arthritis. J Med Genet. 1985 Dec. 22 (6):492-5. [QxMD MEDLINE Link].
- Riveira-Munoz E, He SM, Escaramís G, et al. Meta-Analysis Confirms the LCE3C_LCE3B Deletion as a Risk Factor for Psoriasis in Several Ethnic Groups and Finds Interaction with HLA-Cw6. J Invest Dermatol. 2011 May. 131(5):1105-9. [QxMD MEDLINE Link].
- Hjort G, Schwarz CW, Skov L, Loft N. Clinical Characteristics Associated With Response to Biologics in the Treatment of Psoriasis: A Meta-analysis. JAMA Dermatol. 2024 Jun 18. [QxMD MEDLINE Link].
- Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, Kist J, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005 Jan. 52(1):23-6. [QxMD MEDLINE Link].
- Roman B, Collette S, Smith AM, Theos A. Black and male children have an increased risk of palmoplantar psoriasis compared to White children. Pediatr Dermatol. 2023 Nov-Dec. 40 (6):1071-73. [QxMD MEDLINE Link].
- Klufas DM, Wald JM, Strober BE. Treatment of Moderate to Severe Pediatric Psoriasis: A Retrospective Case Series. Pediatr Dermatol. 2016 Mar-Apr. 33 (2):142-9. [QxMD MEDLINE Link].
- Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007 Dec. 143(12):1493-9. [QxMD MEDLINE Link].
- Harding A. Extent of psoriasis tied to risk of comorbidities. Reuters Health Information. August 15, 2013. [Full Text].
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis Severity and the Prevalence of Major Medical Comorbidity: A Population-Based Study. JAMA Dermatol. 2013 Aug 7. [QxMD MEDLINE Link].
- Riaz S, Emam S, Wang T, Gniadecki R. Negative impact of comorbidities on all-cause mortality of patients with psoriasis is partially alleviated by biologic treatment: A real-world case-control study. J Am Acad Dermatol. 2024 Jul. 91 (1):43-50. [QxMD MEDLINE Link].
- Patel RV, Shelling ML, Prodanovich S, Federman DG, Kirsner RS. Psoriasis and vascular disease-risk factors and outcomes: a systematic review of the literature. J Gen Intern Med. 2011 Sep. 26(9):1036-49. [QxMD MEDLINE Link].
- Li WQ, Han JL, Manson JE, Rimm EB, Rexrode KM, Curhan GC, et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. 2012 Apr. 166(4):811-8. [QxMD MEDLINE Link].
- Piaserico S, Papadavid E, Cecere A, et al. Coronary Microvascular Dysfunction in Asymptomatic Patients with Severe Psoriasis. J Invest Dermatol. 2023 Oct. 143 (10):1929-36.e2. [QxMD MEDLINE Link].
- Henderson D. Psoriasis severity linked to uncontrolled hypertension. Medscape Medical News. October 27, 2014. [Full Text].
- Takeshita J, Wang S, Shin DB, et al. Effect of psoriasis severity on hypertension control: a population-based study in the United Kingdom. JAMA Dermatol. 2015 Feb. 151 (2):161-9. [QxMD MEDLINE Link].
- Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013 Oct 15. 347:f5961. [QxMD MEDLINE Link].
- Laidman J. Moderate and Severe Psoriasis Linked to Higher Kidney Risks. Medscape [serial online]. Available at https://www.medscape.com/viewarticle/812730. Accessed: October 21, 2013.
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010 Aug. 146(8):891-5. [QxMD MEDLINE Link]. [Full Text].
- Oostveen AM, de Jager ME, van de Kerkhof PC, et al. The influence of treatments in daily clinical practice on the Children's Dermatology Life Quality Index in juvenile psoriasis: a longitudinal study from the Child-CAPTURE patient registry. Br J Dermatol. 2012 Jul. 167 (1):145-9. [QxMD MEDLINE Link].
- Lucka TC, Pathirana D, Sammain A, et al. Efficacy of systemic therapies for moderate-to-severe psoriasis: a systematic review and meta-analysis of long-term treatment. J Eur Acad Dermatol Venereol. 2012 Nov. 26 (11):1331-44. [QxMD MEDLINE Link].
- Pettey AA, Balkrishnan R, Rapp SR, Fleischer AB, Feldman SR. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003 Aug. 49(2):271-5. [QxMD MEDLINE Link].
- Sampogna F, Tabolli S, Soderfeldt B, Axtelius B, Aparo U, Abeni D. Measuring quality of life of patients with different clinical types of psoriasis using the SF-36. Br J Dermatol. 2006 May. 154(5):844-9. [QxMD MEDLINE Link].
- Langenbruch A, Radtke MA, Krensel M, Jacobi A, Reich K, Augustin M. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br J Dermatol. 2014 Nov. 171(5):1123-8. [QxMD MEDLINE Link].
- Moadel K, Perry HD, Donnenfeld ED, Zagelbaum B, Ingraham HJ. Psoriatic corneal abscess. Am J Ophthalmol. 1995 Jun. 119(6):800-1. [QxMD MEDLINE Link].
- Durrani K, Foster CS. Psoriatic uveitis: a distinct clinical entity?. Am J Ophthalmol. 2005 Jan. 139(1):106-11. [QxMD MEDLINE Link].
- Takahashi H, Sugita S, Shimizu N, Mochizuki M. A high viral load of Epstein-Barr virus DNA in ocular fluids in an HLA-B27-negative acute anterior uveitis patient with psoriasis. Jpn J Ophthalmol. 2008 Mar-Apr. 52(2):136-8. [QxMD MEDLINE Link].
- Lipper GM. Psoriasis and IBD: Is This Comorbidity for Real?. Medscape Dermatology. Available at https://www.medscape.com/viewarticle/907240. January 11, 2019; Accessed: January 15, 2019.
- Fu Y, Lee CH, Chi CC. Association of Psoriasis With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018 Dec 1. 154 (12):1417-1423. [QxMD MEDLINE Link].
- Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011 Jul. 63 (1):40-6. [QxMD MEDLINE Link].
- Elston DM, Ferringer T, Ko C, Peckham S, High W, DiCaudo D. Dermatopathology. 2nd ed. Philadelphia, Pa: Elsevier Saunders; 2013.
- [Guideline] Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008 May. 58(5):826-50. [QxMD MEDLINE Link].
- [Guideline] Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009 Apr. 60(4):643-59. [QxMD MEDLINE Link].
- [Guideline] Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010 Jan. 62(1):114-35. [QxMD MEDLINE Link].
- [Guideline] American Academy of Dermatology Work Group, Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011 Jul. 65 (1):137-74. [QxMD MEDLINE Link].
- [Guideline] Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021 Feb. 84 (2):432-470. [QxMD MEDLINE Link]. [Full Text].
- Mason AR, Mason J, Cork M, Dooley G, Edwards G. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009 Apr 15. CD005028. [QxMD MEDLINE Link].
- Stern RS, PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012 Apr. 66 (4):553-62. [QxMD MEDLINE Link].
- Carrascosa JM, Plana A, Ferrandiz C. Effectiveness and Safety of Psoralen-UVA (PUVA) Topical Therapy in Palmoplantar Psoriasis: A Report on 48 Patients. Actas Dermosifiliogr. 2013 Mar 6. [QxMD MEDLINE Link].
- Mehta D, Lim HW. Ultraviolet B Phototherapy for Psoriasis: Review of Practical Guidelines. Am J Clin Dermatol. 2016 Apr. 17 (2):125-33. [QxMD MEDLINE Link].
- Stern DK, Creasey AA, Quijije J, Lebwohl MG. UV-A and UV-B Penetration of Normal Human Cadaveric Fingernail Plate. Arch Dermatol. 2011 Apr. 147(4):439-41. [QxMD MEDLINE Link].
- Brown T. Fingernail Psoriasis Data Added to Humira Prescribing Info. Medscape News & Perspective. Available at https://www.medscape.com/viewarticle/877985?src=soc_fb_170405_mscpedt_news_pharm_humira. March 30, 2017; Accessed: April 6, 2017.
- Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between Non-Alcoholic Fatty Liver Disease and Psoriasis: A Novel Hepato-Dermal Axis?. Int J Mol Sci. 2016 Feb 5. 17 (2):[QxMD MEDLINE Link].
- Salvi M, Macaluso L, Luci C, Mattozzi C, Paolino G, Aprea Y, et al. Safety and efficacy of anti-tumor necrosis factors α in patients with psoriasis and chronic hepatitis C. World J Clin Cases. 2016 Feb 16. 4 (2):49-55. [QxMD MEDLINE Link].
- Komrokji RS, Kulasekararaj A, Al Ali NH, Kordasti S, Bart-Smith E, Craig BM, et al. Autoimmune Diseases and Myelodysplastic Syndromes. Am J Hematol. 2016 Feb 13. [QxMD MEDLINE Link].
- Sorensen EP, Algzlan H, Au SC, Garber C, Fanucci K, Nguyen MB, et al. Lower Socioeconomic Status is Associated With Decreased Therapeutic Response to the Biologic Agents in Psoriasis Patients. J Drugs Dermatol. 2016 Feb 1. 15 (2):147-53. [QxMD MEDLINE Link].
- Castaldo G, Galdo G, Rotondi Aufiero F, Cereda E. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis. Obes Res Clin Pract. 2016 May-Jun. 10 (3):348-52. [QxMD MEDLINE Link].
- Barrea L, Balato N, Di Somma C, Macchia PE, Napolitano M, Savanelli MC, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet?. J Transl Med. 2015 Jan 27. 13:18. [QxMD MEDLINE Link].
- Millsop JW, Bhatia BK, Debbaneh M, Koo J, Liao W. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014 Sep. 71 (3):561-9. [QxMD MEDLINE Link].
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, Gutierrez M, Silva JJ, Torres LD, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013 Jan 1. 5 (1):222-34. [QxMD MEDLINE Link].
- Hackethal V. Guidelines on Psoriasis Comorbidity Screening in Kids Issued. Medscape News & Perspective. Available at https://www.medscape.com/viewarticle/880462?nlid=115307_1584&src=WNL_mdplsfeat_170530_mscpedit_derm&uac=106950CX&spon=33&impID=1357759&faf=1#vp_1. May 23, 2017; Accessed: May 31, 2017.
- [Guideline] Smith CH, Yiu ZZN, Bale T, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020 Oct. 183 (4):628-637. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019 Apr. 80 (4):1029-1072. [QxMD MEDLINE Link].
- [Guideline] Berth-Jones J, Exton LS, Ladoyanni E, Mohd Mustapa MF, Tebbs VM, Yesudian PD, et al. British Association of Dermatologists guidelines for the safe and effective prescribing of oral ciclosporin in dermatology 2018. Br J Dermatol. 2019 Jun. 180 (6):1312-1338. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019 Sep. 81 (3):775-804. [QxMD MEDLINE Link]. [Full Text].
- Kui R, Gál B, Gaál M, Kiss M, Kemény L, Gyulai R. Presence of antidrug antibodies correlates inversely with the plasma tumor necrosis factor (TNF)-α level and the efficacy of TNF-inhibitor therapy in psoriasis. J Dermatol. 2016 Sep. 43 (9):1018-23. [QxMD MEDLINE Link].
- Di Lernia V, Bardazzi F. Profile of tofacitinib citrate and its potential in the treatment of moderate-to-severe chronic plaque psoriasis. Drug Des Devel Ther. 2016. 10:533-9. [QxMD MEDLINE Link].
- Lebwohl MG, Stein Gold L, Strober B, Papp KA, Armstrong AW, Bagel J, et al. Phase 3 Trials of Tapinarof Cream for Plaque Psoriasis. N Engl J Med. 2021 Dec 9. 385 (24):2219-2229. [QxMD MEDLINE Link]. [Full Text].
Author
Jacquiline Habashy, DO, MSc Resident Physician, Department of Dermatology, Western University of Health Sciences College of Osteopathic Medicine of the Pacific
Jacquiline Habashy, DO, MSc is a member of the following medical societies: American Osteopathic College of Dermatology
Disclosure: Nothing to disclose.
Coauthor(s)
David T Robles, MD, PhD, FAAD Dermatologist, Oak Tree Dermatology
David T Robles, MD, PhD, FAAD is a member of the following medical societies: American Academy of Dermatology
Disclosure: Nothing to disclose.
Chief Editor
William D James, MD Emeritus Professor, Department of Dermatology, University of Pennsylvania School of Medicine
William D James, MD is a member of the following medical societies: American Academy of Dermatology, American Contact Dermatitis Society, Association of Military Dermatologists, Association of Professors of Dermatology, American Dermatological Association, Women's Dermatologic Society, Medical Dermatology Society, Dermatology Foundation, Society for Investigative Dermatology, Washington DC Dermatological Society, Atlantic Dermatologic Society, Philadelphia Dermatological Society, Pennsylvania Academy of Dermatology, College of Physicians of Philadelphia
Disclosure: Received income in an amount equal to or greater than $250 from: Elsevier
Served as a speaker for various universities, dermatology societies, and dermatology departments.
Additional Contributors
Acknowledgements
Robert Arffa, MD Clinical Assistant Professor, University of Pittsburgh School of Medicine
Robert Arffa, MD is a member of the following medical societies: American Academy of Ophthalmology
Disclosure: Nothing to disclose.
Richard Gordon Jr, MD Staff Physician, Department of Emergency Medicine, Detroit Receiving Hospital University Health Center
Richard Gordon Jr, MD is a member of the following medical societies: American College of Emergency Physicians, American College of Physicians, American Medical Student Association/Foundation, Emergency Medicine Residents Association, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Ryan I Huffman, MD Resident Physician, Department of Ophthalmology, Yale-New Haven Hospital
Disclosure: Nothing to disclose.
Simon K Law, MD, PharmD Clinical Professor of Health Sciences, Department of Ophthalmology, Jules Stein Eye Institute, University of California, Los Angeles, David Geffen School of Medicine
Simon K Law, MD, PharmD is a member of the following medical societies: American Academy of Ophthalmology, American Glaucoma Society, and Association for Research in Vision and Ophthalmology
Disclosure: Nothing to disclose.
Randy Park, MD Chair, Associate Professor, Department of Emergency Medicine, Denton Regional Medical Center
Disclosure: Nothing to disclose.
Brian A Phillpotts, MD Former Vitreo-Retinal Service Director, Former Program Director, Clinical Assistant Professor, Department of Ophthalmology, Howard University College of Medicine
Brian A Phillpotts, MD is a member of the following medical societies: American Academy of Ophthalmology, American Diabetes Association, American Medical Association, and National Medical Association
Disclosure: Nothing to disclose.
Christopher J Rapuano, MD Professor, Department of Ophthalmology, Jefferson Medical College of Thomas Jefferson University; Director of the Cornea Service, Co-Director of Refractive Surgery Department, Wills Eye Institute
Christopher J Rapuano, MD is a member of the following medical societies: American Academy of Ophthalmology, American Society of Cataract and Refractive Surgery, Contact Lens Association of Ophthalmologists, Cornea Society, Eye Bank Association of America, and International Society of Refractive Surgery
Disclosure: Allergan Honoraria Speaking and teaching; Allergan Consulting fee Consulting; Alcon Honoraria Speaking and teaching; RPS Ownership interest Other; Bausch & Lomb Honoraria Speaking and teaching; Merck Consulting fee Consulting; Bausch & Lomb Consulting; Merck Honoraria Speaking and teaching
Adam J Rosh, MD Assistant Professor, Program Director, Emergency Medicine Residency, Department of Emergency Medicine, Detroit Receiving Hospital, Wayne State University School of Medicine
Adam J Rosh, MD is a member of the following medical societies: American Academy of Emergency Medicine, American College of Emergency Physicians, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Hampton Roy Sr, MD Associate Clinical Professor, Department of Ophthalmology, University of Arkansas for Medical Sciences
Hampton Roy Sr, MD is a member of the following medical societies: American Academy of Ophthalmology, American College of Surgeons, and Pan-American Association of Ophthalmology
Disclosure: Nothing to disclose.
Dana A Stearns, MD Assistant Director of Undergraduate Education, Department of Emergency Medicine, Massachusetts General Hospital; Assistant Professor of Surgery, Harvard Medical School
Dana A Stearns, MD is a member of the following medical societies: American College of Emergency Physicians
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment