Pediatric Malabsorption Syndromes: Background, Pathophysiology, Epidemiology (original) (raw)
Overview
Background
Malabsorption syndromes encompass numerous clinical entities that result in chronic diarrhea, abdominal distention, and failure to thrive. [1] Clinical malabsorption can be broken down into several distinct conditions, both congenital and acquired, that affect one or more of the different steps in the intestinal hydrolysis and subsequent transport of nutrients.
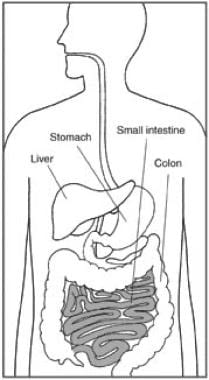
The major site of absorption is the small intestine, as depicted in the illustration below.
The small intestine is a major site of absorption.

Pathophysiology
Carbohydrate, fat, or protein malabsorption is caused by a disorder in the intestinal processes of digestion, transport, or both of these nutrients across the intestinal mucosa into the systemic circulation. Either a congenital abnormality in the digestive or absorptive processes or, more commonly, a secondarily acquired disorder of such processes may result in malabsorption.
Carbohydrates
Of the carbohydrates most commonly present in the diet (starches, sucrose, lactose), only starches require preliminary luminal digestion by salivary and, more importantly, pancreatic amylases. Despite the slow development of pancreatic amylase, whose secretion reaches adult levels during the end of the first year of life, cooked starch malabsorption is rare in infants because of the activity of the brush-border bound glucoamylase, an esoglycosidase that develops early in life.
The final products of amylase digestion include maltose, maltotriose, and higher residues of glucose polymers. The final hydrolysis of disaccharides and oligosaccharides occurs at the brush border of the enterocytes, where sucrase-isomaltase breaks down maltose, isomaltose (to glucose), and sucrose (to glucose and fructose); glucoamylase releases glucose from glucose polymers; and lactase splits lactose into glucose and galactose. Subsequent entry of the final monosaccharides (glucose, galactose, fructose) into the enterocytes through the brush border occurs via carrier molecules. Glucose and galactose share the same carrier, SGLT-1, which transports one molecule of the monosaccharide and one molecule of sodium (Na) in a secondarily active transport, energized by Na-activated and potassium (k)-activated adenosine triphosphatase (NaK ATPase). Instead, fructose is transported by Glut2 and Glut5 transporters across the cell membrane. Although Glut2 can transport both glucose and fructose, Glut5 is a fructose-specific transporter, working only down a concentration gradient (facilitated diffusion).
Disorders of these processes can be congenital (cystic fibrosis and Shwachman-Diamond syndrome, which may cause amylase deficiency; the extremely rare congenital lactase deficiency; glucose-galactose malabsorption; sucrase-isomaltase deficiency; adult-type hypolactasia) or acquired: the most common being lactose intolerance, typically secondary to a damage of the mucosa, such as a viral enteritis or conditions that cause mucosal atrophy, such as celiac disease.
Protein
Proteins are first digested in the stomach, where pepsinogens, which are activated to pepsins by a pH of less than 4, hydrolyze them in large molecular weight peptides. Upon entering the duodenum, the pancreatic proteases (activated by trypsin, secreted by the pancreas as a proenzyme, trypsinogen, which is subsequently activated by the brush border–bound enterokinase) further split them into low molecular weight peptides and free amino acids.
Interestingly, the final breakdown products of intraluminal digestion of protein are composed of low molecular weight peptides (2-6 amino acid residues) for 70% and of free amino acids for 30%. Subsequently, brush border–bound peptidases further hydrolyze peptides to release a mixture of free amino acids and small peptides (2-3 amino acid residues). Finally, free amino acids are taken up by enterocytes through specific Na-linked carrier systems (5 carriers with selective affinities for groups of amino acids are described), whereas dipeptides and tripeptides are translocated into the absorptive epithelial cells by the peptide transporter 1 (PEPT1), which is a carrier with a broad specificity linked to H entry. [2] In the first few months of life, the latter system is much more active than those that transport amino acids and is thought to play a bigger physiological role.
Congenital disorders of protein digestion include conditions such as cystic fibrosis, Shwachman-Diamond syndrome, and enterokinase deficiency, which cause inadequate intraluminal digestion. No congenital defects have been described in any of the brush border–bound peptidases or in the peptide carrier.
Acquired disorders of protein digestion and/or absorption are nonspecific (ie, they also affect the absorption of carbohydrates and lipids) and are found in conditions that result in damage to the absorptive intestinal surface, such as extensive viral enteritis, milk protein allergy enteropathy, and celiac disease.
Lipids
A lingual lipase is responsible for the first partial hydrolysis of triglycerides; this enzyme becomes active in persons with low gastric pH levels and is active even in premature infants. However, the largest part of triglyceride digestion is accomplished in the duodenojejunal lumen because of a complex of pancreatic enzymes, the most important of which is the lipase-colipase complex. Like amylase, these enzymes also develop slowly, and this accounts for the known low capacity of babies to absorb lipids, termed physiologic steatorrhea of the newborn. Additionally, adequate concentrations of intraluminal conjugated bile salts are needed to form micelles, and the secretion of bile acids may also be partially inadequate in very young patients.
Disorders of these processes can be congenital (cystic fibrosis and Shwachman-Diamond syndrome, which cause lipase and colipase deficiency; the uncommon isolated deficiency of lipase and colipase; the extremely rare congenital primary bile acid malabsorption, which results in low bile acids concentrations) or acquired (secondary mostly to disorders of the liver and the biliary tract or to chronic pancreatitis). Clearly, any condition that results in the loss of small intestinal absorptive surface also causes steatorrhea.

Epidemiology
Frequency
Genetically determined syndromes
The prevalence of celiac disease in the United States is around 1% of the general population [3] and has increased over time. [4] Celiac disease in its entirety (ie, including the forms without overt malabsorption) is by far the most common inherited malabsorption syndrome. Cystic fibrosis is the second most common malabsorption syndrome. Other congenital disorders are rare, with the exception of adult-type hypolactasia, which has a prevalence that varies greatly among different ethnic groups.
Acquired syndromes
Cow's milk and soy milk protein allergies are common, especially in infants and young children. [5] The prevalence of milk protein allergy, of which enteropathy is one of the presenting clinical symptoms, is estimated to be around 3%. A transient and common form of malabsorption in infants results from acute-onset enteritis (mostly viral, specifically rotaviral), which causes transient lactose intolerance. Although toddler's (or unspecific) diarrhea accounts for approximately 7.5% of referrals to pediatric gastroenterologists, it should not be considered a malabsorption syndrome because, by definition, no digestive or absorptive processes are impaired.
Secondary malabsorption syndromes that result from liver, pancreas, and intestinal diseases are uncommon. The manifestations vary according to the severity of each disease and the extent of intestinal mucosal injury.
Mortality/Morbidity
Although the morbidity can be severe, and aside from the single entity of cystic fibrosis, common malabsorption syndromes carry low mortality rates. Neonates and young infants, especially those with signs of malnutrition, are particularly at risk. In many of the congenital syndromes, morbidity varies with the particular syndrome and may be associated with systemic manifestations of the disease.
Race
Congenital sucrase-isomaltase deficiency is most common in Canadian Eskimos and natives of Greenland. Deficiency of trehalose, a sugar found almost exclusively in mushrooms, is rare, except in natives of Greenland. Adult-onset lactase deficiency is most common in persons of Asian, African, and Mediterranean descent.
Sex
Celiac disease is slightly more common in females. Autoimmune enteropathy is an X-linked disorder that only affects males in familial cases.
Age
Neonates and young infants with malabsorption syndromes are at particularly high risk for chronic diarrhea and malnutrition. Symptoms of a congenital disease are usually apparent shortly after birth (the exception being adult-onset lactase deficiency, which appears only after age 6-8 y) or after a short hiatus once a particular substance is ingested in substantial amounts. Protein sensitivity syndromes to milk or soy protein usually present in infants younger than 3 months, but solid food protein sensitivity syndromes are also known to occur in older patients.

- Siddiqui Z, Osayande AS. Selected disorders of malabsorption. Prim Care. 2011 Sep. 38(3):395-414; vii. [QxMD MEDLINE Link].
- Ma K, Hu Y, Smith DE. Peptide transporter 1 is responsible for intestinal uptake of the dipeptide glycylsarcosine: studies in everted jejunal rings from wild-type and Pept1 null mice. J Pharm Sci. 2011 Feb. 100 (2):767-74. [QxMD MEDLINE Link].
- Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10. 163(3):286-92. [QxMD MEDLINE Link].
- Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009 Jul. 137(1):88-93. [QxMD MEDLINE Link]. [Full Text].
- Meyer R, Venter C, Fox AT, Shah N. Practical dietary management of protein energy malnutrition in young children with cow's milk protein allergy. Pediatr Allergy Immunol. 2012 Mar 22. [QxMD MEDLINE Link].
- Abenavoli L, Proietti I, Vonghia L, et al. Intestinal malabsorption and skin diseases. Dig Dis. 2008. 26(2):167-74. [QxMD MEDLINE Link].
- Scarpellini E, Giorgio V, Gabrielli M, et al. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: a case-control study. J Pediatr. 2009 Sep. 155(3):416-20. [QxMD MEDLINE Link].
- Bruno MJ, Haverkort EB, Tytgat GN, van Leeuwen DJ. Maldigestion associated with exocrine pancreatic insufficiency: implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. Am J Gastroenterol. 1995 Sep. 90(9):1383-93. [QxMD MEDLINE Link].
- Ford GA, Preece JD, Davies IH, Wilkinson SP. Use of the SeHCAT test in the investigation of diarrhoea. Postgrad Med J. 1992 Apr. 68(798):272-6. [QxMD MEDLINE Link]. [Full Text].
- Wedlake L, A'Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009 Oct. 30(7):707-17. [QxMD MEDLINE Link].
- Mokrowiecka A, Daniel P, Slomka M, Majak P, Malecka-Panas E. Clinical utility of serological markers in inflammatory bowel disease. Hepatogastroenterology. 2009 Jan-Feb. 56(89):162-6. [QxMD MEDLINE Link].
- Ritchie BK, Brewster DR, Davidson GP, Tran CD, McNeil Y, Hawkes JS. 13C-sucrose breath test: novel use of a noninvasive biomarker of environmental gut health. Pediatrics. 2009 Aug. 124(2):620-6. [QxMD MEDLINE Link].
- Yao CK, Tuck CJ. The clinical value of breath hydrogen testing. J Gastroenterol Hepatol. 2017 Mar. 32 Suppl 1:20-22. [QxMD MEDLINE Link].
- Gabrielli M, D'angelo G, Di Rienzo T, Scarpellini E, Ojetti V. Diagnosis of small intestinal bacterial overgrowth in the clinical practice. Eur Rev Med Pharmacol Sci. 2013 Dec. 17 Suppl 2:30-5. [QxMD MEDLINE Link].
- Rizzello CG, De Angelis M, Di Cagno R, et al. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl Environ Microbiol. 2007 Jul. 73(14):4499-507. [QxMD MEDLINE Link].
- [Guideline] Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005 Jan. 40(1):1-19. [QxMD MEDLINE Link].
- Quigley EM, Quera R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology. 2006. 130:S78-90. [QxMD MEDLINE Link].
- Lauritano EC, Gabrielli M, Scarpellini E, et al. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009 Mar-Apr. 13(2):111-6. [QxMD MEDLINE Link].
- Kumpf VJ. Pharmacologic Management of Diarrhea in Patients With Short Bowel Syndrome. JPEN J Parenter Enteral Nutr. 2014 Jan 24. [QxMD MEDLINE Link].
- Volta U, Granito A, Fiorini E, et al. Usefulness of antibodies to deamidated gliadin peptides in celiac disease diagnosis and follow-up. Dig Dis Sci. 2008 Jun. 53(6):1582-8. [QxMD MEDLINE Link].
- Adachi JA, DuPont HL. Rifaximin: a novel nonabsorbed rifamycin for gastrointestinal disorders. Clin Infect Dis. 2006 Feb 15. 42(4):541-7. [QxMD MEDLINE Link].
- Cavataio F, Guandalini S. Cow's milk allergy. Guandalini S, ed. Essential Pediatric Gastroenterology. McGraw-Hill; 2005. 175-92.
- Cole CR, Ziegler TR. Small bowel bacterial overgrowth: a negative factor in gut adaptation in pediatric SBS. Curr Gastroenterol Rep. 2007 Dec. 9(6):456-62. [QxMD MEDLINE Link].
- Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008 Jun. 27(3):328-39. [QxMD MEDLINE Link].
- Fonnesu C, Giovinale M, Verrecchia E, et al. Gastrointestinal amyloidosis: a case of chronic diarrhoea. Eur Rev Med Pharmacol Sci. 2009 Mar. 13 Suppl 1:45-50. [QxMD MEDLINE Link].
- Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006. 130 (2 Suppl 1):S16-28. [QxMD MEDLINE Link].
- Green PH, Jabri B. Celiac disease. Annu Rev Med. 2006. 57:207-21. [QxMD MEDLINE Link].
- Guandalini S, Dincer AP. Nutritional management in diarrhoeal disease. Baillieres Clin Gastroenterol. 1998. 12:697-717. [QxMD MEDLINE Link].
- Gupte GL, Beath SV, Kelly DA, et al. Current issues in the management of intestinal failure. Arch Dis Child. 2006. 91:259-64. [QxMD MEDLINE Link].
- Kneepkens CM, Hoekstra JH. Chronic nonspecific diarrhea of childhood: pathophysiology and management. Pediatr Clin North Am. 1996 Apr. 43(2):375-90. [QxMD MEDLINE Link].
- Longo N, Elsas LJ. Human glucose transporters. Adv Pediatr. 1998. 45:293-313. [QxMD MEDLINE Link].
- Love MW, Dawson PA. New insights into bile acid transport. Curr Opin Lipidol. 1998 Jun. 9(3):225-9. [QxMD MEDLINE Link].
- Maldonado J, Gil A, Narbona E, Molina JA. Special formulas in infant nutrition: a review. Early Hum Dev. 1998 Dec. 53 Suppl:S23-32. [QxMD MEDLINE Link].
- Montalto M, Curigliano V, Santoro L, et al. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006. 12:187-91. [QxMD MEDLINE Link].
- Montalto M, Santoro L, D'Onofrio F, Curigliano V, Visca D, Gallo A, et al. Classification of malabsorption syndromes. Dig Dis. 2008. 26(2):104-11. [QxMD MEDLINE Link].
- Nakamura T, Takeuchi T, Tando Y. Pancreatic dysfunction and treatment options. Pancreas. 1998 Apr. 16(3):329-36. [QxMD MEDLINE Link].
- Robayo-Torres CC, Quezada-Calvillo R, Nichols BL. Disaccharide digestion: clinical and molecular aspects. Clin Gastroenterol Hepatol. 2006. 4:276-87. [QxMD MEDLINE Link].
- Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann''s disease). Orphanet J Rare Dis. 2008 Feb 22. 3:5. [QxMD MEDLINE Link]. [Full Text].
- Weiss B, Skourikhin Y, Modan-Moses D, Broide E, Fradkin A, Bujanover Y. Is adult height of patients with celiac disease influenced by delayed diagnosis?. Am J Gastroenterol. 2008 Jul. 103(7):1770-4. [QxMD MEDLINE Link].
- Zanchi C, Di Leo G, Ronfani L, Martelossi S, Not T, Ventura A. Bone metabolism in celiac disease. J Pediatr. 2008 Aug. 153(2):262-5. [QxMD MEDLINE Link].
- Antunes C, Gossman WG. Whipple Disease. 2017 Jun. [QxMD MEDLINE Link]. [Full Text].
- Tack GJ, van de Water JM, Bruins MJ, Kooy-Winkelaar EM, van Bergen J, Bonnet P, et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: a pilot-study. World J Gastroenterol. 2013 Sep 21. 19 (35):5837-47. [QxMD MEDLINE Link].
- Syage JA, Murray JA, Green PHR, Khosla C. Latiglutenase Improves Symptoms in Seropositive Celiac Disease Patients While on a Gluten-Free Diet. Dig Dis Sci. 2017 Jul 28. [QxMD MEDLINE Link].
- Goel G, King T, Daveson AJ, Andrews JM, Krishnarajah J, Krause R, et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol Hepatol. 2017 Jul. 2 (7):479-493. [QxMD MEDLINE Link].
- The small intestine is a major site of absorption.


Author
Stefano Guandalini, MD, AGAF Founder and Director Emeritus, Celiac Disease Center, University of Chicago; Former Chief, Section of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, University of Chicago Medicine; Professor Emeritus, The University of Chicago Pritzker School of Medicine
Stefano Guandalini, MD, AGAF is a member of the following medical societies: American Gastroenterological Association, European Society for Paediatric Gastroenterology, Hepatology and Nutrition, North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, North American Society for the Study of Celiac Disease
Disclosure: Nothing to disclose.
Coauthor(s)
Richard E Frye, MD, PhD Professor of Child Health, University of Arizona College of Medicine at Phoenix; Chief of Neurodevelopmental Disorders, Director of Autism and Down Syndrome and Fragile X Programs, Division of Neurodevelopmental Disorders, Department of Neurology, Barrow Neurological Institute at Phoenix Children's Hospital
Richard E Frye, MD, PhD is a member of the following medical societies: American Academy of Neurology, American Academy of Pediatrics, Child Neurology Society
Disclosure: Nothing to disclose.
M Akram Tamer, MD Professor, Program Director, Department of Pediatrics, University of Miami, Leonard M Miller School of Medicine
M Akram Tamer, MD is a member of the following medical societies: American Medical Association, Florida Medical Association
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
B UK Li, MD Professor of Pediatrics, Division of Gastroenterology, Hepatology and Nutrition, Medical College of Wisconsin; Attending Gastroenterologist, Director, Cyclic Vomiting Program, Children’s Hospital of Wisconsin
B UK Li, MD is a member of the following medical societies: Alpha Omega Alpha, American Gastroenterological Association, North American Society for Pediatric Gastroenterology, Hepatology and Nutrition
Disclosure: Nothing to disclose.
Chief Editor
Carmen Cuffari, MD Associate Professor, Department of Pediatrics, Division of Gastroenterology/Nutrition, Johns Hopkins University School of Medicine
Carmen Cuffari, MD is a member of the following medical societies: American College of Gastroenterology, American Gastroenterological Association, North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, Royal College of Physicians and Surgeons of Canada
Disclosure: Received honoraria from Prometheus Laboratories for speaking and teaching; Received honoraria from Abbott Nutritionals for speaking and teaching. for: Abbott Nutritional, Abbvie, speakers' bureau.
Additional Contributors