Goodpasture Syndrome: Practice Essentials, Background, Pathophysiology (original) (raw)
Practice Essentials
Goodpasture syndrome is an eponym that has been used to describe the clinical entity of diffuse pulmonary hemorrhage (as seen in the images below) and acute or rapidly progressive glomerulonephritis. Goodpasture disease is a term used to describe glomerulonephritis, with or without pulmonary hemorrhage, and the presence of circulating anti–glomerular basement membrane (anti-GBM) antibodies. The definitions of these terms have not been consistent, however. [1] Anti-GBM disease, a more precise term, should be used to refer to either of the two distinct clinical manifestations of this disorder.
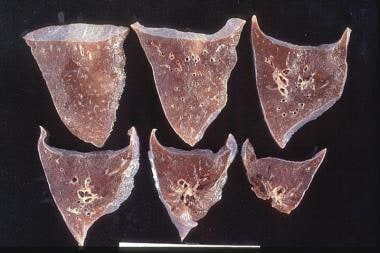
Goodpasture syndrome. A 45-year-old man was admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
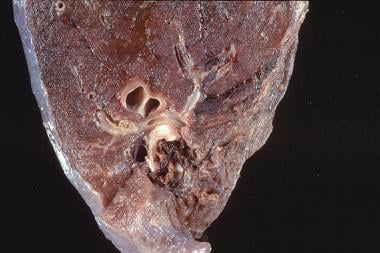
Goodpasture syndrome. Close-up view of gross pathology in a 45-year-old man admitted to the intensive care unit with respiratory failure secondary to massive hemoptysis and acute renal failure. The antiglomerular basement membrane antibodies were strongly positive. The autopsy showed consolidated lung from extensive bleeding, which led to asphyxiation.
Goodpasture syndrome (ie, anti-GBM disease) is an uncommon disorder of complex pathogeneses. Early recognition and treatment of this syndrome are critical because the prognosis for recovery of renal function depends on the initial extent of injury.
The three principles of therapy are as follows (see Treatment) [2] :
- Rapidly remove circulating antibody, primarily by plasmapheresis
- Stop further production of antibodies using immunosuppression with medications
- Remove offending agents that may have initiated the antibody production
Go to Pediatric Anti-GBM Disease (Goodpasture Syndrome) for complete information on this topic.
See Autoimmune Disorders: Making Sense of Nonspecific Symptoms, a Critical Images slideshow, to help identify several diseases that can cause a variety of nonspecific symptoms.

Background
Ernest Goodpasture first described this disorder in 1919. He reported a case of pulmonary hemorrhage and glomerulonephritis during an influenza epidemic. In 1955, Parkin [3] described 3 cases of lung hemorrhage and nephritis that occurred in the absence of arteritis. In 1958, Stanton and Tang [4] reported a series of young men with pulmonary hemorrhage and glomerulonephritis, similar to Goodpasture's original description.
In the 1950s, Krakower and Greenspon [5] identified GBM as the antigen. In 1967, Lerner, Glassock, and Dixon [6] confirmed that the antibodies taken from the diseased kidneys produced nephritis in experimental animals. The discovery of anti-GBM antibodies led to the understanding of the pathogenesis of Goodpasture syndrome.

Pathophysiology
Anti-GBM disease is an autoimmune disorder characterized by autoantibodies directed against the glomerular/alveolar basement membrane. The autoantibodies bind to their reactive epitopes in the basement membranes and activate the complement cascade, resulting in tissue injury. This is a classic type II reaction in the Gell and Coombs classification of antigen-antibody reactions. This binding of antibodies can be visualized as the linear deposition of immunoglobulin along the glomerular basement membrane and, less commonly, the alveolar basement membranes, by direct immunofluorescent techniques.
The basement membranes are complex structures that support layers of endothelium and epithelium. The principal component of basement membrane is type IV collagen, which acts as a support structure and is composed of building blocks that are linked end-to-end. The building blocks are composed of three alpha subunits of collagen, which form a triple helix. Type IV collagen can be expressed as six different chains, alpha1 to alpha6. The alpha chain itself has three structural domains, as follows:
- A short 7S domain at the amino terminus
- A triple helix of three alpha chains, which classically consists of two alpha1 chains and one alpha2 chain, and which ends at the carboxyl terminus
- A noncollagenous domain
In most patients, the autoantibody in Goodpasture syndrome is directed against a 28-kd monomeric subunit present within the noncollagenous domain of the alpha3 chain of type IV collagen (alpha3[IV]NC1). [7, 8] Two conformational epitopes of anti-GBM antibodies have been defined at residues 17-31 and 127-141 of alpha3(IV)NC1, which were named as EA and EB, respectively. The P14 peptide, which has been identified as one of the major linear epitopes recognized by sera from patients with anti-GBM disease, contains the amino acid sequence of EB, as well as one of the T cell epitopes found in anti-GBM disease. [9] Autoantibodies may also be directed against other alpha chains.
Although basement membranes are ubiquitous, only the alveolar and glomerular basement membranes are affected clinically. The preferential binding to the alveolar and glomerular basement membranes appears to be caused by greater accessibility of epitopes and greater expansion of alpha3 collagen units. Furthermore, the alpha3 collagen chains of glomerular and basement membranes are structurally integrated in such a way that they are more accessible to the circulating antibodies.
Under normal conditions, the alveolar endothelium is a barrier to the anti–basement membrane antibodies. However, with increased vascular permeability, antibody binding to the basement membrane occurs in the alveoli. Therefore, for the deposition of antibody, an additional nonspecific lung injury that increases alveolar-capillary permeability is required.
A variety of factors that can result in increased alveolar-capillary permeability have been identified. [10] These include the following:
- Increased capillary hydrostatic pressure
- High concentrations of inspired oxygen
- Bacteremia
- Endotoxemia
- Exposure to volatile hydrocarbons
- Upper respiratory infections
- Tobacco smoking
Strong evidence exists that genetics play an important role. Patients with specific human leukocyte antigen (HLA) types are more susceptible to disease and may have a worse prognosis.
There is an increased prevalence of HLA-DR15 (previously known as HLA-DR2) and DRB1*03, DRB1*04 and a decreased frequency of DRB1*01 and DRB1*07. [11, 12] Goodpasture disease is strongly associated with the DRB1*1501 and to a lesser extent the DRB1*1502 allele. Although a strong association exists between anti-GBM disease and HLA DRB1*1501, this allele is present in as many as one third of individuals in white populations. It is therefore clear that additional factors, either genetic or environmental, are required for disease expression.
Also of note, HLA-B7 is found more frequently and is associated with more severe anti-GBM nephritis.
Although anti-GBM disease is seen as a prototypic autoantibody-mediated disease, T cells have a vital role in disease initiation and progression. T cells enhance B-cell function and antibody production and may play a direct pathogenic role in kidney and lung injury. [13] Autoreactive T cells against alpha3(IV)NC1 are rare in healthy individuals, owing to thymic deletion during fetal development. Autoreactive T cells in patients may form as a consequence of exposure of neoepitopes, altered antigen processing allowing pathogenic epitopes to be presented, or altered T-cell regulation. [12]
Approximately 20%-40% of patients are double-positive for anti-GBM antibodies and antineutrophilic cytoplasmic antibodies (ANCAs). Coexistence of ANCAs (mostly myeloperoxidase [MPO-ANCAs]) with anti-GBM antibodies is thought to occur when the renal involvement in ANCA vasculitis leads to the exposure of antigens from the basement membrane and the formation of anti-GBM antibodies. [14]

Etiology
Like other autoimmune conditions, anti-GBM disease is thought to result from an environmental insult in a person with genetic susceptibility. The human leukocyte antigen (HLA) serotype HLA-DR15 has been strongly asssociated with anti-GBM disease. An initial insult to the pulmonary vasculature is required for exposure of the alveolar capillaries to the anti-GBM antibodies. Environmental factors that may lead to such exposure include the following:
- Exposure to organic solvents or hydrocarbons
- Smoking
- Infection (eg, influenza A2) [15]
- Cocaine inhalation
- Exposure to metal dusts
- Lymphocyte-depletion therapy, such as alemtuzumab
- Extracorporeal shock wave lithotripsy [16]
COVID-19 and COVID-19 vaccinations have been associated with both new and recurrent cases anti-GBM disease. [17, 18, 15]

Epidemiology
Anti-GBM disease is an uncommon disorder. The incidence of anti-GBM disease is estimated to be 0.5-1.8 cases per million per year in both European white and Asian populations. It is responsible for 1-5% of all types of glomerulonephritis and for 10-20% of crescentic glomerulonephritis. [19, 20]
Racial, sexual, and age-related differences in incidence
Anti-GBM disease occurs more commonly in white people than in black people, but it also may be more common in certain ethnic groups, such as the Maoris of New Zealand. The age distribution is bimodal, 20-30 years and 60-70 years. The prevalence of the disease is higher in men in the younger age group and women in the older age subgroup.
A subgroup of patients is double-positive for anti-GBM antibodies and antineutrophilic cytoplasmic antibodies. The peak age incidence for this subgroup is 60-70 years, with a male predominance. [8]

Prognosis
In the past, Goodpasture syndrome was usually fatal. Aggressive therapy with plasmapheresis, corticosteroids, and immunosuppressive agents has dramatically improved prognosis. [21] With this approach, the 5-year survival rate exceeds 80% and fewer than 30% of patients require long-term dialysis. In a study of patients patients admitted to intensive care units for acute manifestation of small-vessel vasculitis, including anti-GBM disease, delayed administration of cyclophosphamide was associated with a higher mortality rate. [22]
Patients presenting with serum creatinine levels greater than 4 mg/dL, oliguria, and more than 50% crescents on renal biopsy rarely recover. They usually progress to end-stage renal failure that requires long-term dialysis. In a retrospective analysis of patients with anti-GBM disease who started renal replacement therapy for end-stage renal disease (ESRD) in Australia and New Zealand (ANZDATA Registry), the median survival rate was 5.93 years with death predicted by older age and history of pulmonary hemorrhage. [20]

- DeVrieze BW, Hurley JA. Goodpasture Syndrome. 2023 Jan. [QxMD MEDLINE Link]. [Full Text].
- McAdoo SP, Pusey CD. Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 2017 Jul 7. 12 (7):1162-1172. [QxMD MEDLINE Link]. [Full Text].
- Parkin TW, Rusted IE, Burchell HB, Edwards JE. Hemorrhagic and interstitial pneumonitis with nephritis. Am J Med. 1955 Feb. 18(2):220-36. [QxMD MEDLINE Link].
- Stanton MC, Tange JD. Goodpasture's syndrome (pulmonary haemorrhage associated with glomerulonephritis). Australas Ann Med. 1958 May. 7(2):132-44. [QxMD MEDLINE Link].
- Krakower CA, Greenspon SA. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun. 51(6):629-39. [QxMD MEDLINE Link].
- Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967 Dec 1. 126(6):989-1004. [QxMD MEDLINE Link]. [Full Text].
- Zhao J, Cui Z, Yang R, Jia XY, Zhang Y, Zhao MH. Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity. Kidney Int. 2009 Nov. 76(10):1108-15. [QxMD MEDLINE Link].
- Cui Z, Zhao MH. Advances in human antiglomerular basement membrane disease. Nat Rev Nephrol. 2011 Jul 19. 7(12):697-705. [QxMD MEDLINE Link].
- Jia XY, Cui Z, Li JN, Hu SY, Zhao MH. Identification of critical residues of linear B cell epitope on Goodpasture autoantigen. PLoS One. 2015. 10 (4):e0123277. [QxMD MEDLINE Link]. [Full Text].
- Chan AL, Louie S, Leslie KO, Juarez MM, Albertson TE. Cutting edge issues in Goodpasture's disease. Clin Rev Allergy Immunol. 2011 Oct. 41(2):151-62. [QxMD MEDLINE Link].
- Yang R, Cui Z, Zhao J, Zhao MH. The role of HLA-DRB1 alleles on susceptibility of Chinese patients with anti-GBM disease. Clin Immunol. 2009 Nov. 133(2):245-50. [QxMD MEDLINE Link].
- Peto P, Salama AD. Update on antiglomerular basement membrane disease. Curr Opin Rheumatol. 2011 Jan. 23(1):32-7. [QxMD MEDLINE Link].
- Cui Z, Zhao J, Jia XY, Zhu SN, Zhao MH. Clinical features and outcomes of anti-glomerular basement membrane disease in older patients. Am J Kidney Dis. 2011 Apr. 57(4):575-82. [QxMD MEDLINE Link].
- Thongprayoon C, Kaewput W, Boonpheng B, Ungprasert P, Bathini T, Srivali N, et al. Impact of ANCA-Associated Vasculitis on Outcomes of Hospitalizations for Goodpasture's Syndrome in the United States: Nationwide Inpatient Sample 2003-2014. Medicina (Kaunas). 2020 Mar 1. 56 (3):[QxMD MEDLINE Link]. [Full Text].
- Asim M, Akhtar M. Epidemiology, Impact, and Management Strategies of Anti-Glomerular Basement Membrane Disease. Int J Nephrol Renovasc Dis. 2022. 15:129-138. [QxMD MEDLINE Link]. [Full Text].
- Cranfield A, Mathavakkannan S. Goodpasture's disease following extracorporeal shock wave lithotripsy: a case report & literature review. Clin Case Rep. 2015 Mar. 3 (3):160-4. [QxMD MEDLINE Link]. [Full Text].
- Prendecki M, Clarke C, Cairns T, Cook T, Roufosse C, Thomas D, et al. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020 Sep. 98 (3):780-781. [QxMD MEDLINE Link]. [Full Text].
- Prema KSJ, Kurien A. Incidence of anti-glomerular basement membrane disease during the COVID-19 pandemic. Clin Kidney J. 2022 Jan. 15 (1):180-181. [QxMD MEDLINE Link]. [Full Text].
- Kluth DC, Rees AJ. Anti-glomerular basement membrane disease. J Am Soc Nephrol. 1999 Nov. 10(11):2446-53. [QxMD MEDLINE Link].
- Tang W, McDonald SP, Hawley CM, et al. Anti-glomerular basement membrane antibody disease is an uncommon cause of end-stage renal disease. Kidney Int. 2013 Mar. 83(3):503-10. [QxMD MEDLINE Link].
- Shah MK, Hugghins SY. Characteristics and outcomes of patients with Goodpasture's syndrome. South Med J. 2002 Dec. 95(12):1411-8. [QxMD MEDLINE Link].
- Kimmoun A, Baux E, Das V, Terzi N, Talec P, Asfar P, et al. Outcomes of patients admitted to intensive care units for acute manifestation of small-vessel vasculitis: a multicenter, retrospective study. Crit Care. 2016 Jan 26. 20:27. [QxMD MEDLINE Link].
- Weber MF, Andrassy K, Pullig O, Koderisch J, Netzer K. Antineutrophil-cytoplasmic antibodies and antiglomerular basement membrane antibodies in Goodpasture's syndrome and in Wegener's granulomatosis. J Am Soc Nephrol. 1992 Jan. 2(7):1227-34. [QxMD MEDLINE Link].
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med. 2004 Sep. 25(3):583-92, vii. [QxMD MEDLINE Link].
- Sinico RA, Radice A, Corace C, Sabadini E, Bollini B. Anti-glomerular basement membrane antibodies in the diagnosis of Goodpasture syndrome: a comparison of different assays. Nephrol Dial Transplant. 2006 Feb. 21(2):397-401. [QxMD MEDLINE Link].
- Yang R, Hellmark T, Zhao J, et al. Levels of epitope-specific autoantibodies correlate with renal damage in anti-GBM disease. Nephrol Dial Transplant. 2009 Jun. 24(6):1838-44. [QxMD MEDLINE Link].
- Liang D, Liang S, Xu F, Zhang M, Li X, Tu Y, et al. Clinicopathological features and outcome of antibody-negative anti-glomerular basement membrane disease. J Clin Pathol. 2019 Jan. 72 (1):31-37. [QxMD MEDLINE Link].
- Zhong Z, Tan J, Tang Y, Li Z, Qin W. Goodpasture syndrome manifesting as nephrotic-range proteinuria with anti-glomerular basement membrane antibody seronegativity: A case report. Medicine (Baltimore). 2020 Sep 25. 99 (39):e22341. [QxMD MEDLINE Link]. [Full Text].
- Olson SW, Arbogast CB, Baker TP, et al. Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol. 2011 Oct. 22(10):1946-52. [QxMD MEDLINE Link]. [Full Text].
- Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004 Oct. 66(4):1535-40. [QxMD MEDLINE Link].
- Rutgers A, Slot M, van Paassen P, van Breda Vriesman P, Heeringa P, Tervaert JW. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005 Aug. 46(2):253-62. [QxMD MEDLINE Link].
- de Joode AA, Roozendaal C, van der Leij MJ, Bungener LB, Sanders JS, Stegeman CA. Performance of two strategies for urgent ANCA and anti-GBM analysis in vasculitis. Eur J Intern Med. 2014 Feb. 25(2):182-6. [QxMD MEDLINE Link].
- Zhao J, Yan Y, Cui Z, Yang R, Zhao MH. The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity. Hum Immunol. 2009 Jun. 70(6):425-9. [QxMD MEDLINE Link].
- Frankel SK, Cosgrove GP, Fischer A, Meehan RT, Brown KK. Update in the diagnosis and management of pulmonary vasculitis. Chest. 2006 Feb. 129(2):452-65. [QxMD MEDLINE Link].
- Johnson JP, Moore J Jr, Austin HA 3rd, Balow JE, Antonovych TT, Wilson CB. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore). 1985 Jul. 64(4):219-27. [QxMD MEDLINE Link].
- Levy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001 Jun 5. 134(11):1033-42. [QxMD MEDLINE Link].
- Apaydin S. The treatment of ANCA-associated rapidly-progressive glomerulonephritis and Goodpasture syndrome with therapeutic apheresis. Transfus Apher Sci. 2018 Feb. 57 (1):8-12. [QxMD MEDLINE Link].
- Zhang YY, Tang Z, Chen DM, Gong DH, Ji DX, Liu ZH. Comparison of double filtration plasmapheresis with immunoadsorption therapy in patients with anti-glomerular basement membrane nephritis. BMC Nephrol. 2014 Aug 3. 15:128. [QxMD MEDLINE Link]. [Full Text].
- Shah Y, Mohiuddin A, Sluman C, et al. Rituximab in anti-glomerular basement membrane disease. QJM. 2012 Feb. 105(2):195-7. [QxMD MEDLINE Link].
- Syeda UA, Singer NG, Magrey M. Anti-glomerular basement membrane antibody disease treated with rituximab: A case based review. Semin Arthritis Rheum. 2013 Jan 24. [QxMD MEDLINE Link].
- Kashtan CE. Renal transplantation in patients with Alport syndrome. Pediatr Transplant. 2006 Sep. 10(6):651-7. [QxMD MEDLINE Link].
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct. 100 (4S):S1-S276. [QxMD MEDLINE Link]. [Full Text].
Author
Pranay Kathuria, MD, MACP, FASN, FNKF Professor of Medicine, Gussman Chair in Internal Medicine, Director, Division of Nephrology and Hypertension, Program Director, Nephrology Fellowship Program, University of Oklahoma School of Community Medicine, University of Oklahoma College of Medicine
Pranay Kathuria, MD, MACP, FASN, FNKF is a member of the following medical societies: American College of Physicians-American Society of Internal Medicine, American Society of Hypertension, American Society of Nephrology, National Kidney Foundation, International Society for Peritoneal Dialysis
Disclosure: Nothing to disclose.
Coauthor(s)
Frazier T Stevenson, MD Associate Professor of Clinical Medicine and Director of Education Development, University of California Davis School of Medicine
Disclosure: Nothing to disclose.
Sat Sharma, MD, FRCPC Professor and Head, Division of Pulmonary Medicine, Department of Internal Medicine, University of Manitoba Faculty of Medicine; Site Director, Respiratory Medicine, St Boniface General Hospital, Canada
Sat Sharma, MD, FRCPC is a member of the following medical societies: American Academy of Sleep Medicine, American College of Chest Physicians, American College of Physicians-American Society of Internal Medicine, American Thoracic Society, Canadian Medical Association, Royal College of Physicians and Surgeons of Canada, Royal Society of Medicine, Society of Critical Care Medicine, World Medical Association
Disclosure: Nothing to disclose.
Chief Editor
Vecihi Batuman, MD, FASN Professor of Medicine, Section of Nephrology-Hypertension, Deming Department of Medicine, Tulane University School of Medicine
Vecihi Batuman, MD, FASN is a member of the following medical societies: American College of Physicians, American Society of Hypertension, American Society of Nephrology, Southern Society for Clinical Investigation
Disclosure: Nothing to disclose.
Acknowledgements
Eleanor Lederer, MD Professor of Medicine, Chief, Nephrology Division, Director, Nephrology Training Program, Director, Metabolic Stone Clinic, Kidney Disease Program, University of Louisville School of Medicine; Consulting Staff, Louisville Veterans Affairs Hospital
Eleanor Lederer, MD is a member of the following medical societies: American Association for the Advancement of Science, American Federation for Medical Research, American Society for Biochemistry and Molecular Biology, American Society for Bone and Mineral Research, American Society of Nephrology, American Society of Transplantation, International Society of Nephrology, Kentucky Medical Association, National Kidney Foundation, and Phi Beta Kappa
Disclosure: Dept of Veterans Affairs Grant/research funds Research
James W Lohr, MD Professor, Department of Internal Medicine, Division of Nephrology, Fellowship Program Director, University of Buffalo State University of New York School of Medicine and Biomedical Sciences
James W Lohr, MD is a member of the following medical societies: American College of Physicians, American Heart Association, American Society of Nephrology, and Central Society for Clinical Research
Disclosure: Genzyme Honoraria Speaking and teaching
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Mauro Verrelli, MD, FRCP(C), FACP Assistant Professor, Department of Medicine, Section of Nephrology, University of Manitoba, Canada
Mauro Verrelli, MD, FRCP(C), FACP is a member of the following medical societies: American College of Physicians-American Society of Internal Medicine, American Society of Nephrology, Canadian Medical Association, and Royal College of Physicians and Surgeons of Canada
Disclosure: Nothing to disclose.