Nephrotic Syndrome: Practice Essentials, Pathophysiology, Etiology (original) (raw)
Overview
Practice Essentials
Nephrotic syndrome is the combination of nephrotic-range proteinuria with a low serum albumin level and edema. Nephrotic-range proteinuria is the loss of 3 grams or more per day of protein into the urine or, on a single spot urine collection, the presence of 2 g of protein per gram of urine creatinine.
Nephrotic syndrome has many causes, including primary kidney diseases such as minimal-change disease, focal segmental glomerulosclerosis, and membranous glomerulonephritis. Nephrotic syndrome can also result from systemic diseases that affect other organs in addition to the kidneys, such as diabetes, amyloidosis, and lupus erythematosus. [1]
Nephrotic syndrome may affect adults and children of both sexes and of any race. It may occur in typical form, or in association with nephritic syndrome. The latter term connotes glomerular inflammation, with hematuria and impaired kidney function.
The first sign of nephrotic syndrome in children is usually swelling of the face; this is followed by swelling of the entire body. Adults can present with dependent edema. Fatigue and loss of appetite are common symptoms. (See Presentation.)
Classification
Nephrotic syndrome can be primary, being a disease specific to the kidneys, or it can be secondary, being a renal manifestation of a systemic general illness. In all cases, injury to glomeruli is an essential feature. Kidney diseases that affect tubules and interstitium, such as interstitial nephritis, will not cause nephrotic syndrome.
Primary causes of nephrotic syndrome include the following, in approximate order of frequency:
- Minimal-change nephropathy
- Focal glomerulosclerosis
- Membranous nephropathy
- Hereditary nephropathies
Secondary causes include the following, again in order of approximate frequency:
- Diabetes mellitus
- Lupus erythematosus
- Viral infections (eg, hepatitis B, hepatitis C, human immunodeficiency virus [HIV] )
- Amyloidosis and paraproteinemias
- Preeclampsia
- Allo-antibodies from enzyme replacement therapy
Nephrotic-range proteinuria may occur in other kidney diseases, such as IgA nephropathy. In that common glomerular disease, one third of patients may have nephrotic-range proteinuria. [2]
Nephrotic syndrome may occur in persons with sickle cell disease and evolve to renal failure. Membranous nephropathy may complicate bone marrow transplantation, in association with graft versus host disease.
From a therapeutic perspective, nephrotic syndrome may be classified as steroid sensitive, steroid resistant, steroid dependent, or frequently relapsing.
The above causes of nephrotic syndrome are largely those for adults, and this article will concentrate primarily on adult nephrotic syndrome. However, nephrotic syndrome in infancy and childhood is an important entity. For discussion of this topic, see Pediatric Nephrotic Syndrome.

Pathophysiology
In a healthy individual, less than 0.1% of plasma albumin may traverse the glomerular filtration barrier. [3] Controversy exists regarding the sieving of albumin across the glomerular permeability barrier. On the basis of studies in experimental animals, it has been proposed that ongoing albumin passage into the urine occurs in many grams per day, with equivalent substantial tubular uptake of albumin, the result being that the urine contains 80 mg or less of albumin per day. [4]
However, studies of humans with tubular transport defects suggest that the glomerular urinary space albumin concentration is 3.5 mg/L. [5] At this concentration, and a normal daily glomerular filtration rate (GFR) of 150 liters, one would expect at most 525 mg per day of albumin in the final urine. In health, urine albumin is less than 50 mg/day, because most of the filtered albumin is re-absorbed by the tubules. Amounts above 500 mg/day point to glomerular disease.
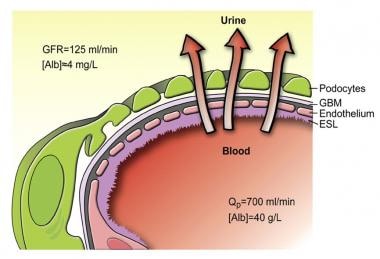
The glomerular capillaries are lined by a fenestrated endothelium that sits on the glomerular basement membrane, which in turn is covered by glomerular epithelium, or podocytes, which envelops the capillaries with cellular extensions called foot processes. In between the foot processes are the filtration slits. These three structures—the fenestrated endothelium, glomerular basement membrane, and glomerular epithelium—are the glomerular filtration barrier. A schematic drawing of the glomerular barrier is provided in the image below.
Schematic drawing of the glomerular barrier. Podo = podocytes; GBM = glomerular basement membrane; Endo = fenestrated endothelial cells; ESL = endothelial cell surface layer (often referred to as the glycocalyx). Primary urine is formed through the filtration of plasma fluid across the glomerular barrier (arrows); in humans, the glomerular filtration rate (GFR) is 125 mL/min. The plasma flow rate (Qp) is close to 700 mL/min, with the filtration fraction being 20%. The concentration of albumin in serum is 40 g/L, while the estimated concentration of albumin in primary urine is 4 mg/L, or 0.1% of its concentration in plasma. Courtesy of the American Physiological Society (www.the-aps.org) and reproduced from Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008 Apr;88(2):451-87.
Filtration of plasma water and solutes is extracellular and occurs through the endothelial fenestrae and filtration slits. The importance of the podocytes and the filtration slits is shown by genetic diseases. In congenital nephrotic syndrome of the Finnish type, the gene for nephrin, a protein of the filtration slit, is mutated, leading to nephrotic syndrome in infancy_._ Similarly, podocin, a protein of the podocytes, may be abnormal in a number of children with steroid-resistant focal glomerulosclerosis.
The glomerular structural changes that may cause proteinuria are damage to the endothelial surface, the glomerular basement membrane, or the podocytes. One or more of these mechanisms may be seen in any one type of nephrotic syndrome. Albuminuria alone may occur or, with greater injury, leakage of all plasma proteins (ie, proteinuria) may take place.
Proteinuria that is more than 85% albumin is selective proteinuria. Albumin has a net negative charge, and it is proposed that loss of glomerular membrane negative charges could be important in causing albuminuria. Nonselective proteinuria, being a glomerular leakage of all plasma proteins, would not involve changes in glomerular net charge but rather a generalized defect in permeability. This construct does not permit clear-cut separation of causes of proteinuria, except in minimal-change nephropathy, in which proteinuria is selective.
Pathogenesis of edema
There are two current hypotheses for the formation of edema in nephrotic syndrome. The underfill hypothesis holds that the loss of albumin leading to lower plasma colloid pressure is the cause. The overfill hypothesis states that the edema is due to primary renal sodium retention.
Underfill hypothesis
An increase in glomerular permeability leads to albuminuria and eventually to hypoalbuminemia. In turn, hypoalbuminemia lowers the plasma colloid osmotic pressure, causing greater transcapillary filtration of water throughout the body and thus the development of edema.
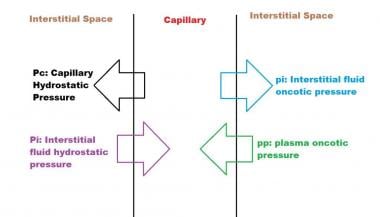
Capillary hydrostatic pressure and the gradient of plasma to interstitial fluid oncotic pressure determine the movement of fluid from the vascular compartment to the interstitium. The oncotic pressure is mainly determined by the protein content. The flux of water across the capillary wall can be expressed by the following formula:
Qw = K ([Pc - pp] - [Pi - pi]
In this formula, Qw is net flux of water, K is the capillary filtration coefficient, Pc is capillary hydrostatic pressure, and pp is the plasma oncotic pressure, while Pi is the interstitial fluid hydrostatic pressure and pi is the interstitial fluid oncotic pressure, shown schematically below.
Forces determining capillary filtration
With a high enough capillary hydrostatic pressure or a low enough intravascular oncotic pressure, the amount of fluid filtered exceeds the maximal lymphatic flow, and edema occurs. In patients with nephrotic syndrome, this causes a reduction in plasma volume, with a secondary increase of sodium and water retention by the kidneys.
Overfill Hypothesis
An alternative hypothesis is an intrinsic defect in the renal tubules which cause a decrease in sodium excretion. This could occur if the filtered intraluminal protein directly stimulated renal epithelial sodium reabsorption. [6] Two facts support this hypothesis: (1) sodium retention is observed even before the serum albumin level starts falling, and (2) intravascular volume is normal or even increased in most patients with nephrotic syndrome.
A third possible mechanism is an enhanced peripheral capillary permeability to albumin, as shown by radioisotopic technique in human studies of 60 patients with nephrotic syndrome. [7] This would then lead to increased tissue oncotic pressure and fluid retention in the peripheral tissues.
Metabolic consequences of proteinuria
Metabolic consequences of the nephrotic syndrome include the following:
- Infection
- Hyperlipidemia and atherosclerosis
- Hypocalcemia and bone abnormalities
- Hypercoagulability
- Hypovolemia
Acute kidney injury may indicate an underlying glomerulonephritis but is more often precipitated by hypovolemia or sepsis. Edema of the kidneys that causes a pressure-mediated reduction in the GFR has also been proposed.
Additional consequences include the following:
- Hypertension related to fluid retention and reduced kidney function may occur
- Edema of the gut may cause defective absorption, leading to malnutrition
- Ascites and pleural effusions may develop
Infection
Infection is a major concern in nephrotic syndrome. Both gram positive and gram negative bacterial infect. Varicella infection is also common. The most common infectious complications are bacterial sepsis, cellulitis, pneumonia, and peritonitis.
Proposed explanations for the increased infection risk include the following:
- Urinary immunoglobulin losses
- Edema fluid acting as a culture medium
- Protein deficiency
- Decreased bactericidal activity of the leukocytes
- Immunosuppressive therapy
- Decreased perfusion of the spleen caused by hypovolemia
- Urinary loss of a complement factor (properdin factor B) that opsonizes certain bacteria
Hyperlipidemia and atherosclerosis
Hyperlipidemia is a classic feature of the nephrotic syndrome, rather than a mere complication. It is related to the hypoproteinemia and low serum oncotic pressure of nephrotic syndrome, which then leads to reactive hepatic protein synthesis, including of lipoproteins. [8] In addition, reduced plasma levels of lipoprotein lipase results in diminution of lipid catabolism. Some of the elevated serum lipoproteins are filtered at the glomeruli, leading to lipiduria and the classic findings of oval fat bodies and fatty casts in the urine sediment.
Atherosclerotic vascular disease appears to occur in greater frequency in persons with nephrotic syndrome than in healthy persons of the same age. Curry and Roberts showed that the frequency and extent of coronary artery stenoses were greater in patients with nephrotic syndrome than in non-nephrotic control subjects. [9]
When their study was published, in 1977, lipid-lowering treatments were less widely used than they are today. Accordingly, the average highest serum total cholesterol in this series was over 400 mg/dL. That is in the range of serum cholesterol seen in familial hypercholesterolemia, a disease that predisposes individuals to myocardial infarction.
Hypocalcemia
Hypocalcemia is common in the nephrotic syndrome, but rather than being a true hypocalcemia, it is usually caused by a low serum albumin level. Nonetheless, low bone density and abnormal bone histology are reported in association with nephrotic syndrome. This could be caused by urinary losses of vitamin D–binding proteins, with consequent hypovitaminosis D and, as a result, reduced intestinal calcium absorption. [10]
Tessitore et al reported that when the GFR was normal, persons with the nephrotic syndrome had no consistent calcium or bony abnormalities. [11] Yet in that same study, when the GFR was reduced, bone mineralization defects were found by biopsy. A later study found osteomalacia on bone biopsy in over half of adults who had longstanding nephrotic syndrome but whose GFR was preserved. [10]
Low bone mass may be found in relation to cumulative steroid dose. [12] However, intermittent corticosteroid treatment of childhood steroid-sensitive nephrotic syndrome was not associated with bone mineral deficits in one study. [13] It is possible that long duration of either the nephrotic syndrome or treatments for it are the important risk factors for bone disease in these patients.
Hypercoagulability
Venous thrombosis and pulmonary embolism are well-known complications of the nephrotic syndrome. Hypercoagulability in these cases appears to derive from urinary loss of anticoagulant proteins, such as antithrombin III and plasminogen, along with the simultaneous increase in clotting factors, especially factors I, VII, VIII, and X.
A study by Mahmoodi et al of almost 300 patients with nephrotic syndrome confirmed that the annual incidence of venous thromboembolism (VTE) was almost 10 times higher in these persons than in the normal population (1% vs 0.1 to 0.2%). [14] Moreover, that risk appeared especially elevated during the first 6 months of nephrotic syndrome, being at almost 10%. This high incidence may justify the routine use of preventive anticoagulation treatment during the first 6 months of a persistent nephrotic syndrome.
Mahmoodi et al's study also showed an increased risk of arterial thrombotic events in subjects with nephrotic syndrome, including coronary and cerebrovascular ones. Unlike the risk of VTE, which was related to proteinuria, this arterial risk was related to usual risk factors for arterial disease, such as hypertension, diabetes, smoking, and reduced GFR.
Hypovolemia
Hypovolemia occurs when hypoalbuminemia decreases the plasma oncotic pressure, resulting in a loss of plasma water into the interstitium and causing a decrease in circulating blood volume. Hypovolemia is generally observed only when the patient's serum albumin level is less than 1.5 g/dL. Symptoms include vomiting, abdominal pain, and diarrhea. The signs include cold hands and feet, delayed capillary filling, oliguria, and tachycardia. Hypotension is a late feature.

Etiology
Common primary causes of nephrotic syndrome include kidney diseases such as minimal-change nephropathy, membranous nephropathy, and focal glomerulosclerosis. Secondary causes include systemic diseases such as diabetes mellitus, lupus erythematosus, and amyloidosis. Congenital and hereditary focal glomerulosclerosis may result from mutations of genes that code for podocyte proteins, including nephrin, podocin, or the cation channel 6 protein. [15] Nephrotic syndrome can result from drugs of abuse, such as heroin.
The proposed mechanisms of membranous nephropathy are as follows:
- Immune complex deposition from the circulation
- In-situ formation of immune complexes through the reaction of circulating autoantibodies to a native antigen
- In-situ formation of immune complexes with a non-native (extrinsic) antigen that is bound to the podocytes or glomerular basement membrane
The first mechanism could explain the secondary membranous nephropathy of systemic lupus erythematosus.
The second mechanism appears to explain 70% of idiopathic membranous nephropathy. M-type phospholipase A2 receptor (PLA2R) antibodies are found in about 70% of patients who have idiopathic membranous glomerular nephropathy. [16] These IgG antibodies are found both circulating in the plasma and deposited on the glomerular basement membranes.
The third mechanism may explain the rare occurrence of nephrotic syndrome in subjects treated with enzyme replacement therapy for genetic enzyme deficiency diseases such as Pompe or Fabry disease [17, 18] This may result from allo-antibodies to the infused enzyme that are deposited on the glomerular basement membrane, with ensuing secondary membranous nephropathy.
Nephrotic-range proteinuria occurring in the third trimester of pregnancy is the classical finding of preeclampsia. It may occur de novo or it may be superimposed on another chronic kidney disease. In the latter case, the patient will have had preexisting proteinuria that worsened during pregnancy.
In steroid-resistant nephrotic syndrome (SNRS), about 30% of childhood-onset cases and 10%-15% of adult-onset cases are hereditary, resulting from an alteration in one of roughly 60 genes. Genetic SRNS can occur on its own (nonsyndromic) or as part of a syndrome, in association with additional signs and symptoms (eg, Alport syndrome, primary coenzyme Q10 deficiency). [19]
Medication can cause nephrotic syndrome. This includes the very infrequent occurrence of minimal-change nephropathy with use of nonsteroidal anti-inflammatory drugs (NSAIDs), and the occurrence of membranous nephropathy with use of gold and penicillamine, which are older drugs used for rheumatic diseases. Focal glomerulosclerosis can occur in association with intravenous bisphosphonates. Lithium and interferon therapy have been associated with focal glomerulosclerosis of the collapsing type.
Nephrotic-range proteinuria could occur with the use of anticancer agents, such as bevacizumab, that inhibit vascular endothelial growth factor (VEGF). [20] However, the clinical picture of this complication is of a thrombotic microangiopathy rather than of nephrotic syndrome per se.
The association of membranous nephropathy with cancer is a clinical dilemma. This association presumably results from immune complex injury to the glomeruli caused by cancer antigens. While about 6000 new cases of membranous nephropathy occur each year in the United States, 1.5 million new cases of non-skin cancer are diagnosed. Therefore, from the oncologist’s standpoint, the problem of paraneoplastic membranous nephropathy is trivial. However, a carefully performed analysis from France suggested that the cancer rate is approximately 10-fold higher in persons with membranous nephropathy than in the general population, especially in individuals over age 65 years. [21] In that study, 50% of membranous nephropathy cases were diagnosed before the diagnosis of cancer. Thus, in some patients with membranous nephropathy one should consider the possibility of an undiagnosed cancer.
Nephrotic syndrome has been reported following both COVID-19 infection and vaccination, most commonly related to a collapsing form of focal segmental glomerulosclerosis. [22]

Epidemiology
United States statistics
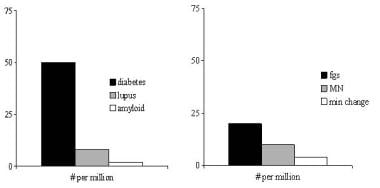
The figure below shows the incidence per million population of important causes of nephrotic syndrome. Diabetic nephropathy with nephrotic syndrome is most common, at an estimated rate of at least 50 cases per million population. In children, nephrotic syndrome may occur at a rate of 20 cases per million children. [23]
Incidence of important causes of nephrotic syndrome, in number per million population. The left panel shows systemic causes, and the right panel lists primary renal diseases that can cause nephrotic syndrome. fgs = focal glomerulosclerosis, MN = membranous nephropathy, min change = minimal-change nephropathy. Data are in part from Swaminathan et al and Bergesio et al.
International statistics
Biopsy studies in children with nephrotic syndrome have shown similar types of histology in India and Turkey, compared with what one would expect in Western countries. [24, 25] In Pakistani adults with nephrotic syndrome, the spectrum of histologies of kidney biopsies is similar to that seen in western countries. [26]
In parts of Africa and the Middle East (eg, Egypt), glomerular disease may be associated with urogenital schistosomal infection. [27] However, so-called tropical nephrotic syndrome from parasitic diseases such as schistosomiasis or malaria may not be a true entity.
Doe et al reviewed causes of nephrotic syndrome in African children; kidney biopsy most often showed typical histologic findings (focal and segmental glomerulosclerosis and minimal change disease). [28]
The connection of nephrotic syndrome to quartan malaria is not well-established. Indeed, Pakasa and Sumaili call attention to the apparent decline of parasite-associated nephrotic syndrome in the Congo. [29, 30] It is possible that the perceived association between nephrotic syndrome and parasitic infections was coincidental, as supported by the ongoing and probably increasing occurrence of chronic kidney disease in the Congo. [30]
Race-, sex-, and age-related demographics
Because diabetes is major cause of nephrotic syndrome, American Indians, Hispanics, and African Americans have a higher incidence of nephrotic syndrome than do white persons. HIV nephropathy is a complication of HIV infection that is unusual in whites; it is seen with greater frequency in African Americans, because of their much greater prevalence of the ApoL1 risk alleles. [31] Focal glomerulosclerosis appears to be overrepresented as a cause of nephrotic syndrome in African-American as compared with white children. [32]
There is a male predominance in the occurrence of nephrotic syndrome, as for chronic kidney disease in general. This male overrepresentation is also seen in paraneoplastic membranous nephropathy. [21] But lupus nephritis affects mostly women.
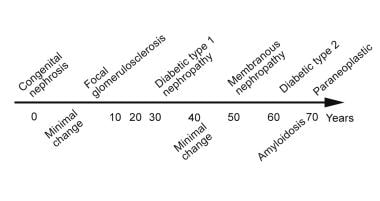
The image below shows typical ages at which a given cause of nephrotic syndrome may occur. It does not show every possible cause of nephrotic syndrome, such as lupus nephritis, which typically affects young black women. The ages shown are averages.
A schema of the average patient age at presentation in various common forms of nephrotic syndrome. (Timeline not to scale.)

Prognosis
In the pre-antibiotic era, infection was a major factor in the mortality rate among patients with nephrotic syndrome. [33] Treatments for nephrotic syndrome and its complications have reduced the morbidity and mortality once associated with the syndrome. Currently, the prognosis for patients with primary nephrotic syndrome depends on its cause.
Infants with congenital nephrotic syndrome have a dismal prognosis: survival beyond several months is possible only with dialysis and kidney transplantation.
Children with idiopathic nephrotic syndrome generally have an excellent prognosis. The majority of these cases (90%) are steroid sensitive and approximately a quarter of these children will have no further exacerbations after completing the initial course of therapy. Although the remainder may experience relapsing disease, kidney failure is rare. [34]
Only approximately 20% of patients with focal glomerulosclerosis undergo remission of proteinuria; an additional 10% improve but remain proteinuric. Many patients experience frequent relapses, become steroid-dependent, or become steroid-resistant. End-stage renal disease (ESRD) develops in 25-30% of patients with focal segmental glomerulosclerosis by 5 years and in 30-40% of these patients by 10 years.
The prognosis for patients with minimal-change nephropathy is very good. Most children respond to steroid therapy; still, about 50% of children have one or two relapses within 5 years and approximately 20% of them continue to relapse 10 years after diagnosis. Only 30% of children never have a relapse after the initial episode. Approximately 3% of patients who initially respond to steroids become steroid-resistant.
Adults with minimal-change nephropathy have a burden of relapse similar to that of children. However, the long-term prognosis for kidney function in patients with this disease is excellent, with little risk of renal failure.
Analysis of 441 adult and pediatric patients by the Nephrotic Syndrome Study Network (NEPTUNE) found that complete remission of proteinuria occurred in 45% of cases, while 5% progressed to ESRD. The following were inversely associated with complete remission of proteinuria [35] :
- Higher pre-biopsy proteinuria level.
- Pathology diagnosis of focal segmental glomerulosclerosis [FSGS] versus minimal-change disease
Poor patient response to steroid therapy may predict a poor outcome. Children who present with hematuria and hypertension are more likely to be steroid-resistant and have a poorer prognosis than those without hematuria or hypertension. In a retrospective study of pediatric patients with steroid-resistant nephrotic syndrome, 13 of 16 patients achieved remission with calcineurin inhibitor therapy. However, three of the 13 experienced recurrences and progressed to ESRD. [36]
Of patients with nephrotic syndrome due to membranous nephropathy, approximately 30% experience spontaneous remission. Of those with persistent nephrotic syndrome, 40% to 50% progress to ESRD over a period of 10 years. [37]
Donadio et al reported 140 patients with idiopathic membranous nephropathy, 89 of whom received no treatment with corticosteroids or immunosuppressive drugs and 51 of whom were treated primarily with short-term courses of prednisone alone, and found that survival rates in these patients were the same as those expected for the general population. [38]
The prognosis may worsen because of (1) an increased incidence of renal failure and the complications secondary to nephrotic syndrome, including thrombotic episodes and infection, or (2) treatment-related conditions, such as infectious complications of immunosuppressive drug therapy.
In secondary nephrotic syndromes, morbidity and mortality are related to the primary disease process (eg, diabetes, lupus, amyloidosis). In diabetic nephropathy, however, the magnitude of proteinuria itself relates directly to mortality. [39]
In diabetic nephropathy with nephrotic syndrome, patients usually have a good response to angiotensin blockade, with reduction of proteinuria and some slowing of the loss of renal function. True remission is uncommon, however. Cardiovascular morbidity and mortality increase as kidney function declines, and some patients will eventually need dialysis or a kidney transplant.
In primary amyloidosis, prognosis is not good, even with intensive chemotherapy. In secondary amyloidosis, remission of the underlying cause, such as rheumatoid arthritis, is followed by remission of the amyloidosis and its associated nephrotic syndrome.

Patient Education
Pediatric nephrotic syndrome is a chronic illness characterized by relapses and remissions, which can extend throughout childhood. There will be illness from the disease and from its treatment. Parents may monitor their child's urine and record the results in a diary. The diary can also be used to write down an agreed-upon plan for the management of relapses. Information booklets should be given to the family. Peer support and psychological counseling may be helpful.
Nephrotic syndrome in adults can also wax and wane, with the complications as reviewed above.
Progression to renal failure will require preparation for dialysis and/or kidney transplantation.

- Tapia C, Bashir K. Nephrotic Syndrome. 2023 Jan. [QxMD MEDLINE Link]. [Full Text].
- Wu MY, Chen CS, Yiang GT, Cheng PW, Chen YL, Chiu HC, et al. The Emerging Role of Pathogenesis of IgA Nephropathy. J Clin Med. 2018 Aug 20. 7 (8):716-21. [QxMD MEDLINE Link]. [Full Text].
- Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008 Apr. 88(2):451-87. [QxMD MEDLINE Link].
- Russo LM, Bakris GL, Comper WD. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis. 2002 May. 39(5):899-919. [QxMD MEDLINE Link].
- Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 2001 Nov. 60(5):1885-92. [QxMD MEDLINE Link].
- Hamm LL, Batuman V. Edema in the nephrotic syndrome: new aspect of an old enigma. J Am Soc Nephrol. 2003 Dec. 14(12):3288-9. [QxMD MEDLINE Link].
- Rostoker G, Behar A, Lagrue G. Vascular hyperpermeability in nephrotic edema. Nephron. 2000 Jul. 85(3):194-200. [QxMD MEDLINE Link].
- Appel GB, Blum CB, Chien S, Kunis CL, Appel AS. The hyperlipidemia of the nephrotic syndrome. Relation to plasma albumin concentration, oncotic pressure, and viscosity. N Engl J Med. 1985 Jun 13. 312(24):1544-8. [QxMD MEDLINE Link].
- Curry RC Jr, Roberts WC. Status of the coronary arteries in the nephrotic syndrome. Analysis of 20 necropsy patients aged 15 to 35 years to determine if coronary atherosclerosis is accelerated. Am J Med. 1977 Aug. 63(2):183-92. [QxMD MEDLINE Link].
- Mittal SK, Dash SC, Tiwari SC, Agarwal SK, Saxena S, Fishbane S. Bone histology in patients with nephrotic syndrome and normal renal function. Kidney Int. 1999 May. 55(5):1912-9. [QxMD MEDLINE Link].
- Tessitore N, Bonucci E, D'Angelo A, Lund B, Corgnati A, Lund B, et al. Bone histology and calcium metabolism in patients with nephrotic syndrome and normal or reduced renal function. Nephron. 1984. 37(3):153-9. [QxMD MEDLINE Link].
- Gulati S, Godbole M, Singh U, Gulati K, Srivastava A. Are children with idiopathic nephrotic syndrome at risk for metabolic bone disease?. Am J Kidney Dis. 2003 Jun. 41(6):1163-9. [QxMD MEDLINE Link].
- Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med. 2004 Aug 26. 351(9):868-75. [QxMD MEDLINE Link].
- Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008 Jan 15. 117(2):224-30. [QxMD MEDLINE Link].
- Rood IM, Deegens JKJ, Lugtenberg D, Bongers EMHF, Wetzels JFM. Nephrotic Syndrome With Mutations in NPHS2: The Role of R229Q and Implications for Genetic Counseling. Am J Kidney Dis. 2018 Sep 18. [QxMD MEDLINE Link].
- Glassock RJ. Antiphospholipase A2 receptor autoantibody guided diagnosis and treatment of membranous nephropathy: a new personalized medical approach. Clin J Amer Soc Nephrol. 2014. 9:1341-3. [QxMD MEDLINE Link].
- Hunley TE, Corzo D, Dudek M, Kishnani P, Amalfitano A, Chen YT, et al. Nephrotic syndrome complicating alpha-glucosidase replacement therapy for Pompe disease. Pediatrics. 2004. 114:e532-5. [QxMD MEDLINE Link].
- Lathara Z, Ambruzs JM, Cohen EP. Alloimmune Membranous Nephropathy in Fabry Disease. J Am Soc Nephrol Abstract Supplement. 2015. SA-PO004. [Full Text].
- Lipska-Ziętkiewicz BS, Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, et al. Genetic Steroid-Resistant Nephrotic Syndrome Overview. August 26, 2021. [QxMD MEDLINE Link]. [Full Text].
- George BA, Zhou XJ, Toto R. Nephrotic syndrome after bevacizumab: case report and literature review. Am J Kidney Dis. 2007 Feb. 49(2):e23-9. [QxMD MEDLINE Link].
- Lefaucheur C, Stengel B, Nochy D, et al. Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 2006 Oct. 70(8):1510-7. [QxMD MEDLINE Link].
- Cancarevic I, Nassar M, Medina L, Sanchez A, Parikh A, Hosna A, et al. Nephrotic Syndrome in Adult Patients With COVID-19 Infection or Post COVID-19 Vaccine: A Systematic Review. Cureus. 2022 Sep. 14 (9):e29613. [QxMD MEDLINE Link]. [Full Text].
- Wong W. Idiopathic nephrotic syndrome in New Zealand children, demographic, clinical features, initial management and outcome after twelve-month follow-up: results of a three-year national surveillance study. J Paediatr Child Health. 2007 May. 43(5):337-41. [QxMD MEDLINE Link].
- Kumar J, Gulati S, Sharma AP, Sharma RK, Gupta RK. Histopathological spectrum of childhood nephrotic syndrome in Indian children. Pediatr Nephrol. 2003 Jul. 18(7):657-60. [QxMD MEDLINE Link].
- Ozkaya N, Cakar N, Ekim M, Kara N, Akkök N, Yalçinkaya F. Primary nephrotic syndrome during childhood in Turkey. Pediatr Int. 2004 Aug. 46(4):436-8. [QxMD MEDLINE Link].
- Kazi JI, Mubarak M. Pattern of glomerulonephritides in adult nephrotic patients--report from SIUT. J Pak Med Assoc. 2007 Nov. 57(11):574. [QxMD MEDLINE Link].
- Barsoum R. The changing face of schistosomal glomerulopathy. Kidney Int. 2004. 66:2472-2484.
- Doe JY, Funk M, Mengel M, et al. Nephrotic syndrome in African children: lack of evidence for 'tropical nephrotic syndrome'?. Nephrol Dial Transplant. 2006. 21:672-676.
- Pakasa NM, Sumaili EK. The nephrotic syndrome in the Democratic Republic of Congo. N Engl J Med. 2006 Mar 9. 354(10):1085-6. [QxMD MEDLINE Link].
- Sumaili EK, Krzesinski JM, Zinga CV, Cohen EP, Delanaye P, Munyanga SM, et al. Prevalence of chronic kidney disease in Kinshasa: results of a pilot study from the Democratic Republic of Congo. Nephrol Dial Transplant. 2009 Jan. 24(1):117-22. [QxMD MEDLINE Link].
- Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, et al. APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol. 2012. 23:343-50. [QxMD MEDLINE Link].
- Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999 May. 55(5):1885-90. [QxMD MEDLINE Link].
- Arneil GC, Lam CN. Long-term assessment of steroid therapy in childhood nephrosis. Lancet. 1966 Oct 15. 2(7468):819-21. [QxMD MEDLINE Link].
- McKay AM, Parekh RS, Noone D. Therapeutic trials in difficult to treat steroid sensitive nephrotic syndrome: challenges and future directions. Pediatr Nephrol. 2023 Jan. 38 (1):17-34. [QxMD MEDLINE Link]. [Full Text].
- Gipson DS, Troost JP, Lafayette RA, et al. Complete Remission in the Nephrotic Syndrome Study Network. Clin J Am Soc Nephrol. 2016. 11:81-9. [QxMD MEDLINE Link].
- Beins NT, Dell KM. Long-Term Outcomes in Children with Steroid-Resistant Nephrotic Syndrome Treated with Calcineurin Inhibitors. Front Pediatr. 2015. 3:104. [QxMD MEDLINE Link].
- Harrison P. MENTOR: Rituximab Is a Winner in Membranous Nephropathy. Medscape Medical News. Available at https://www.medscape.com/viewarticle/915207. July 3, 2019; Accessed: September 25, 2023.
- Donadio JV Jr, Torres VE, Velosa JA, Wagoner RD, Holley KE, Okamura M. Idiopathic membranous nephropathy: the natural history of untreated patients. Kidney Int. 1988 Mar. 33(3):708-15. [QxMD MEDLINE Link].
- Jude EB, Anderson SG, Cruickshank JK, et al. Natural history and prognostic factors of diabetic nephropathy in type 2 diabetes. Quart J Med. 2002. 95:371-7. [QxMD MEDLINE Link].
- Varghese SA, Powell TB, Budisavljevic MN, et al. Urine biomarkers predict the cause of glomerular disease. J Am Soc Nephrol. 2007. 18:913-22. [QxMD MEDLINE Link].
- Cohen EP, Lemann J. The role of the laboratory in evaluation of kidney function. Clin Chem. 1991. 37:785-796.
- Gupta K, Iskandar SS, Daeihagh P, et al. Distribution of pathologic findings in individuals with nephrotic proteinuria according to serum albumin. Nephrol Dial Transplant. 2008 May. 23(5):1595-9. [QxMD MEDLINE Link].
- Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009 Jul 2. 361(1):11-21. [QxMD MEDLINE Link]. [Full Text].
- Hofstra JM, Beck LH Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011 Jun. 6(6):1286-91. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct. 100 (4S):S1-S276. [QxMD MEDLINE Link]. [Full Text].
- Palmer SC, Nand K, Strippoli GF. Interventions for minimal change disease in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2008 Jan 23. CD001537. [QxMD MEDLINE Link].
- Moret L, Ganea A, Dao M, Hummel A, Knebelman B, Subra JF, et al. Apheresis in Adult With Refractory Idiopathic Nephrotic Syndrome on Native Kidneys. Kidney Int Rep. 2021 Aug. 6 (8):2134-2143. [QxMD MEDLINE Link]. [Full Text].
- Vaziri ND. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int. 2016 Jul. 90 (1):41-52. [QxMD MEDLINE Link].
- Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. 2018 Jan. 14 (1):57-70. [QxMD MEDLINE Link].
- Kallash M, Smoyer WE, Mahan JD. Rituximab Use in the Management of Childhood Nephrotic Syndrome. Front Pediatr. 2019. 7:178. [QxMD MEDLINE Link]. [Full Text].
- Mühlig AK, Lee JY, Kemper MJ, Kronbichler A, Yang JW, Lee JM, et al. Levamisole in Children with Idiopathic Nephrotic Syndrome: Clinical Efficacy and Pathophysiological Aspects. J Clin Med. 2019 Jun 16. 8 (6):[QxMD MEDLINE Link]. [Full Text].
- Sinha A, Puraswani M, Kalaivani M, Goyal P, Hari P, Bagga A. Efficacy and safety of mycophenolate mofetil versus levamisole in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Kidney Int. 2019 Jan. 95 (1):210-218. [QxMD MEDLINE Link].
- Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM. Anti-CD20 Antibodies for Idiopathic Nephrotic Syndrome in Children. Clin J Am Soc Nephrol. 2015 Nov 19. 160 (5 Pt 1):1062-7. [QxMD MEDLINE Link].
- Ruggenenti P, et al; Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014 Apr. 25 (4):850-63. [QxMD MEDLINE Link].
- Maxted AP, Dalrymple RA, Chisholm D, McColl J, Tse Y, Christian MT, et al. Low-dose rituximab is no less effective for nephrotic syndrome measured by 12-month outcome. Pediatr Nephrol. 2019 May. 34 (5):855-863. [QxMD MEDLINE Link]. [Full Text].
- Okutsu M, Kamei K, Sato M, Kanamori T, Nishi K, Ishiwa S, et al. Prophylactic rituximab administration in children with complicated nephrotic syndrome. Pediatr Nephrol. 2021 Mar. 36 (3):611-619. [QxMD MEDLINE Link].
- Chan EY, Yu ELM, Angeletti A, et al. Long-Term Efficacy and Safety of Repeated Rituximab to Maintain Remission in Idiopathic Childhood Nephrotic Syndrome: An International Study. J Am Soc Nephrol. 2022 Jun. 33 (6):1193-1207. [QxMD MEDLINE Link]. [Full Text].
- Bonanni A, Rossi R, Murtas C, Ghiggeri GM. Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep. 2015 Sep 16. 2015:[QxMD MEDLINE Link].
- Hibino S, Uemura O, Nagai T, Yamakawa S, Iwata N, Ito H, et al. Three year outcome of childhood idiopathic nephrotic syndrome under a unified immunosuppressive protocol. Pediatr Int. 2014 Sep 15. [QxMD MEDLINE Link].
- Hahn D, Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev. 2015 Mar 18. CD001533. [QxMD MEDLINE Link].
- Christian MT, Webb NJA, Mehta S, Woolley RL, Afentou N, Frew E, et al. Evaluation of Daily Low-Dose Prednisolone During Upper Respiratory Tract Infection to Prevent Relapse in Children With Relapsing Steroid-Sensitive Nephrotic Syndrome: The PREDNOS 2 Randomized Clinical Trial. JAMA Pediatr. 2022 Mar 1. 176 (3):236-243. [QxMD MEDLINE Link]. [Full Text].
- Yadav M, Sinha A, Khandelwal P, Hari P, Bagga A. Efficacy of low-dose daily versus alternate-day prednisolone in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Pediatr Nephrol. 2018 Sep 7. 6 (1):63-9. [QxMD MEDLINE Link].
- Dufek S, Holtta T, Trautmann A, et al. Management of children with congenital nephrotic syndrome: challenging treatment paradigms. Nephrol Dial Transplant. 2018 Jun 21. [QxMD MEDLINE Link].
- [Guideline] Boyer O, Schaefer F, Haffner D, Bockenhauer D, Hölttä T, Bérody S, et al. Management of congenital nephrotic syndrome: consensus recommendations of the ERKNet-ESPN Working Group. Nat Rev Nephrol. 2021 Apr. 17 (4):277-289. [QxMD MEDLINE Link].
- Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007 May. 2(3):445-53. [QxMD MEDLINE Link].
- du Buf-Vereijken PW, Branten AJ, Wetzels JF. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis. 2005 Dec. 46(6):1012-29. [QxMD MEDLINE Link].
- Fervenza FC, et al; MENTOR Investigators. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N Engl J Med. 2019 Jul 4. 381 (1):36-46. [QxMD MEDLINE Link].
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011 Jan 28. 60 (2):1-64. [QxMD MEDLINE Link]. [Full Text].
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012 Oct 12. 61 (40):816-9. [QxMD MEDLINE Link]. [Full Text].
- Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep. 2016 Aug 26. 65 (5):1-54. [QxMD MEDLINE Link].
- Cohen JI. Strategies for herpes zoster vaccination of immunocompromised patients. J Infect Dis. 2008 Mar 1. 197 Suppl 2:S237-41. [QxMD MEDLINE Link].
- Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013 Aug 15. 9 (1):30. [QxMD MEDLINE Link]. [Full Text].
- Chen Y, Schieppati A, Chen X, Cai G, Zamora J, Giuliano GA, et al. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2014 Oct 16. 10:CD004293. [QxMD MEDLINE Link].
- Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol. 2010 Dec. 5(12):2207-12. [QxMD MEDLINE Link]. [Full Text].
- Bomback AS, Tumlin JA, Baranski J, et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther. 2011 Mar 14. 5:147-53. [QxMD MEDLINE Link]. [Full Text].
- Bomback AS, Canetta PA, Beck LH Jr, Ayalon R, Radhakrishnan J, Appel GB. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012. 36(1):58-67. [QxMD MEDLINE Link].
- Schematic drawing of the glomerular barrier. Podo = podocytes; GBM = glomerular basement membrane; Endo = fenestrated endothelial cells; ESL = endothelial cell surface layer (often referred to as the glycocalyx). Primary urine is formed through the filtration of plasma fluid across the glomerular barrier (arrows); in humans, the glomerular filtration rate (GFR) is 125 mL/min. The plasma flow rate (Qp) is close to 700 mL/min, with the filtration fraction being 20%. The concentration of albumin in serum is 40 g/L, while the estimated concentration of albumin in primary urine is 4 mg/L, or 0.1% of its concentration in plasma. Courtesy of the American Physiological Society (www.the-aps.org) and reproduced from Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008 Apr;88(2):451-87.
- Incidence of important causes of nephrotic syndrome, in number per million population. The left panel shows systemic causes, and the right panel lists primary renal diseases that can cause nephrotic syndrome. fgs = focal glomerulosclerosis, MN = membranous nephropathy, min change = minimal-change nephropathy. Data are in part from Swaminathan et al and Bergesio et al.
- A schema of the average patient age at presentation in various common forms of nephrotic syndrome. (Timeline not to scale.)
- Forces determining capillary filtration


Author
Ramapriya Sinnakirouchenan, MD, MBBS Assistant Professor, Department of Medicine, Division of Nephrology, Medical College of Wisconsin; Medical Director of Dialysis Unit, Fresenius Kidney Care
Ramapriya Sinnakirouchenan, MD, MBBS is a member of the following medical societies: American Society of Nephrology
Disclosure: Nothing to disclose.
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Eleanor Lederer, MD, FASN Professor of Medicine, Chief, Nephrology Division, Director, Nephrology Training Program, Director, Metabolic Stone Clinic, Kidney Disease Program, University of Louisville School of Medicine; Consulting Staff, Louisville Veterans Affairs Hospital
Eleanor Lederer, MD, FASN is a member of the following medical societies: American Association for the Advancement of Science, American Society for Bone and Mineral Research, American Society of Nephrology, American Society of Transplantation, International Society of Nephrology, Kentucky Medical Association, National Kidney Foundation
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: American Society of Nephrology
Received income in an amount equal to or greater than $250 from: Healthcare Quality Strategies, Inc.
Chief Editor
Vecihi Batuman, MD, FASN Professor of Medicine, Section of Nephrology-Hypertension, Deming Department of Medicine, Tulane University School of Medicine
Vecihi Batuman, MD, FASN is a member of the following medical societies: American College of Physicians, American Society of Hypertension, American Society of Nephrology, Southern Society for Clinical Investigation
Disclosure: Nothing to disclose.
Additional Contributors
Laura Lyngby Mulloy, DO, FACP Professor of Medicine, Chief, Section of Nephrology, Hypertension, and Transplantation Medicine, Glover/Mealing Eminent Scholar Chair in Immunology, Medical College of Georgia, Georgia Regents University
Disclosure: Nothing to disclose.