Sinonasal Manifestations of Cystic Fibrosis: Overview, Diagnostic Considerations, Clinical Evaluation (original) (raw)
Overview
Cystic fibrosis (CF) is a chronic, multisystem disorder characterized by recurrent endobronchial infections, progressive obstructive pulmonary disease, and pancreatic insufficiency with intestinal malabsorption. Mutations in the CF transmembrane conductance regulator (CFTR) gene lead to viscous secretions and compromised functioning of the upper and lower airways, including the nose. Due to the chronicity and severity of sinonasal disease, children with CF often require the attention of an otolaryngologist. Other pediatric head and neck problems, such as the ear, laryngeal, and adenotonsillar disease, appear to be similar in prevalence and pathophysiology to those in patients without CF.
Historically, middle ear disease was found to be less prevalent in patients with CF; [1] this led to searches for genetic or anatomic “protections” in these children, which were never truly fruitful. Interestingly, subsequent incidence studies have shown equivalent rates. Likely reasons for this change include the use of more stringent methods of otitis media surveillance, including pneumatic otoscopy and tympanometry, as well as expansion of CF genetic testing and diagnostic criteria, bringing a larger, less severely affected patient population into contemporary CF clinics. [2]
Sinonasal disease remains the most common problem of the head and neck in patients with CF and will therefore be the focus of this article. [3]
See also Cystic Fibrosis and Thoracic Cystic Fibrosis Imaging.

Diagnostic Considerations
Most patients already have a diagnosis of CF upon presentation to the otolaryngologist and are being referred for consideration of sinus surgery. A diagnosis of CF may occasionally be made by the otolaryngologist based on the presence of nasal polyposis in an otherwise healthy-seeming child. Patients sometimes tolerate mild forms of this disease, thereby escaping early diagnosis. Segal found one new CF patient out of 16 healthy children with nasal polyps. [4]
Sweat chloride testing is recommended in any child with nasal polyps. Evidence of CFTR dysfunction on a sweat chloride test remains the most definitive way to diagnose CF. Genetic testing is also considered a valuable diagnostic tool and is now recommended for all individuals who meet the sweat chloride criteria for CF or whose results are inconclusive. [5]
Non–cystic fibrosis-related polyps may be caused by severe allergic rhinitis, inflammation associated with the Samter triad (asthma, aspirin insensitivity, nasal polyposis), Kartagener syndrome (situs inversus, ciliary dysmotility), and other immunologic disorders. These conditions can usually be differentiated by a complete history and physical examination, along with sweat chloride testing and cilia biopsy, as indicated.

Clinical Evaluation
It is important to elicit a thorough history of sinonasal symptoms from patients with CF, because most clinical decisions are based on history over physical or radiographic findings.
History
Symptoms to inquire about include the following:
- Nasal obstruction
- Worsening nasal discharge
- Facial pain
- Worsening cough
- Fever
In addition, question patients regarding their recent pulmonary status. Chronic sinusitis seems to be closely associated with endobronchial bacterial infections and may impact pulmonary reactivity and chronicity of disease within the sinobronchial tract. [6] Decreasing exercise tolerance also often correlates with acute sinus exacerbations or worsening chronic disease.
Since about the turn of the 21st century, a number of validated instruments for correlating history and disease burden with outcomes have been developed; rhinology has been a prolific field for these instruments. For example, the Sino-Nasal Outcome Test (SNOT-22) is validated for children and can be used for objective history taking and even tracking of symptoms over time. [7]
Physical assessment
As always, a thorough physical examination should be performed to evaluate the nose and sinuses, as well as to uncover other conditions that may exacerbate sinus disease. Facial evaluation may reveal a widened nasal bridge due to chronic polyposis. Sometimes, polyps may even protrude from the nares.
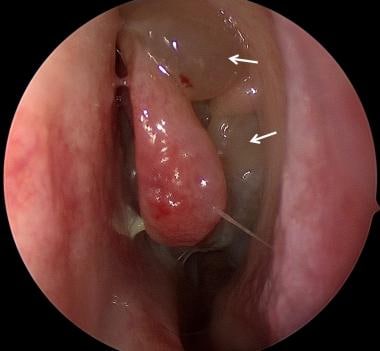
Swollen turbinates, purulent nasal discharge, and nasal polyps may be visible on anterior rhinoscopy (see the image below). Even if polyps are not readily visible on physical exam, a SNOT-22 score above 11 is predictive of subclinical nasal polyps and can be considered an indication for nasal endoscopy in children with CF. [7] Rigid nasal endoscopy is best for complete nasal examination but may not be tolerated by young children. Flexible endoscopy can be performed when the rigid instrument cannot be used; movement can be tolerated for flexible endoscopy, but significant movement during a rigid exam can be unacceptably traumatic to the nasal tissue.
Endoscopic view of nasal polyps (arrows) protruding from the middle meatus of the left nasal cavity. Courtesy of Christopher R Grindle, MD.
Nasal polyps may be found in 6-67% of children with cystic fibrosis. This variability is explained by several factors. For example, if anterior rhinoscopy alone is used, smaller and more posterior polyps will be missed and numbers will be lower. In addition, rigid nasal endoscopy has been shown to find polyps in 33-67% of cases. [8, 9] Nasal polyps also clearly increase with age, with one study finding that up to 45% of adolescent patients with CF had endoscopically notable polyps, compared with 19% of patients under age 6 years. [10] Although it may seem logical that the frequency of nasal polyps would vary with the specific genetic mutation, this is not the case. [11]
During nasal endoscopy, the nasopharynx must also be considered. Adenoid hypertrophy and inflammation may be present in younger patients and may play a role in nasal obstruction and rhinosinusitis. Adenoidectomy can play a big part in controlling sinonasal disease in patients with CF, as well as in non-CF patients. [12]

CT Scanning of the Sinuses
More than 90% of patients with CF exhibit radiographic evidence of chronic sinusitis. [6] Despite this, there is poor correlation between the severity of symptoms and computed tomography (CT) scan findings. [13] Clinically symptomatic sinusitis, as evidenced by pain, discharge, fever, or postnasal drip, is found in only about 10% of patients with CF. [3] Thus, most patients with radiologically evident disease do not report symptoms. This phenomenon may represent a truly asymptomatic disease state, or it may suggest that patients with this chronic disease have psychologically adapted to their symptoms. [3, 6]
Given the above discrepancy, clinical history is weighed more heavily than radiographic evaluation when treatment decisions are made. However, this does not mean that imaging is not helpful in the management of patients with CF; it is a critical adjunct to surgical management in this population.
Indications for CT scanning (axial and coronal views without contrast) include evaluation of the extent of disease and preparation for operative intervention. However, there are legitimate concerns with regard to radiation exposure in growing children; furthermore, CT scans are often specially formatted for image-guided surgery. For these reasons, it is ideal to have a discussion with the treating otolaryngologist prior to ordering CT scans in patients who may require surgery.
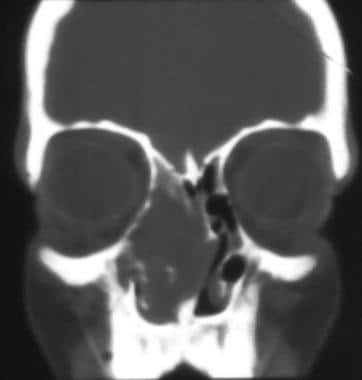
The most common sinus abnormality seen on radiographic imaging is sinus opacification. In addition, 70% of children with CF will have medial displacement of the lateral nasal wall of the middle meatus, along with uncinate process demineralization; these are the results of bony remodeling from the slow, constant pressure of sinus secretions, as well as polyposis. [14, 15] This phenomenon has been long described subjectively but was verified objectively in 2019 by CT scan measurements of lateral wall angles. [16, 17] This finding can be somewhat difficult for the novice to appreciate on CT scans when it occurs bilaterally, as is most common in CF, but it can be very obvious when seen unilaterally, as demonstrated in the image below.
This image demonstrates unilateral nasal polyposis with medial bulging of the lateral nasal wall and viscid secretions in the maxillary sinus. This feature is often observed bilaterally in patients with cystic fibrosis (CF) and represents a mucocele-like state (but is easier to appreciate when seen unilaterally, as shown here).
Chronic sinusitis in CF often causes poor pneumatization, hypoplasia of the maxillary and ethmoid sinuses, and poor development of the frontal sinuses. [18] These findings do not differ significantly as patients grow older. Adolescent patients with CF often have no frontal sinus cavity present on CT scan evaluation. [15]

Bacteriology
Sinus aspirates, used for culturing, are extremely helpful for the detection and appropriate management of infections in patients with CF but may only be obtainable under general anesthesia. Operative cultures, as well as large-scale studies, are informative for physicians for treatment decisions. Differences exist in organisms and sensitivities from different sinus cavities of a single CF patient; therefore, when applicable, physicians should try to obtain specimens from all accessible sinuses. [19] Unfortunately, easily accessible cultures, like nasopharyngeal, throat, or sputum cultures, are not predictive of identities and sensitivities of sinus organisms. [20, 21]
The first landmark bacteriology study, in 1982, involved transantral maxillary sinus aspirates of 20 patients with CF; it showed Pseudomonas species (65%), nontypeable Haemophilus influenzae (50%), alpha-hemolytic streptococci (25%), and anaerobes such as Peptostreptococcus and Bacteroides species (25%). [20] Newer data points to the changing pattern in the microbiology of upper airways in persons with CF. A study of sinus cultures from pediatric patients with CF found methicillin-sensitive Staphylococcus aureus (MSSA) to be the most common isolate at 25%, followed by methicillin-resistant S aureus (22%) and Pseudomonas aeruginosa (19%). [22] Other studies have found similar trends in both upper and lower respiratory tract samples in patients with CF. [23, 24] In addition, P aeruginosa species isolated from the airways of patients with CF showed differing sensitivities compared with those from individuals who did not have CF, demonstrating greater resistance to aminoglycosides, trimethoprim/sulfamethoxazole, and fluoroquinolones. [25, 26]
Non-CF patients do have a different bacterial load from sinusitis. Acute sinusitis usually is caused by Pneumococcus species, nontypeable H influenzae, and Moraxella catarrhalis. Chronic sinusitis is often caused by those organisms in addition to S aureus (most commonly implicated) and anaerobes such as Peptostreptococcus, Bacteroides, Veillonella, and Fusobacterium. [27, 28]
Evidence of fungal sinus disease has also been seen in patients with CF. One study found positive fungal sinus cultures in a third of samples taken from patients with CF, most frequently Candida albicans. While a small number of these patients had concomitant allergic fungal sinusitis, most cases seemed to represent asymptomatic colonization. [29]

Management
Aggressive medical management is always performed before surgical intervention. Patients may report chronic purulent nasal discharge or cough, and antibiotic therapy is used whenever they experience a subjective increase in nasal obstruction, cough, or drainage.
Medical management with aggressive nasal toilet, appropriate use of antibiotics against Pseudomonas species and staphylococci, and nasal steroid agents will improve symptoms and lengthen the interval between surgeries.

Pharmacotherapy
Antimicrobial therapy
As noted above, the bacteriology of sinonasal disease in patients with CF differs from that in patients without CF, which affects antibiotic choices. The most notable difference is the presence of Pseudomonas species in patients with CF. As a result, although studies on oral antibiotics for managing sinus disease in CF patients are still lacking, empiric treatment with oral ciprofloxacin is reasonable for treating rhinosinusitis accompanying CF. In severe cases or in those with concomitant pulmonary exacerbations, intravenous antibiotic treatment may also be needed.
Although quinolones are well-known for their activity against Pseudomonas species, they are generally used with caution in the pediatric population due to their potential adverse effects on tendons and joints; however, they are used quite frequently in patients with CF. [26, 30] Macrolides are an alternative option that has gained more attention in chronic rhinosinusitis management. Their therapeutic benefits are likely derived from their anti-inflammatory properties, so these agents are effective in _Pseudomonas_-colonized upper airways. [31, 32]
Some authors advocate irrigations with antipseudomonal antibiotics such as tobramycin. Nebulization with such medications is clearly beneficial for lung infections and was shown in one study to decrease bacterial colonization of paranasal sinuses and improve sinonasal symptoms. [33] Nebulized agents can increase the medication's concentration at the site of infection and decrease systemic exposure to toxins.
Other nebulized formulations in development include amikacin, levofloxacin, and ciprofloxacin. [34] Serial tobramycin irrigation of the maxillary sinuses, which can be done in an office setting in a compliant patient, may significantly decrease the intensity and frequency of infections, especially when performed after sinus surgery. [35]
Nasal toilet
Because mucociliary clearance is chronically impaired in CF, irrigations are critical and should be performed as a daily routine as patients begin to develop sinonasal symptoms. Nasal saline irrigations serve to decrease bacterial colonization, wash away inspissated secretions that lead to obstruction, and temporarily aid in vasoconstriction. Irrigation is also required after any surgical intervention, because surgery enlarges sinus ostia but does not address underlying defects in mucociliary clearance.
Nasal steroidal and decongestant agents
Nasal steroids are generally helpful, both for acute exacerbations and for long-term therapy, to decrease mucosal edema and promote mucus clearance. Short-term bursts of systemic steroids may improve acute exacerbations; these may also help if administered preoperatively to decrease intraoperative bleeding in patients with nasal polyposis. [36] One randomized, controlled study showed that betamethasone nasal spray twice daily for 6 weeks reduced the size of nasal polyps in patients with CF. [37] Other studies have also shown improvement in polyp size and sinus symptomatology in association with topical inhaled corticosteroid use. [36, 38]
Systemic decongestants are of variable benefit and have cardiovascular side effects, as well as a potential for misuse, making them less effective choices for chronic management. However, short-term usage during illness may be helpful. Topical decongestants like oxymetazoline or phenylephrine may also be useful for brief periods of illness or for postoperative control, but their propensity for causing rhinitis medicamentosa makes them unsuitable for any long-term utility. Antihistamines are generally not beneficial and, because of their drying effect on secretions, may actually be detrimental.
One big advance in pharmacology over the past 20 years for patients with CF has been the development of recombinant human deoxyribonuclease I (dornase alfa, or Pulmozyme) for pulsating nasal inhalation. This can reduce sinonasal symptoms even without sinus surgery and can also improve quality of life, being useful with regard to mucosal edema and polyp control after sinus surgery. [36, 39] Other mucolytics are generally ineffective in persons with CF.

Surgical Indications and Contraindications
Selecting candidates for surgery
Objective, evidence-based indications for surgery in patients with CF are lacking. Settings in which surgery should be considered include the following:
- Persistent nasal obstruction associated with nasal polyposis and/or medial bowing of the lateral nasal wall following intensive medical management
- Medialization of the lateral nasal wall, as demonstrated by endoscopy or CT scanning, even without subjective nasal obstruction, because of the high prevalence of mucocele-like formations
- Pulmonary exacerbations that appear to correlate with sinonasal disease exacerbations, worsening of pulmonary status, or diminished activity level, despite appropriate medical management
- Facial pain or headaches that have no other apparent cause and that adversely affect quality of life
- Desire for improvement in symptom profile beyond that which medical management has achieved in a patient with significant nasal cavity and paranasal sinus symptoms [6]
Contraindications to surgery include the following:
- Severe obstructive pulmonary disease - This condition may make the risk of anesthesia unacceptable.
- Vitamin K deficiency or other coagulopathies - Patients with cystic fibrosis are at risk for vitamin K deficiency from pancreatic insufficiency, hepatobiliary disease, or both, and if unsupplemented, they may have a bleeding diathesis. [40] A prolonged prothrombin time (PT) preoperatively should prompt correction and performance of surgery after the PT is normalized.
- Hypoplastic sinus cavities - This may be a relative contraindication. Delay in pneumatization and in development of the maxillary, ethmoid, and frontal sinuses may occur in patients with CF, and hypoplastic sinus cavities are often encountered. CT scans must be carefully evaluated and surgeons should be experienced, as these anatomic abnormalities can make surgery more hazardous.
Studies show that patients with CF who have chronic sinusitis demonstrate improvement in symptoms and in quality-of-life measures, following surgical treatment. [41, 42] However, surgical intervention for individuals with CF should still be carefully considered. These patients may develop severe mucus plugging during general anesthesia, and this risk increases with the duration of intubation.
A study by Tumin et al indicated that endoscopic sinus surgery can be safely performed in children with CF. While the results showed a higher rate of prolonged hospital stays in children with CF than in those without the disease who underwent such surgery (30% vs 9%, respectively), the rates of readmission and reoperation between the two groups were similar. [43]
A retrospective study by Do et al indicated that when used together, Lund-Mackay and modified Lund-Mackay scores can be used to predict which pediatric patients with CF will need sinus surgery. The Lund-Mackay scoring system is a validated tool that interprets radiographic findings to evaluate the severity of rhinosinusitis, with higher scores indicative of more severe sinus disease. The study found that using the Lund-Mackay system, the optimal threshold score of 13 best predicted the need for sinus surgery, with a sensitivity and specificity of 89.3% and 69.2%, respectively. The optimal score using the modified Lund-Mackay was 19, which carried a sensitivity and specificity of 67.7% and 84.6%, respectively. [44]
Multidisciplinary evaluation and safety considerations
Patients with CF should be cared for by a team of physicians familiar with their needs. Surgery on these patients must be undertaken in a cautious manner, with attention given to their pulmonary status. Preoperative communication with the pediatric, pulmonary, and anesthesia teams is therefore essential.
The decision and timing of surgery must be carefully planned with the pulmonary team. The surgical procedure may be performed under local anesthesia for appropriately selected patients, but for children, general anesthesia is commonly used. (However, severe obstructive pulmonary disease may make the risk of anesthesia unacceptable.) The anesthesia team may consider arterial hypotension to a systolic pressure of 85-90 mmHg to allow faster and safer surgery in a relatively bloodless field. Prior to surgery, the nose should be prepared with a topical vasoconstrictive agent such as oxymetazoline and injected with lidocaine or bupivacaine with epinephrine.
In this manner, systemic narcotics, with their deleterious effects on postoperative respiration, are used minimally or avoided entirely. Deep extubation is preferred, if possible, to avoid airway irritation, hypertension, and coughing on emergence. [45]
Informed consent for sinus surgery is generally similar to that for sinus surgery in patients without CF, except for the following:
- Anesthetic risks may be higher for patients with CF, depending on their pulmonary status.
- These patients may require several sinus procedures throughout their lives, and as in patients without CF, surgical risks are higher with revision surgery. Patients and their families should be informed of this increased risk. Surgeons should keep this in mind and attempt to preserve landmarks to facilitate subsequent surgery.

Procedural Concerns
Poor ciliary function and thickened mucus are hallmarks of CF and currently are not curable in patients with the disease. The surgeon’s goal, then, is to decompress, relieve obstruction, and improve drainage and access. [3]
It is ideal to perform the required surgery in patients with CF in less than 1 hour to avoid respiratory compromise. However, the length of the operation varies according to the extent of disease, blood loss, number of previous procedures, and experience of the surgeon. Safety and avoidance of complications are the primary concerns.
If blood obscures the field, performing repeated irrigations and packing the nose with hemostatic and decongestant pledgets can allow the procedure to progress safely, as in patients without CF.
Initially, sinus surgeons used a minimalist approach, performing polypectomy alone to improve the airway. It was long ago noted, however, that recurrences were fewer and symptom-free intervals longer when polypectomy was combined with a more long-term sinus drainage procedure. [46]
General guidelines now state that polyps should be removed as completely as is safe but with an understanding that recurrence is likely. This procedure is generally performed for improvement, not cure, and it is most wise to leave residual polyps if adequate landmarks are unavailable for safe progress.
Powered endoscopic sinus surgery with a microdebrider allows for safe and rapid removal of tissue, especially polyps, under direct visualization. This technique is particularly efficacious in patients with CF, because it allows rapid and precise removal of diseased tissue, with preservation of landmarks. Consequently, this should allow safer surgery both in terms of anesthetic time and decreased complications in patients who are likely to need multiple revisions. [47, 48]
Image-guided surgery
Image-guided surgery is useful for its real-time correlation between patient anatomy and preoperative CT images. Intraoperatively, the surgeon utilizes a probe to correlate the precise location of the instrument on axial, coronal, and sagittal CT scans. This provides rapid and accurate intraoperative localization of surrounding structures. One case series of 20 CF patients, evaluating the efficacy of CT-assisted navigation in endoscopic sinus surgery, cited the benefits of imaging with regard to decreased operative time, improved precision, and more extensive removal of disease. [48] Thus, the use of image guidance may increase the safety of sinus surgery, particularly given the altered sinus anatomy commonly seen in patients with CF.
Many surgeons prefer image-guided procedures in cases of revision surgery, the obscuring of anatomic landmarks by significant disease, sphenoidal/frontal sinus disease, or disease abutting the skull base. It is still advisable to contact the otolaryngologist before ordering a CT scan, so that proper films are obtained and repeat radiation is avoided.
Extent of surgery
Most surgeons currently advocate endoscopic procedures such as wide middle-meatal antrostomies, anterior and posterior ethmoidectomies, and removal of polypoid disease in the frontal recess. One study of sinus surgery involving polypectomy, middle-meatal antrostomy, and total ethmoidectomy in 26 pediatric and young adult patients with CF reported improvement in symptoms at 23-month follow-up. [42] Some experienced centers are now recommending even wider opening of sinuses to allow for egress of thick secretions and for aggressive nasal toilet. Endoscopic fronto-spheno-ethmoidectomies and modified endoscopic medial maxillectomies seem to decrease sinus and lung colonization and to decrease hospital admissions for pulmonary exacerbations. [38, 49]
Generally, a conservative approach to sinus surgery is employed first, with removal of polypoid tissue, generous maxillary antrostomies, and total ethmoidectomy, with more aggressive maneuvers employed for revision procedures and older patients. Sinus surgeons know well that patients with CF usually require multiple procedures over time; therefore, surgical landmarks are specifically left in place.

Postoperative Details
While almost all other sinus surgery is conducted on an outpatient basis, patients with CF who undergo endoscopic sinus surgery may require a prolonged postoperative hospital stay. This may be related to their poorer baseline pulmonary function and the need for intravenous antibiotics after surgery. [43, 50]
There are no postoperative dietary restrictions, and patients are generally advised to avoid strenuous activity for about 10 days following the procedure to avoid bleeding.
Aggressive postoperative nasal irrigations are helpful, and most patients who undergo surgery are old enough and mature enough to tolerate this intervention. In general, these chronically ill patients and their families become well-educated about their disease and are generally very compliant.
Postoperative cleaning with suctioning and crust removal may help to prevent synechia (scar or contracture) formation. Young patients who cannot tolerate this procedure can briefly return to the operating room 2-3 weeks later for such cleaning, if desired.
For chronic postoperative monitoring, most patients can be periodically re-assessed with office nasal endoscopy. If symptoms return, medical therapy, as discussed above, is reinstated before returning to the operating room.

Complications
Common, minor complications of sinus surgery include bleeding and synechia formation. Rare and more serious complications include cerebrospinal fluid leak, eye injury, and anosmia. [43] Although perioperative medical management may be more complex in patients with CF, surgical complications specifically related to endoscopic sinus surgery do not occur more frequently in these individuals than in those without the disease. [51] However, patients with CF are more likely to require readmission following surgery, due to pulmonary exacerbation, which is likely a result of poorer baseline lung function. Therefore, as noted above, a longer postoperative hospital stay may be warranted for these individuals. [51, 52]
It should be noted that disease recurrence may be considered by some to be a complication, but such recurrence is actually the norm in persons with CF.
As in patients without CF, revision cases with decreased landmarks can be more hazardous. This fact, as noted above, must be considered in patients with CF, and surgical landmarks are left in place, if at all possible.

Outcomes
Because many patients with CF need a subsequent procedure in response to polyp regrowth after sinus surgery, such operations should be viewed as treatments and not necessarily cures; [53] revision surgery is necessary in up to 89% of individuals. [38] However, surgery can provide considerable improvement, and for significant periods of time. Multiple studies have shown that sinonasal symptoms, including obstruction, olfactory dysfunction, purulent nasal discharge, and headache, are all significantly improved after endoscopic sinus surgery. [42, 54]
In a retrospective study of adult patients with CF, Ji et al found that following endoscopic sinus surgery, the SNOT-22 score improved by an overall mean value of 9.42 points. However, less sinonasal score improvement was found in patients who on culture were found to be positive for Pseudomonas infection. [55]
One clinical dictum remains true across the preoperative and postoperative period: clinical disease does not correlate with radiographic findings for patients with CF. It is counterintuitive, but true, that even postoperatively, sinus appearance on imaging does not improve, despite clear symptomatic improvement. [9, 41, 56] Therefore, although repeat CT scanning may be required for operative planning when symptoms return, keep in mind that postoperatively, CT scans remain abnormal even though symptoms improve. This means that for patients who have had sinus surgery, just like for those who have not yet undergone an operation, decisions regarding revision surgery must heavily weigh clinical symptoms over CT scan results.
Although many surgeons and physicians have believed that pulmonary function is improved after sinus surgery in patients with CF, this has not consistently been supported by data. Some small, older studies showed a correlation between sinus disease quality of life and forced expiratory volume in 1 second (FEV1) in children and adults following endoscopic sinus surgery. [57, 58] In contrast, a 2011 study of 41 children demonstrated no improvement in pulmonary function test (PFT) results after sinus surgery. [59]
Two systematic reviews concluded that endoscopic sinus surgery does not consistently improve PFT results in children and adults. [41, 54] However, the studies that have been performed are either retrospective or small. Larger, prospective studies could yield a definitive conclusion, but such research may not occur, because decisions to undergo major surgery are highly individualized, and prospective, randomized surgical trials are uncommon. At this point in time, decisions for surgery are based clearly on a goal to improve sinonasal symptoms, as opposed to an intent to improve pulmonary function.

Future Directions
Steroid nasal irrigation
Investigations have been ongoing to determine the benefit of adding steroid to saline nasal irrigation in the treatment of chronic rhinosinusitis. The idea that use of a high-volume, low-pressure system will improve delivery of the medication to the sinus cavities is promising. A randomized, controlled trial by Tait et al studied the effect of budesonide nasal irrigation in adults with chronic rhinosinusitis, with or without nasal polyposis. Although the treatment improved SNOT-22 scores in these patients, the results were not statistically significant when compared with saline nasal irrigation. [60]
However, when compared with steroid nasal spray, this new delivery method may be more effective. This was demonstrated in one randomized, controlled trial involving adult patients with chronic rhinosinusitis with nasal polyposis who had undergone endoscopic sinus surgery. The results showed improved SNOT-22 scores with postoperative budesonide atomizer therapy, compared with fluticasone nasal spray. [61] Steroid nasal irrigation is still a fairly novel concept, and more studies are needed to thoroughly assess its efficacy in managing sinonasal disease, specifically in the pediatric population or in patients with CF.
14- and 15-membered ring macrolide antibiotics
Long-term therapy with low-dose 14- and 15-membered ring macrolide antibiotics seems to promote and sustain reparative processes in the chronically inflamed upper and lower respiratory tract. [62] One randomized, controlled trial showed a statistically significant improvement in SNOT-20 scores in non-CF patients with chronic rhinosinusitis after 12 weeks of low-dose roxithromycin treatment. [31] The benefit of macrolide therapy is thought to extend beyond its direct antimicrobial activity, and the tissue reparative effects are seen regardless of the presence of P aeruginosa. [32] In the future, this may lead to better control of sinonasal disease in patients with CF.
Inhibition of biofilm formation
Pseudomonas colonization in patients with CF probably represents a biofilm state, whereby the bacteria exist in an organized community with attachment to surfaces in the thick mucus in airways. Bacteria in biofilms exhibit increased antibiotic resistance and are less susceptible to host clearance mechanisms, thereby making them harder to clear with conventional antibiotic regimens. [63] Low-dose, long-term macrolide therapy can potentially counteract this challenge, with one of the treatment's proposed mechanisms of action being disruption of the integrity of biofilm formation and reduction of airway adhesion, which is critical against _P aeruginosa–_colonized airways. [3] In addition, because biofilm growth is dependent on iron, iron chelators are being investigated as potential targets to disrupt and prevent biofilms. Indeed, iron chelators combined with tobramycin have been associated with decreased biofilm formation in vitro. [64]
Heart-lung/double-lung transplantation
The only definitive treatment for patients with cystic fibrosis accompanied by advanced lung disease is heart-lung or double-lung transplant. Survival is similar to that for lung transplant recipients without cystic fibrosis and is most adversely affected by rejection or complications of immunosuppression. Performance of this procedure in patients with advanced cystic fibrosis is only limited by the availability of donor organs. [65]
Gene therapy
Gene therapy for CF involves the insertion of DNA encoding a normal CFTR gene into respiratory cells. Vectors generally consist of viruses or liposomes. Multiple clinical trials of such treatment are under way. Preliminary results of randomized, controlled studies have not shown adverse events; unfortunately, the therapy has also not demonstrated statistically significant measurable improvements. [66]
Increasingly, medications that modify the CFTR gene and its protein are being developed. These agents aim to compensate for the defective CFTR protein by increasing alternative chloride channel activity, inhibiting sodium reabsorption, or increasing chloride conductance. [34, 64]
One CFTR modulator therapy that shows promise for improving sinonasal symptoms in patients with CF is ivacaftor. This drug works as a CFTR potentiator by increasing the transport of chloride ions across epithelial cells. It is currently FDA-approved for CF lung disease in patients with certain genetic mutations. Moreover, clinical studies have shown that this agent is effective for the treatment of chronic rhinosinusitis in CF. For instance, Sheikh et al found evidence of radiologic improvement in sinus disease for patients on ivacaftor. Although the improvement in CT scan findings may not correlate well with improvement in clinical symptoms, the study also found that the use of ivacaftor therapy resulted in lower sweat chloride levels, thus helping to correct the underlying pathologic process in CF. [67] McCormick et al showed that ivacaftor improves the SNOT-20 score in the rhinologic (at 1, 3, and 6 months), psychologic (at 1, 3, and 6 months), and sleep (at 1 and 3 months) domains. [68]
In 2019, the FDA approved elexacaftor-tezacaftor-ivacaftor as the first triple therapy for patients with cystic fibrosis aged 12 years or older who have one or more F508del mutations in the CFTR gene. [69] Elexacaftor and tezacaftor are CFTR correctors (as opposed to potentiators).
Using the Questionnaire of Olfactory Disorders (QOD), which measures olfactory-specific quality of life, a study by Miller et al indicated that the elexacaftor-tezacaftor-ivacaftor combination can improve severe olfactory dysfunction in persons with cystic fibrosis. The investigators found that in patients with cystic fibrosis who had nasal polyps and the poorest baseline scores on the QOD, the drug combination led to clinically significant change. In patients with better baseline QOD scores, however, the treatment tended to produce qualitative score improvements that were nonetheless not clinically meaningful. [70]
A study by Di Gioia et al indicated that in patients with cystic fibrosis who have chronic rhinosinusitis, the elexacaftor-tezacaftor-ivacaftor combination can reduce acute exacerbations of the rhinosinusitis and lead to its significant endoscopically and radiologically assessed improvement. The investigators found that prior to treatment, the mean annual number of acute exacerbations of chronic rhinosinusitis was 0.55, compared with 0.35 during treatment. The average pretreatment score on the Lund-Kennedy scale was 4.21 points, but this dropped to 1.5 points after therapy began. Also before the start of treatment, the average and modified Lund-Mackay scores were 14.6 and 16.45 points, respectively; after therapy commenced, these fell to 5.87 and 6.73 points, respectively. [71]
As more is learned about the molecular structure and function of the CFTR protein, as well as modifying genes, more avenues for effective therapy will be opened. [34, 64]

- Haddad J Jr, Gonzalez C, Kurland G, Orenstein DM, Casselbrant ML. Ear disease in children with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 1994 May. 120 (5):491-3. [QxMD MEDLINE Link].
- McCoy JL, Kaffenberger TM, Yang TS, Shaffer AD, Dohar JE. Middle ear disease in CF? It's not just about the sinuses anymore!. Int J Pediatr Otorhinolaryngol. 2020 Jul. 134:110032. [QxMD MEDLINE Link].
- Le C, McCrary HC, Chang E. Cystic Fibrosis Sinusitis. Adv Otorhinolaryngol. 2016. 79:29-37. [QxMD MEDLINE Link].
- Segal N, Gluk O, Puterman M. Nasal polyps in the pediatric population. B-ENT. 2012. 8 (4):265-7. [QxMD MEDLINE Link].
- [Guideline] Farrell PM, White TB, Ren CL, et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017 Feb. 181S:S4-S15.e1. [QxMD MEDLINE Link]. [Full Text].
- Nishioka GJ, Cook PR. Paranasal sinus disease in patients with cystic fibrosis. Otolaryngol Clin North Am. 1996 Feb. 29 (1):193-205. [QxMD MEDLINE Link].
- Thamboo A, Santos RC, Naidoo L, Rahmanian R, Chilvers MA, Chadha NK. Use of the SNOT-22 and UPSIT to appropriately select pediatric patients with cystic fibrosis who should be referred to an otolaryngologist: cross-sectional study. JAMA Otolaryngol Head Neck Surg. 2014 Oct. 140 (10):934-9. [QxMD MEDLINE Link]. [Full Text].
- Yung MW, Gould J, Upton GJ. Nasal polyposis in children with cystic fibrosis: a long-term follow-up study. Ann Otol Rhinol Laryngol. 2002 Dec. 111 (12 Pt 1):1081-6. [QxMD MEDLINE Link].
- Gergin O, Kawai K, MacDougall RD, et al. Sinus Computed Tomography Imaging in Pediatric Cystic Fibrosis: Added Value?. Otolaryngol Head Neck Surg. 2016 Jul. 155 (1):160-5. [QxMD MEDLINE Link].
- Schraven SP, Wehrmann M, Wagner W, Blumenstock G, Koitschev A. Prevalence and histopathology of chronic polypoid sinusitis in pediatric patients with cystic fibrosis. J Cyst Fibros. 2011 May. 10 (3):181-6. [QxMD MEDLINE Link]. [Full Text].
- Cimmino M, Cavaliere M, Nardone M, et al. Clinical characteristics and genotype analysis of patients with cystic fibrosis and nasal polyposis. Clin Otolaryngol Allied Sci. 2003 Apr. 28 (2):125-32. [QxMD MEDLINE Link].
- Brietzke SE, Brigger MT. Adenoidectomy outcomes in pediatric rhinosinusitis: a meta-analysis. Int J Pediatr Otorhinolaryngol. 2008 Oct. 72 (10):1541-5. [QxMD MEDLINE Link].
- Sakano E, Ribeiro AF, Barth L, Condino Neto A, Ribeiro JD. Nasal and paranasal sinus endoscopy, computed tomography and microbiology of upper airways and the correlations with genotype and severity of cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2007 Jan. 71 (1):41-50. [QxMD MEDLINE Link].
- April MM, Zinreich SJ, Baroody FM, Naclerio RM. Coronal CT scan abnormalities in children with chronic sinusitis. Laryngoscope. 1993 Sep. 103 (9):985-90. [QxMD MEDLINE Link].
- Berkhout MC, Klerx-Melis F, Fokkens WJ, Nuijsink M, van Aalderen WM, Heijerman HG. CT-abnormalities, bacteriology and symptoms of sinonasal disease in children with Cystic Fibrosis. J Cyst Fibros. 2016 Nov. 15 (6):816-24. [QxMD MEDLINE Link]. [Full Text].
- Brihaye P, Clement PA, Dab I, Desprechin B. Pathological changes of the lateral nasal wall in patients with cystic fibrosis (mucoviscidosis). Int J Pediatr Otorhinolaryngol. 1994 Jan. 28 (2-3):141-7. [QxMD MEDLINE Link].
- Hervochon R, Teissier N, Blondeau JR, et al. Computed Tomography Description of the Uncinate Process Angulation in Patients With Cystic Fibrosis and Comparison With Primary Ciliary Dyskinesia, Nasal Polyposis, and Controls. Ear Nose Throat J. 2019 Feb. 98 (2):89-93. [QxMD MEDLINE Link]. [Full Text].
- Ledesma-Medina J, Osman MZ, Girdany BR. Abnormal paranasal sinuses in patients with cystic fibrosis of the pancreas. Radiological findings. Pediatr Radiol. 1980 Feb. 9 (2):61-4. [QxMD MEDLINE Link].
- Brook I. Discrepancies in the recovery of bacteria from multiple sinuses in acute and chronic sinusitis. J Med Microbiol. 2004 Sep. 53 (Pt 9):879-85. [QxMD MEDLINE Link]. [Full Text].
- Shapiro ED, Milmoe GJ, Wald ER, Rodnan JB, Bowen AD. Bacteriology of the maxillary sinuses in patients with cystic fibrosis. J Infect Dis. 1982 Nov. 146 (5):589-93. [QxMD MEDLINE Link].
- Muhlebach MS, Miller MB, Moore C, Wedd JP, Drake AF, Leigh MW. Are lower airway or throat cultures predictive of sinus bacteriology in cystic fibrosis?. Pediatr Pulmonol. 2006 May. 41 (5):445-51. [QxMD MEDLINE Link].
- Sobin L, Kawai K, Irace AL, et al. Microbiology of the Upper and Lower Airways in Pediatric Cystic Fibrosis Patients. Otolaryngol Head Neck Surg. 2017 Aug. 157 (2):302-8. [QxMD MEDLINE Link].
- Digoy GP, Dunn JD, Stoner JA, Christie A, Jones DT. Bacteriology of the paranasal sinuses in pediatric cystic fibrosis patients. Int J Pediatr Otorhinolaryngol. 2012 Jul. 76 (7):934-8. [QxMD MEDLINE Link].
- Salsgiver EL, Fink AK, Knapp EA, et al. Changing Epidemiology of the Respiratory Bacteriology of Patients With Cystic Fibrosis. Chest. 2016 Feb. 149 (2):390-400. [QxMD MEDLINE Link]. [Full Text].
- Bosso JA, Mauldin PD, Steed LL. Consequences of combining cystic fibrosis- and non-cystic fibrosis-derived Pseudomonas aeruginosa antibiotic susceptibility results in hospital antibiograms. Ann Pharmacother. 2006 Nov. 40 (11):1946-9. [QxMD MEDLINE Link].
- Qin X, Zhou C, Zerr DM, et al. Heterogeneous Antimicrobial Susceptibility Characteristics in Pseudomonas aeruginosa Isolates from Cystic Fibrosis Patients. mSphere. 2018 Mar-Apr. 3 (2):e00615-17. [QxMD MEDLINE Link]. [Full Text].
- Wald ER. Rhinitis and acute and chronic sinusitis. Pediatr Otolaryngol. 1996. 2:843-58.
- Brook I. Microbiology of chronic rhinosinusitis. Eur J Clin Microbiol Infect Dis. 2016 Jul. 35 (7):1059-68. [QxMD MEDLINE Link]. [Full Text].
- Wise SK, Kingdom TT, McKean L, DelGaudio JM, Venkatraman G. Presence of fungus in sinus cultures of cystic fibrosis patients. Am J Rhinol. 2005 Jan-Feb. 19 (1):47-51. [QxMD MEDLINE Link].
- Briggs EC, Nguyen T, Wall MA, MacDonald KD. Oral antimicrobial use in outpatient cystic fibrosis pulmonary exacerbation management: a single-center experience. Clin Respir J. 2012 Jan. 6(1):56-64. [QxMD MEDLINE Link]. [Full Text].
- Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006 Feb. 116 (2):189-93. [QxMD MEDLINE Link].
- Cervin A, Wallwork B. Anti-inflammatory effects of macrolide antibiotics in the treatment of chronic rhinosinusitis. Otolaryngol Clin North Am. 2005 Dec. 38 (6):1339-50. [QxMD MEDLINE Link].
- Mainz JG, Schadlich K, Schien C, et al. Sinonasal inhalation of tobramycin vibrating aerosol in cystic fibrosis patients with upper airway Pseudomonas aeruginosa colonization: results of a randomized, double-blind, placebo-controlled pilot study. Drug Des Devel Ther. 2014. 8:209-17. [QxMD MEDLINE Link]. [Full Text].
- Anderson P. Emerging therapies in cystic fibrosis. Ther Adv Respir Dis. 2010 Jun. 4 (3):177-85. [QxMD MEDLINE Link]. [Full Text].
- Moss RB, King VV. Management of sinusitis in cystic fibrosis by endoscopic surgery and serial antimicrobial lavage. Reduction in recurrence requiring surgery. Arch Otolaryngol Head Neck Surg. 1995 May. 121 (5):566-72. [QxMD MEDLINE Link].
- Crockett DJ, Wilson KF, Meier JD. Perioperative strategies to improve sinus surgery outcomes in patients with cystic fibrosis: a systematic review. Otolaryngol Head Neck Surg. 2013 Jul. 149 (1):30-9. [QxMD MEDLINE Link].
- Hadfield PJ, Rowe-Jones JM, Mackay IS. A prospective treatment trial of nasal polyps in adults with cystic fibrosis. Rhinology. 2000 Jun. 38 (2):63-5. [QxMD MEDLINE Link].
- Virgin FW, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy. 2012 Jan-Feb. 26 (1):70-5. [QxMD MEDLINE Link]. [Full Text].
- Mainz JG, Schien C, Schiller I, et al. Sinonasal inhalation of dornase alfa administered by vibrating aerosol to cystic fibrosis patients: a double-blind placebo-controlled cross-over trial. J Cyst Fibros. 2014 Jul. 13 (4):461-70. [QxMD MEDLINE Link].
- Rashid M, Durie P, Andrew M, et al. Prevalence of vitamin K deficiency in cystic fibrosis. Am J Clin Nutr. 1999 Sep. 70 (3):378-82. [QxMD MEDLINE Link]. [Full Text].
- Hughes A, Adil EA. What is the role of endoscopic sinus surgery in adult patients with cystic fibrosis?. Laryngoscope. 2015 Sep. 125 (9):2018-20. [QxMD MEDLINE Link].
- Keck T, Rozsasi A. Medium-term symptom outcomes after paranasal sinus surgery in children and young adults with cystic fibrosis. Laryngoscope. 2007 Mar. 117 (3):475-9. [QxMD MEDLINE Link].
- Tumin D, Hayes D Jr, Kirkby SE, Tobias JD, McKee C. Safety of endoscopic sinus surgery in children with cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2017 Jul. 98:25-8. [QxMD MEDLINE Link].
- Do BA, Lands LC, Mascarella MA, et al. Lund-Mackay and modified Lund-Mackay score for sinus surgery in children with cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2015 Aug. 79 (8):1341-5. [QxMD MEDLINE Link].
- Davidson TM, Murphy C, Mitchell M, Smith C, Light M. Management of chronic sinusitis in cystic fibrosis. Laryngoscope. 1995 Apr. 105 (4 Pt 1):354-8. [QxMD MEDLINE Link].
- Crockett DM, McGill TJ, Healy GB, Friedman EM, Salkeld LJ. Nasal and paranasal sinus surgery in children with cystic fibrosis. Ann Otol Rhinol Laryngol. 1987 Jul-Aug. 96 (4):367-72. [QxMD MEDLINE Link].
- Mendelsohn MG, Gross CW. Soft-tissue shavers in pediatric sinus surgery. Otolaryngol Clin North Am. 1997 Jun. 30 (3):443-9. [QxMD MEDLINE Link].
- Fuchsmann C, Ayari S, Reix P, et al. Contribution of CT-assisted navigation and microdebriders to endoscopic sinus surgery in cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2008 Mar. 72 (3):343-9. [QxMD MEDLINE Link].
- Vital D, Hofer M, Boehler A, Holzmann D. Posttransplant sinus surgery in lung transplant recipients with cystic fibrosis: a single institutional experience. Eur Arch Otorhinolaryngol. 2013 Jan. 270 (1):135-9. [QxMD MEDLINE Link]. [Full Text].
- Do BA, Lands LC, Saint-Martin C, et al. Effect of the F508del genotype on outcomes of endoscopic sinus surgery in children with cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2014 Jul. 78 (7):1133-7. [QxMD MEDLINE Link].
- Albritton FD, Kingdom TT. Endoscopic sinus surgery in patients with cystic fibrosis: an analysis of complications. Am J Rhinol. 2000 Nov-Dec. 14 (6):379-85. [QxMD MEDLINE Link].
- McKeon M, Medina G, Kawai K, Cunningham M, Adil E. Readmissions following ambulatory pediatric endoscopic sinus surgery. Laryngoscope. 2019 Dec. 129 (12):2681-6. [QxMD MEDLINE Link].
- Cuyler JP. Follow-up of endoscopic sinus surgery on children with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 1992 May. 118 (5):505-6. [QxMD MEDLINE Link].
- Macdonald KI, Gipsman A, Magit A, et al. Endoscopic sinus surgery in patients with cystic fibrosis: a systematic review and meta-analysis of pulmonary function. Rhinology. 2012 Dec. 50 (4):360-9. [QxMD MEDLINE Link].
- Ji KSY, Frank-Ito D, Abi Hachem R, et al. Endoscopic Sinus Surgery for Cystic Fibrosis: Variables Influencing Sinonasal and Pulmonary Outcomes. Am J Rhinol Allergy. 2021 Nov 20. 19458924211059606. [QxMD MEDLINE Link].
- McMurphy AB, Morriss C, Roberts DB, Friedman EM. The usefulness of computed tomography scans in cystic fibrosis patients with chronic sinusitis. Am J Rhinol. 2007 Nov-Dec. 21 (6):706-10. [QxMD MEDLINE Link].
- Friedman EM, Stewart M. An assessment of sinus quality of life and pulmonary function in children with cystic fibrosis. Am J Rhinol. 2006 Nov-Dec. 20 (6):568-72. [QxMD MEDLINE Link].
- Halvorson DJ, Dupree JR, Porubsky ES. Management of chronic sinusitis in the adult cystic fibrosis patient. Ann Otol Rhinol Laryngol. 1998 Nov. 107 (11 Pt 1):946-52. [QxMD MEDLINE Link].
- Osborn AJ, Leung R, Ratjen F, James AL. Effect of endoscopic sinus surgery on pulmonary function and microbial pathogens in a pediatric population with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 2011 Jun. 137 (6):542-7. [QxMD MEDLINE Link]. [Full Text].
- Tait S, Kallogjeri D, Suko J, Kukuljan S, Schneider J, Piccirillo JF. Effect of Budesonide Added to Large-Volume, Low-pressure Saline Sinus Irrigation for Chronic Rhinosinusitis: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2018 Jul 1. 144 (7):605-12. [QxMD MEDLINE Link]. [Full Text].
- Neubauer PD, Schwam ZG, Manes RP. Comparison of intranasal fluticasone spray, budesonide atomizer, and budesonide respules in patients with chronic rhinosinusitis with polyposis after endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016 Mar. 6(3):233-7. [QxMD MEDLINE Link]. [Full Text].
- Garey KW, Alwani A, Danziger LH, Rubinstein I. Tissue reparative effects of macrolide antibiotics in chronic inflammatory sinopulmonary diseases. Chest. 2003 Jan. 123(1):261-5. [QxMD MEDLINE Link]. [Full Text].
- Murray TS, Egan M, Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr. 2007 Feb. 19(1):83-8. [QxMD MEDLINE Link]. [Full Text].
- Mogayzel PJ Jr, Flume PA. Update in cystic fibrosis 2009. Am J Respir Crit Care Med. 2010 Mar 15. 181 (6):539-44. [QxMD MEDLINE Link]. [Full Text].
- Rubin BK. Emerging therapies for cystic fibrosis lung disease. Chest. 1999 Apr. 115 (4):1120-6. [QxMD MEDLINE Link].
- Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007 Aug. 18 (8):726-32. [QxMD MEDLINE Link].
- Sheikh SI, Long FR, McCoy KS, Johnson T, Ryan-Wenger NA, Hayes D Jr. Ivacaftor improves appearance of sinus disease on computerised tomography in cystic fibrosis patients with G551D mutation. Clin Otolaryngol. 2015 Feb. 40 (1):16-21. [QxMD MEDLINE Link].
- McCormick J, Cho DY, Lampkin B, et al. Ivacaftor improves rhinologic, psychologic, and sleep-related quality of life in G551D cystic fibrosis patients. Int Forum Allergy Rhinol. 2019 Mar. 9 (3):292-7. [QxMD MEDLINE Link]. [Full Text].
- US Food and Drug Administration. FDA approves new breakthrough therapy for cystic fibrosis. FDA. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-new-breakthrough-therapy-cystic-fibrosis. October 21, 2019; Accessed: March 4, 2024.
- Miller JE, Taylor-Cousar JL, Overdevest JB, et al. Determining the minimal clinically important difference for the questionnaire of olfactory disorders in people with cystic fibrosis and factors associated with improvement after highly effective modulator therapy. Int Forum Allergy Rhinol. 2023 Dec 25. [QxMD MEDLINE Link].
- Di Gioia S, Lucca F, Venditto L, et al. Efficacy of Elexacaftor-Tezacaftor-Ivacaftor on chronic rhinosinusitis in cystic fibrosis. Am J Otolaryngol. 2024 Feb 24. 45 (3):104236. [QxMD MEDLINE Link].
Author
Nicole Murray, MD Director, Connecticut Children's Center for Airway, Voice, and Swallowing; Assistant Director of Pediatric Otolaryngology, Connecticut Children's Medical Center
Nicole Murray, MD is a member of the following medical societies: American Academy of Otolaryngology-Head and Neck Surgery
Disclosure: Nothing to disclose.
Coauthor(s)
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Chief Editor
Arlen D Meyers, MD, MBA Emeritus Professor of Otolaryngology, Dentistry, and Engineering, University of Colorado School of Medicine
Arlen D Meyers, MD, MBA is a member of the following medical societies: American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology-Head and Neck Surgery, American Head and Neck Society
Disclosure: Serve(d) as a director, officer, partner, employee, advisor, consultant or trustee for: Cerescan; Neosoma; MI10;Invitrocaptal,Medtechsyndicates
Received income in an amount equal to or greater than $250 from: Neosoma; Cyberionix (CYBX);MI10;Invitrocaptal;MTS
Received ownership interest from Cerescan for consulting for: Neosoma, MI10 advisor.
Additional Contributors
Acknowledgements
Tiffiny Ainsworth, MD Resident Physician, Department of Otolaryngology-Head and Neck Surgery, University of Connecticut Health Center
Tiffiny Ainsworth, MD, is a member of the following medical societies: American Academy of Otolaryngology-Head and Neck Surgery, American Medical Association, and Connecticut State Medical Society
Disclosure: Nothing to disclose.
Karla R Brown, MD Assistant Professor, Department of Otolaryngology, Tulane University Medical Center
Disclosure: Nothing to disclose.