Erythrocyte Alloimmunization and Pregnancy: Overview, Background, Pathophysiology (original) (raw)
Pregnancy in the nonalloimmunized Rh D–negative patient
The primary goal of caring for an Rh D-negative pregnant woman who is nonimmunized is prevention of alloimmunization. As already discussed, every patient should have her ABO blood group, Rh type, and antibody screen (indirect Coombs test) checked at the first prenatal visit of each pregnancy. Patients who are found to be Rh D negative with concurrent negative results from antibody screens are candidates for anti-D IgG prophylaxis unless the Rh status of the father of the baby is negative and the paternity certain. In theory, if the Rh D status of the father is known, the patient can be counseled regarding the risk of the fetus having the Rh D antigen; however, because of a 3-5% rate of unknown or false paternity, discussing this issue privately with the patient is of the utmost importance. Anti-D IgG is absolutely contraindicated only in those patients with a documented hypersensitivity to anti-D IgG.
Exogenous administration of IgG to suppress an immune response, as in the case of anti-D IgG prophylaxis, is known as antibody-mediated immune suppression. Although several theories have been proposed to explain the mechanism of action of antibody-mediated immune suppression, the most likely mechanism is via central inhibition, wherein Rh D IgG coats fetal erythrocytes, which are then sequestered in the spleen and lymph nodes. The local increase in antigen-antibody complexes interrupts the commitment of B cells to plasma cell clones, thereby suppressing the primary immune response. Additionally, these antigen-antibody complexes stimulate the release of cytokines by immune effector cells that inhibit the proliferation of antigen-specific B cells.
The standard dosing regimen of anti-D IgG is 300 mcg intramuscularly at both 28 weeks' gestation (range, 26-30 weeks' gestation) and again postpartum within 72 hours of delivery unless the neonate is confirmed to be Rh D negative. [14] The 300-mcg dose was determined in 1963 by Pollack et al following experiments in which male volunteers who received Rh D positive erythrocytes were administered varying doses of anti-D IgG to prevent alloimmunization. In the early days of anti-D IgG prophylaxis, most fetomaternal hemorrhages were known to occur during delivery, and thus, anti-D IgG was administered only in the postpartum period. This continues to be the regimen used in many other countries.
Although this produced a dramatic decrease in the prevalence of alloimmunization, Bowman et al observed that despite this postpartum anti-D IgG prophylaxis, 1-2% of susceptible women continued to become sensitized. [15] They concluded that these women were experiencing fetomaternal hemorrhages prior to delivery, and they conducted experiments in which antepartum doses were added to the prophylaxis regimen. This resulted in a reduction in the number of sensitized women from 1.8% to 0.1% and eventually led to a regimen that includes the additional dose at approximately 28 weeks' gestation, which is used today in the United States.
In settings in which the volume of a fetomaternal hemorrhage can be calculated, 10 mcg of anti-D IgG should be administered for every milliliter of fetal blood in the maternal circulation. Thus, the 300-mcg dose is more than adequate for a typical fetomaternal hemorrhage and covers hemorrhage volumes of up to 30 mL of whole fetal blood. In the less than 1% of cases in which the volume of fetomaternal hemorrhage exceeds 30 mL, using the Kleihauer-Betke test or flow cytometry to quantify the volume of fetomaternal hemorrhage and administering the appropriate amount of anti-D IgG (ie, 10 mcg/mL fetal blood) is necessary.
Following delivery, if the infant is found to be Rh D negative, the postpartum dose may be omitted. However, if any doubt remains concerning whether to administer anti-D IgG, always administer unless contraindicated. If the fetus if found to be Rh positive, administer anti-D IgG and take care to screen for excess fetomaternal hemorrhage, particularly if cesarean delivery or manual removal of the placenta occurred because both increase the risk and volume of fetomaternal hemorrhage.
Because delivery of an Rh D–positive fetus is not the only means by which transplacental passage of fetal blood can occur, anti-D IgG prophylaxis for nonimmunized women who are Rh D–negative is also warranted in the following scenarios: first- and second-trimester bleeding, spontaneous or elective abortion, prior to any invasive procedure (eg, amniocentesis), evidence of subchorionic or retroplacental hematoma on ultrasonography, external cephalic version and intrauterine fetal death. Patients who experience antepartum bleeding or intrauterine fetal death in the third trimester should have a Kleihauer-Betke test or flow cytometry to determine whether more RhoGAM (ie, after the prophylactic dose at 28 weeks' gestation) is necessary. If so, patients can be given 10 mcg anti-D IgG per estimated milliliter of whole fetal blood in the maternal circulation.
Because fetal Rh antigens are present as early as the 30th day after conception, anti-D IgG is indicated with ectopic pregnancy and with therapeutic and spontaneous abortions. The risk of alloimmunization in susceptible women undergoing therapeutic or spontaneous abortion is 4-5% and 1.5-2%, respectively. For pregnancies less than or equal to 12 weeks' gestation, 50 mcg of anti-D IgG (also known as MICRhoGAM) is sometimes administered because the entire blood volume of the fetus is usually less than 5 mL. However, pregnancies exceeding 12 weeks' gestation or pregnancies in which the gestational age is unknown should receive the full 300-mcg dose.
The Society of Family Planning advises against routine Rh testing and administration of Rh immunoglobulin before 12 weeks’ gestation for patients who undergo spontaneous, medication, or uterine aspiration abortion. [1] The World Health Organization also recommends against routine Rh immunoglobulin administration for both medical and surgical abortion at less than 12 weeks’ gestation. [16]
Invasive obstetric procedures, such as CVS and amniocentesis (with respective risks of alloimmunization of at least 14% and 7-15%), also necessitate anti-D IgG prophylaxis at the time of the procedure. While a dose of 50 mcg is adequate for first-trimester procedures, the 300-mcg dose should be used for both second- and third-trimester procedures. Additionally, if amniocentesis is performed within 72 hours of delivery, as is often the case with fetal lung maturity determinations, withholding the postpartum anti-D IgG until the fetal Rh status is established postpartum is possible. However, if delivery is to be delayed for more than 72 hours, anti-D IgG should be administered.
Although not an invasive obstetric procedure, external cephalic version is associated with fetomaternal hemorrhage in 2-6% of cases, irrespective of procedure success. In this situation, administering an additional dose or checking for antibody status from the 28-week gestation prophylactic dose and performing a Kleihauer-Betke or flow cytometry test shortly afterward to check for fetomaternal hemorrhage is reasonable. Provided delivery occurs within 3 weeks of the administration of anti-D IgG, a repeat dose is only necessary in the event of a large fetomaternal hemorrhage at delivery.
Although the above guidelines have been dramatically successful in reducing the prevalence of Rh D alloimmunization since the introduction of anti-D IgG prophylaxis in 1968, improper management of nonimmunized Rh D–negative patients continues to be problematic. Potential errors and oversights that can result in the patient becoming Rh D alloimmunized include failing to type every patient with the potential for transfusion or fetomaternal hemorrhage, not screening for fetomaternal hemorrhage, not administering anti-D IgG when indicated, or administering an inadequate dosage of anti-D IgG.
Fetal assessment in the alloimmunized pregnancy
As discussed above, assessment of fetal Rh D status is critical in determining whether a pregnancy in an alloimmunized woman is at risk for the development of hemolytic disease of the fetus and newborn. Once a fetus is found to be at risk (ie, Rh D positive), the goals of managing the alloimmunized pregnancy are 2-fold. First is the detection of fetal anemia prior to the occurrence of fetal compromise. After detection, the goal is to minimize fetal morbidity and mortality by correcting this anemia until fetal lung maturity and delivery can be achieved. Because of the potential need for invasive diagnostic and therapeutic procedures, pregnancies complicated by erythrocyte alloimmunization should be managed by maternal-fetal medicine specialists.
The introduction of measures to both predict the severity of fetal disease and treat it has greatly reduced perinatal mortality rates and augmented survival rates. The decision to use specific interventions should be dictated by the degree of fetal disease. Consider fetal D antigen status, maternal antibody titers, and prior obstetric history to individualize and optimize management of an alloimmunized pregnancy.
First affected pregnancy
Although not reliably accurate in predicting the severity of fetal disease, past obstetric history can be somewhat prognostic. In general, the severity of fetal disease in a particular pregnancy tends to be similar to, if not more severe than, that of prior pregnancies. Additionally, with a history of a prior hydropic fetus, the chance that the next Rh D–incompatible fetus will also become hydropic if untreated is greater than 80%.
In the first affected pregnancy, maternal antibody titer determination is necessary to assess the risk to the fetus and to guide the decision-making process. In general, women with titers higher than 1:4 should be considered Rh alloimmunized. However, the threshold for invasive fetal testing (ie, the critical titer) varies at different institutions and is generally 1:16 or higher because these titers have been associated with fetal hydrops. Titers tend to correlate more reliably with the severity of fetal disease in the first sensitized pregnancy than in subsequent pregnancies. As such, first sensitized pregnancies in which antibody titers are 1:8 or lower can be managed by serially monitoring maternal antibody titers (every 2-4 weeks). If titers remain below the critical titer, delivery can occur at term.
Should titers rise to 1:16 or higher and fetal Rh antigen is D+ or unknown, fetal assessment is indicated via middle cerebral artery peak systolic velocity (MCA-PSV) Doppler ultrasonography or serial amniocentesis for delta OD450 if the former is not available.
History of a previously affected fetus or infant
An anamnestic maternal antibody response typically increases the severity of fetal disease in subsequent pregnancies. In patients with a prior history of a severely affected fetus or neonate, maternal antibody titers are not predictive of the severity of fetal anemia. Instead, fetal assessment for the development of anemia is initiated at 18 weeks.
Ultrasonography
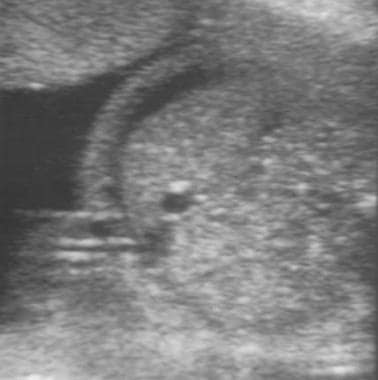
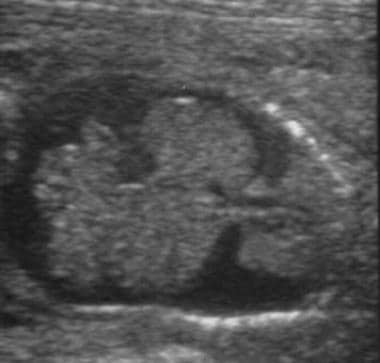
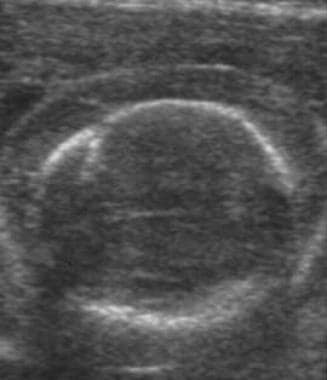
Ultrasonographic evaluation of the fetus is a significant part of managing an Rh-incompatible pregnancy. Accurate determination of gestational age via ultrasonography is critical because management is guided in large part by gestational age. Although it cannot help predict the impending development of hydrops, ultrasonography can help unequivocally diagnose the presence of hydrops—a diagnosis that would greatly affect the course of treatment. The sonographic findings consistent with hydrops include ascites, pleural and pericardial effusions, and edema. See the following images.
Ultrasound images of hydrops. Transverse image of fetal abdomen with ascites.
Hydropic fetus. Ultrasound image of coronal view of fetus with ascites and bilateral pleural effusions.
Fetal hydrops. Ultrasound image of scalp edema in a hydropic fetus.
Several other sonographic findings have been proposed as possible indicators of the future development of hydrops. These include polyhydramnios, increased placental thickness (>4 cm), dilation of the cardiac chambers, dilation of the umbilical vein, chronic enlargement of the spleen and liver, and visualization of both sides of the fetal bowel wall. However, none of these findings has proven predictive. Ultrasonography can be used in the management of Rh-incompatible pregnancies to assess fetal well-being; diagnose hydrops; and guide amniocenteses, fetal blood sampling (FBS), and intrauterine transfusions (IUTs). In this capacity, ultrasonography has improved both the safety and success rate of invasive procedures and has helped to minimize invasive testing.
Middle cerebral artery Doppler for MCA-PSV
The use of Doppler flow measurements in various fetal blood vessels has been studied to help predict the severity of fetal anemia. The anemic fetus attempts to enhance oxygenation by increasing cardiac output, thus increasing the velocity of blood flow. Anemia also results in decreased blood viscosity, which in turn results in increased velocity of blood flow. In their studies of the middle cerebral artery (MCA), Mari et al demonstrated that increases in peak velocity of systolic blood flow in the MCA can be used to detect moderate and severe anemia in nonhydropic fetuses. [17] The MCA is used because it can be evaluated using a minimal angle of insonation. Generally, a threshold value of 1.5 multiples of the median (MoMs) is used to determine when FBS with possible IUT should be used. Serial MCA Doppler studies are obtained every 1-2 weeks depending on the trend.
Reliable MCA-PSV values can be obtained as early as 18 weeks' gestation, but care should be taken after 35 weeks' gestation, after which time the false-positive rate of MCA-PSV increases, and consideration should be given to converting to amniocentesis for surveillance and assessment of fetal lung maturity. If MCA Doppler studies are routinely used, an estimated 70% of invasive testing could be avoided.
In a prospective multicenter study of alloimmunized pregnancies and antigen-positive fetuses, MCA-PSV Doppler studies were more sensitive than spectrophotometric analysis of amniotic fluid when using the Liley curve for the prediction of severe fetal anemia. However, MCA-PSV values were not significantly more sensitive when using the Queenan curve. Because amniocentesis is an invasive procedure, MCA Doppler ultrasonography should be preferentially used when available. [18]
Doppler ultrasonography of the MCA had a sensitivity of 88%, specificity of 82% an accuracy of 85%. Comparatively, the amniotic-fluid deltaOD450 had a sensitivity of 76%, a specificity of 77%, and an accuracy of 76%. Based on this, dopplers were more sensitive by 12 percentage points and more accurate by 9 percentage points than the amniotic fluid assessment. [13]
Amniocentesis for delta OD
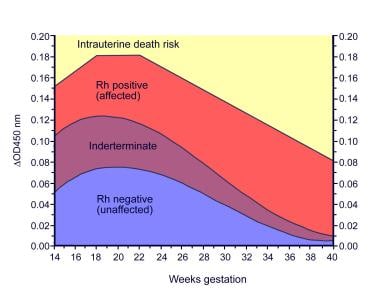
Prior to the development of MCA-PSV Doppler ultrasonography for fetal assessment, spectrophotometric analysis of amniotic fluid was routinely performed to detect the presence and severity of fetal hemolysis and anemia. Amniotic fluid containing high levels of bilirubin, such as that found in fetuses with severe hemolytic disease, is yellowish-brown. This observation led to the eventual development by Liley in 1961 of a method to predict the severity of fetal hemolysis using spectrophotometric measurements of bilirubin in amniotic fluid. Because the wavelength at which bilirubin absorbs light is 420-460 nm, the amount of shift in optical density from linearity at 450 nm (delta OD450) in amniotic fluid samples can be used to estimate the degree of fetal hemolysis. The Queenan curve (see the image below) is a modification of the Liley curve to adjust for the relative inaccuracy of delta OD450 readings in the early-to-middle secondtrimester.IncontrasttotheLileycurve,whichhas3zonesoffetal anemia, the Queenan curve has 4 zones and has been shown to be more predictive for fetal anemia compared with the Liley curve.
Queenan curve. Adapted from Queenan et al, Am J Obstet Gynecol, 1993.
Traditional management of alloimmunized patients with serial amniocenteses (as depicted below) was based on which zone the delta OD450 measurement falls into on the Liley or Queenan curves. Evidence from several studies, including Liley's original work, indicates that mild or no hemolytic disease occurs in zone 1; intermediate disease occurs in zone 2 (transitional between mild and severe hemolysis); and severe disease, including the development of hydrops within the week, occurs in zone 3.
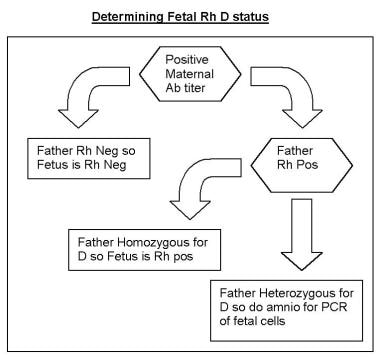
Determining fetal Rh D status.
Based on this evidence, once serial measurements are started, if a zone 1 reading is obtained, monitoring the delta OD450 approximately every 3 weeks is reasonable. However, with a trend into zone 2, the frequency of testing should increase to every 1-2 weeks depending on the steepness of the slope of the curve and the closeness of the measurement to zone 3.
Fetal blood sampling
The previously discussed predictive measures are indirect measures of fetal disease. The only definitive means of diagnosing fetal anemia and acidosis is via fetal blood sampling (FBS), also known as cordocentesis or percutaneous umbilical blood sampling (PUBS), which was first performed in the early 1980s. FBS helps provide direct and accurate diagnosis of anemia and fetal acidosis.
The other advantage of FBS is that by providing direct access to the umbilical vein, the same procedure can be used to transfuse the fetus if indicated by fetal hematocrits less than 30%. Despite the wealth of information afforded by FBS, routine use for screening for fetal anemia is not universal because of concerns regarding fetal and maternal complications. These include fetomaternal hemorrhage, fetal loss (0.5-2%per procedure), placental abruption, acute refractory fetal distress, and amnionitis with maternal adult respiratory distress syndrome.
Intrauterine transfusions
Once the MCA Doppler peak systolic velocity reaches more than 1.5 MOM for gestational age (or the delta OD450 measurements have entered high zone 2 or zone 3, or the fetus has been diagnosed with hydrops based on ultrasonographic findings), a decision should be made to perform an FBS with possible IUT if the hematocrit is less than 30%. Alternatively, if beyond 34 weeks' gestation, the decision is to proceed towards delivery (32 wk with mature lung indices). Numerous important factors must be considered when preparing a patient for FBS and possible IUT, including gestational age, the possibility of delivery, fetal maturation with corticosteroids, and the likelihood of transfusion.
If a patient is beyond viability (24 wk), a discussion regarding the management of fetal bradycardia should occur. Even though 24 weeks' gestation is considered the cusp of viability, the outcomes are likely to be even more dismal if the fetus is hydropic. In this setting, consultation with a neonatologist is important, as is fully informing the patient of the description and probabilities of different outcomes.
If the fetus is at a gestational age at which the patient desires immediate delivery if signs of fetal distress occurred, the procedure may be performed with the patient under epidural or spinal anesthesia to avoid the risks associated with general anesthesia and to decrease the amount of time required to deliver the fetus. In addition, also consider regional anesthesia in the setting of likely transfusion because this procedure can be time consuming and uncomfortable for the patient. Because of the possibility of immediate delivery, a 48-hour course of betamethasone to accelerate fetal maturity, particularly the lungs, is usually administered before the first 2 procedures.
The first IUTs were performed intraperitoneally in 1963. However, since the advent of FBS and intravenous transfusions into the umbilical vein, use of the intraperitoneal transfusion (IPT) has diminished in the management of anemic fetuses. Benefits of IVT over IPT include direct measurement of the fetal hemoglobin and acid-base status, lower failure rate (particularly in hydropic fetuses), lower rates of procedure-related morbidity and mortality, and better efficacy at earlier gestational ages. The only benefit offered by IPT is the ability to drain fetal ascites during the procedure, but this is of minimal benefit in hydropic fetuses. IPT may additionally be used in the severely anemic early second trimester fetus (18-24 weeks' gestation) due to technical imitations in accessing the umbilical vein (small caliber).
When preparing to perform an FBS, a number of issues must be addressed before the procedure itself. As mentioned previously, the patient must have a consultation with a neonatologist and anesthesiologist. The blood for possible transfusion must be typed and cross-matched against the mother's serum to help rule out any other possible hemolytic antibodies. Additionally, the unit of red blood cells should be cytomegalovirus-negative, irradiated, less than 5 days old, and with a hematocrit level between 75-80%. This can require several hours. The large variety of equipment, such as transfusion tubing, a blood warmer, heparinized syringes, and an accurate machine to obtain a rapid hematocrit value, must be prepared and calibrated. Unless the procedure is being performed in a unit that routinely performs these procedures, these issues should be discussed and reviewed in detail with the team of nurses, physicians, and technologists involved in the procedure.
The amount to be transfused can be calculated once the hematocrit/hemoglobin results are returned. In general, 30-60 mL/kg of nonhydropic fetal weight is transfused because volumes higher than this may be difficult for the fetus to tolerate. Once the transfusion is accomplished, a final blood sample is often taken to estimate the final hematocrit value of the fetus, although this measurement is an underestimation of the hematocrit secondary to the large volume infused during the transfusion. The fetus should be monitored with continuous fetal monitoring for the ensuing 4 hours (both during the procedure and immediately after because decelerations of the fetal heart rate are common and must be managed cautiously).
If the initial hematocrit value was extremely low, a repeat procedure may be necessary as soon as within a week. Otherwise, the procedure can be performed every 2-4 weeks based on the post-transfusion hemoglobin value. Because the goal is to maintain the fetal hemoglobin value at greater than 9 g/dL and because it drops at approximately 1 g/dL every 3 days, these values can be used to calculate how much time can be allotted until the next procedure. Alternatively, the MCA peak systolic velocity may be used to time the second IUT, typically using a threshold of 1.32 MOM, though this is not well established. Serial IUTs are usually performed until 34 weeks' gestation, beyond which time the risk of the procedure likely outweighs the benefits. This leads to delivery of the fetus at 34-37 weeks' gestation and, occasionally, even earlier if fetal lung maturity is documented.
Antenatal testing
Additional fetal assessment via antenatal testing with nonstress tests or biophysical profiles should begin at 32 week’ gestation in all gestations complicated by erythrocyte alloimmunization.
Timing of delivery
In determining the optimal delivery time, gestational age, severity of fetal anemia, and fetal lung maturity should all be considered. If fetal surveillance remains reassuring (ie, serial MCA-PSV studies remain < 1.5 MoMs or spectrophotometric analysis does not indicate severe fetal anemia), labor should be induced at 37–38 weeks of gestation after confirmation of fetal lung maturity to allow fetal hepatic maturation.
After 35 weeks’ gestation, the rate of false-positive results from MCA-PSV studies is increased. Thus, fetal assessment should be performed via amniocentesis, which has the additional benefit of allowing assessment of fetal lung maturity. Between 36-38 weeks’ gestation, if fetal lung maturity has not been achieved but the fetus is found to be in an upper affected zone, consideration should be given to administration of oral phenobarbital (30 mg tid for 7 d) to accelerate hepatic maturity in an effort to minimize hyperbilirubinemia, and labor should be induced in 1 week. However, many clinicians may opt for immediate delivery at this point to minimize risk from further hemolysis or additional invasive procedures.