Esophageal Atresia With or Without Tracheoesophageal Fistula: Practice Essentials, Pathophysiology, Etiology (original) (raw)
Practice Essentials
Esophageal atresia refers to a congenitally interrupted esophagus. [1] One or more fistulae may be present between the anomalous esophagus and the trachea. The lack of esophageal patency prevents swallowing. In addition to preventing normal feeding, this problem may cause infants to aspirate and literally drown in their own saliva, which quickly overflows the upper pouch of the obstructed esophagus. If a tracheoesophageal fistula (TEF) is present, fluid (either saliva from above or gastric secretions from below) may flow directly into the tracheobronchial tree.
The condition was first described anecdotally in the 17th century. In 1670, Durston described the first case of esophageal atresia in one conjoined twin. In 1696, Gibson provided the first description of esophageal atresia with a distal TEF. In 1862, Hirschsprung (a famous pediatrician from Copenhagen) described 14 cases of esophageal atresia. In 1898, Hoffman attempted primary repair of the defect but was not successful and resorted to the placement of a gastrostomy.
At the start of the 20th century, surgeons were theorizing about how the lesion could be repaired. In 1939 and 1940, Ladd of Boston and Lever of Minnesota first achieved surgical success in stages; success meant that the affected children survived and skin-lined pharyngogastric conduits were eventually constructed. In 1941, Haight of Michigan successfully repaired esophageal atresia in a 12-day-old baby using a primary single-stage left-side extrapleural approach. Subsequent to that child's survival and with advances in surgical and anesthetic techniques, esophageal atresia is now regarded as an eminently correctable congenital lesion.
The treatment plan for each baby must be individualized. (See Treatment.) Prognostic classifications (eg, the Waterston, Spitz, and Poenaru prognostic classification systems) can provide guidance in patients with multiple problems and help determine the indications for and timing of surgical repair, but early and decisive identification of the most life-threatening anomaly is essential. Surgical approaches to treatment vary according to surgeons' preferences and variations in pathologic anatomy.
For patient education resources, see the Esophagus, Stomach, and Intestine Center and Procedures Center, as well as Choking and Bronchoscopy.

Pathophysiology
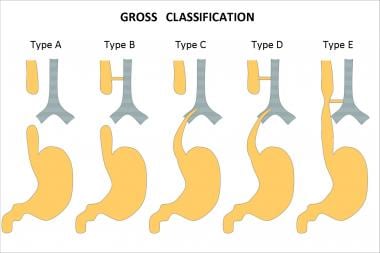
The variants of esophageal atresia have been described using many anatomic classification systems. To avoid ambiguity, the clinician should use a narrative description. Nevertheless, Gross of Boston described the classification system that is most often cited (see the image below). [2]
Esophageal atresia classification according to Gross.
According to the system formulated by Gross, the types of esophageal atresia and their approximate incidence in all infants born with esophageal anomalies are as follows:
- Type A - Esophageal atresia without fistula or so-called pure esophageal atresia (10%)
- Type B - Esophageal atresia with proximal TEF (< 1%)
- Type C - Esophageal atresia with distal TEF (85%)
- Type D - Esophageal atresia with proximal and distal TEFs (< 1%)
- Type E - TEF without esophageal atresia or so-called H-type fistula (4%)
- Type F - Congenital esophageal stenosis (< 1%) (not discussed in this article)
A fetus with esophageal atresia cannot effectively swallow amniotic fluid, especially when TEF is absent. [3] In a fetus with esophageal atresia and a distal TEF, some amniotic fluid presumably flows through the trachea and down the fistula to the gut. Polyhydramnios may be the result of this change in the recycling of amniotic fluid through the fetus. Polyhydramnios, in turn, may lead to premature labor. The fetus also appears to derive some nutritional benefit from the ingestion of amniotic fluid; thus, fetuses with esophageal atresia may be small for their gestational age.
The neonate with esophageal atresia cannot swallow and drools copious amounts of saliva. Aspiration of saliva or milk, if the baby is allowed to suckle, can lead to an aspiration pneumonitis. In a baby with esophageal atresia and a distal TEF, the lungs may be exposed to gastric secretions. Also, air from the trachea can pass down the distal fistula when the baby cries, strains, or receives ventilation. [4] This condition can lead to an acute gastric perforation, which is often lethal.
Prerepair esophageal manometric studies have revealed that the distal esophagus in esophageal atresia is essentially dysmotile, with poor or absent propagating peristaltic waves. This condition results in variable degrees of dysphagia after the repair and contributes to gastroesophageal reflux (GER).
The trachea is also affected by the disordered embryogenesis in esophageal atresia. The membranous part of the trachea, the pars membranacea, is often wide and imparts a cross-sectional D shape to the trachea, as opposed to the usual C shape. These changes cause secondary anteroposterior structural weakening of the trachea, or tracheomalacia.
This weakening can result in a sonorous cough as the intrathoracic trachea resonates and partially collapses with forceful expiration. Secretions can be difficult to clear and may lead to frequent pneumonias. Also, the trachea can partially collapse during feeding, after repair, or with episodes of GER; this partial collapse can lead to ineffective respiration; hypoxia; and, somewhat inexplicably, apnea.

Etiology
No human teratogens that cause esophageal atresia are known. Esophageal atresia that occurs in families has been reported. A 2% risk of recurrence is present when a sibling is affected. The occasional association of esophageal atresia with trisomies 21, 13, and 18 further suggests genetic causation. Also, twinning occurs about six times more frequently in patients with esophageal atresia than in those without the condition.
Most authorities believe that the development of esophageal atresia has a nongenetic basis. [5] Debate about the embryopathologic process of this condition continues, and little about it is known. The old His theory that lateral infoldings divide the foregut into the esophagus and trachea is attractively simple, but findings from human embryology studies do not support this theory.
In 1984, O'Rahilly proposed that a fixed cephalad point of tracheoesophageal separation is present, with the tracheobronchial and esophageal elements elongating in a caudal direction from this point. [6] This theory does not easily account for esophageal atresia but explains TEF as a deficiency or breakdown of esophageal mucosa, which occurs as the linear growth of the organ exceeds the cellular division of the esophageal epithelium.
In a 1987 report, Kluth eschewed the concept that tracheoesophageal septation has a key role in the development of esophageal atresia. [7] Instead, he based the embryopathologic process on the faulty development of the early, but already differentiated, trachea and esophagus, in which a dorsal fold comes to lie too far ventrally; thus, the early tracheoesophagus remains undivided. He also suggested that esophageal vascular events, ischemic events, or both may be causes in cases of esophageal atresia without fistula.
In 2001, Orford et al postulated that the ectopic, ventrally displaced location of the notochord in an embryo at 21 days' gestation can lead to a disruption of the gene locus, sonic hedgehog-signaled apoptosis in the developing foregut, and variants of esophageal atresia. [8] This situation may be due to various early gestation teratogenic influences such as twinning, toxin exposure, or possible abortion.
In 2003, Spilde et al reported esophageal atresia-TEF formations in the embryos of rat models of doxorubicin-induced teratogenesis. [9] Specific absences of certain fibroblast growth factor (FGF) elements have been reported, specifically FGF1 and the IIIb splice variant of the FGF2R receptor. [10] These specific FGF-signaling absences are postulated to allow the nonbranching development of the fistulous tract from the foregut, which then establishes continuity with the developing stomach.

Epidemiology
The incidence of esophageal atresia is 1 case in 3000-4500 births. This frequency may be decreasing for unknown reasons. [11] Internationally, the highest incidence of this disorder is reported in Finland, where it is 1 case in 2500 births.

Prognosis
Statistics regarding mortality in esophageal atresia are constantly changing and improving. [12, 13, 14, 15] One must consider the classification system used in reporting such statistics.
Mortality relative to the Montreal classification is as follows [16] :
- Class I - Mortality of 7.3%
- Class II - Mortality of 69.2%
Mortality relative to the Spitz grouping is as follows [17] :
- Group I - Mortality of 3%
- Group II - Mortality of 41%
- Group III - Mortality of 78%
Mortality relative to the Waterston categorization is as follows [18] :
- Category A - Mortality of 0%
- Category B - Mortality of 4%
- Category C - Mortality of 11%
Fetuses with antenatal diagnoses of esophageal atresia seem to have a worse prognosis. [19] The cohort of babies in whom esophageal atresia is detected antenatally has a 75% mortality, whereas the cohort of babies in whom esophageal atresia is not detected antenatally has a 21% mortality. Babies who survive have varied morbidities related to any of the associated anomalies and complications. However, most children who undergo a successful repair of esophageal atresia are relatively healthy.

- van Lennep M, Singendonk MMJ, Dall'Oglio L, Gottrand F, Krishnan U, Terheggen-Lagro SWJ, et al. Oesophageal atresia. Nat Rev Dis Primers. 2019 Apr 18. 5 (1):26. [QxMD MEDLINE Link].
- Gross RE. The Surgery of Infancy and Childhood. Philadelphia: WB Saunders; 1953. 76.
- Beasley SW, Hutson JM, Auldist AW. Essential Paediatric Surgery. Oxford, England: Oxford University Press; 1996.
- Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Philadelphia: Elsevier; 2021.
- Moore KL, Persaud TVN, Torchia MG. The Developing Human: Clinically Oriented Embryology. 11th ed. Philadelphia: Elsevier; 2020.
- O'Rahilly R, Müller F. Chevalier Jackson lecture. Respiratory and alimentary relations in staged human embryos. New embryological data and congenital anomalies. Ann Otol Rhinol Laryngol. 1984 Sep-Oct. 93 (5 Pt 1):421-9. [QxMD MEDLINE Link].
- Kluth D, Steding G, Seidl W. The embryology of foregut malformations. J Pediatr Surg. 1987 May. 22 (5):389-93. [QxMD MEDLINE Link].
- Orford J, Manglick P, Cass DT, Tam PP. Mechanisms for the development of esophageal atresia. J Pediatr Surg. 2001 Jul. 36 (7):985-94. [QxMD MEDLINE Link].
- Spilde TL, Bhatia AM, Marosky JK, Preuett B, Kobayashi H, Hembree MJ, et al. Fibroblast growth factor signaling in the developing tracheoesophageal fistula. J Pediatr Surg. 2003 Mar. 38 (3):474-7; discussion 474-7. [QxMD MEDLINE Link].
- Crisera CA, Maldonado TS, Longaker MT, Gittes GK. Defective fibroblast growth factor signaling allows for nonbranching growth of the respiratory-derived fistula tract in esophageal atresia with tracheoesophageal fistula. J Pediatr Surg. 2000 Oct. 35 (10):1421-5. [QxMD MEDLINE Link].
- Ashcraft KW, Holder TM. Pediatric Esophageal Surgery. Orlando, FL: Grune and Stratton; 1996.
- Clark DC. Esophageal atresia and tracheoesophageal fistula. Am Fam Physician. 1999 Feb 15. 59 (4):910-6, 919-20. [QxMD MEDLINE Link].
- Encinas JL, Luis AL, Avila LF, Martinez L, Guereta L, Lassaletta L, et al. Impact of preoperative diagnosis of congenital heart disease on the treatment of esophageal atresia. Pediatr Surg Int. 2006 Feb. 22 (2):150-3. [QxMD MEDLINE Link].
- Jolley SG. A longitudinal study of children treated with the most common form of esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2007 Sep. 42 (9):1632-3; author reply 1633. [QxMD MEDLINE Link].
- Lopes MF, Botelho MF. Midterm follow-up of esophageal anastomosis for esophageal atresia repair: long-gap versus non-long-gap. Dis Esophagus. 2007. 20 (5):428-35. [QxMD MEDLINE Link].
- Poenaru D, Laberge JM, Neilson IR, Guttman FM. A new prognostic classification for esophageal atresia. Surgery. 1993 Apr. 113 (4):426-32. [QxMD MEDLINE Link].
- Spitz L, Kiely EM, Morecroft JA, Drake DP. Oesophageal atresia: at-risk groups for the 1990s. J Pediatr Surg. 1994 Jun. 29 (6):723-5. [QxMD MEDLINE Link].
- Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR 3rd. Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Arch Surg. 1995 May. 130 (5):502-8; discussion 508-9. [QxMD MEDLINE Link].
- Stringer MD, McKenna KM, Goldstein RB, Filly RA, Adzick NS, Harrison MR. Prenatal diagnosis of esophageal atresia. J Pediatr Surg. 1995 Sep. 30 (9):1258-63. [QxMD MEDLINE Link].
- Shaw-Smith C. Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. J Med Genet. 2006 Jul. 43 (7):545-54. [QxMD MEDLINE Link]. [Full Text].
- Kassif E, Miller T TE, Tsur A, Trozky Y, Gur T, De Castro H, et al. Dynamic Esophageal Patency Assessment: An effective method for prenatally diagnosing esophageal atresia. Am J Obstet Gynecol. 2021 Jun 16. [QxMD MEDLINE Link].
- Diaz LK, Akpek EA, Dinavahi R, Andropoulos DB. Tracheoesophageal fistula and associated congenital heart disease: implications for anesthetic management and survival. Paediatr Anaesth. 2005 Oct. 15 (10):862-9. [QxMD MEDLINE Link].
- FDA authorizes use of new device to treat esophageal birth defect in babies. US Food and Drug Administration. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm558241.htm. May 12, 2017; Accessed: April 10, 2023.
- van der Zee DC, Vieirra-Travassos D, Kramer WL, Tytgat SH. Thoracoscopic elongation of the esophagus in long gap esophageal atresia. J Pediatr Surg. 2007 Oct. 42 (10):1785-8. [QxMD MEDLINE Link].
- Spitz L, Kiely E, Brereton RJ. Esophageal atresia: five year experience with 148 cases. J Pediatr Surg. 1987 Feb. 22 (2):103-8. [QxMD MEDLINE Link].
- Spitz L. Esophageal atresia: past, present, and future. J Pediatr Surg. 1996 Jan. 31 (1):19-25. [QxMD MEDLINE Link].
- Saxena AK, Ainoedhofer H, Höllwarth ME. Culture of ovine esophageal epithelial cells and in vitro esophagus tissue engineering. Tissue Eng Part C Methods. 2010 Feb. 16 (1):109-14. [QxMD MEDLINE Link].
- Saxena AK. Tissue engineering and regenerative medicine research perspectives for pediatric surgery. Pediatr Surg Int. 2010 Jun. 26 (6):557-73. [QxMD MEDLINE Link].
- Saxena AK. Congenital anomalies of soft tissues: birth defects depending on tissue engineering solutions and present advances in regenerative medicine. Tissue Eng Part B Rev. 2010 Oct. 16 (5):455-66. [QxMD MEDLINE Link].
- Model L, Wiesel O. A narrative review of esophageal tissue engineering and replacement: where are we?. Ann Transl Med. 2021 May. 9 (10):910. [QxMD MEDLINE Link]. [Full Text].
- Saxena AK, Ainoedhofer H, Höllwarth ME. Esophagus tissue engineering: in vitro generation of esophageal epithelial cell sheets and viability on scaffold. J Pediatr Surg. 2009 May. 44 (5):896-901. [QxMD MEDLINE Link].
- Saxena AK, Baumgart H, Komann C, Ainoedhofer H, Soltysiak P, Kofler K, et al. Esophagus tissue engineering: in situ generation of rudimentary tubular vascularized esophageal conduit using the ovine model. J Pediatr Surg. 2010 May. 45 (5):859-64. [QxMD MEDLINE Link].
- Dunn JC, Fonkalsrud EW, Atkinson JB. Simplifying the Waterston's stratification of infants with tracheoesophageal fistula. Am Surg. 1999 Oct. 65 (10):908-10. [QxMD MEDLINE Link].
- Randolph JG, Newman KD, Anderson KD. Current results in repair of esophageal atresia with tracheoesophageal fistula using physiologic status as a guide to therapy. Ann Surg. 1989 May. 209 (5):526-30; discussion 530-1. [QxMD MEDLINE Link].
- Konkin DE, O'hali WA, Webber EM, Blair GK. Outcomes in esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2003 Dec. 38 (12):1726-9. [QxMD MEDLINE Link].
- Schlottmann F, Patti MG. Esophagus and diaphragm. Doherty GM, ed. Current Surgical Diagnosis and Treatment. 15th ed. New York: McGraw-Hill; 2020. 455-84.
- McCollum MO, Rangel SJ, Blair GK, Moss RL, Smith BM, Skarsgard ED. Primary reversed gastric tube reconstruction in long gap esophageal atresia. J Pediatr Surg. 2003 Jun. 38 (6):957-62. [QxMD MEDLINE Link].
- Firriolo JM, Nuzzi LC, Ganske IM, Hamilton TE, Smithers CJ, Ganor O, et al. Supercharged Jejunal Interposition: A Reliable Esophageal Replacement in Pediatric Patients. Plast Reconstr Surg. 2019 Jun. 143 (6):1266e-1276e. [QxMD MEDLINE Link].
- Skarsgard ED. Dynamic esophageal lengthening for long gap esophageal atresia: experience with two cases. J Pediatr Surg. 2004 Nov. 39 (11):1712-4. [QxMD MEDLINE Link].
- Foker JE, Linden BC, Boyle EM Jr, Marquardt C. Development of a true primary repair for the full spectrum of esophageal atresia. Ann Surg. 1997 Oct. 226 (4):533-41; discussion 541-3. [QxMD MEDLINE Link].
- Foker JE, Kendall TC, Catton K, Khan KM. A flexible approach to achieve a true primary repair for all infants with esophageal atresia. Semin Pediatr Surg. 2005 Feb. 14 (1):8-15. [QxMD MEDLINE Link].
- Allal H, Kalfa N, Lopez M, Forgues D, Guibal MP, Raux O, et al. Benefits of the thoracoscopic approach for short- or long-gap esophageal atresia. J Laparoendosc Adv Surg Tech A. 2005 Dec. 15 (6):673-7. [QxMD MEDLINE Link].
- Bax KM, van Der Zee DC. Feasibility of thoracoscopic repair of esophageal atresia with distal fistula. J Pediatr Surg. 2002 Feb. 37 (2):192-6. [QxMD MEDLINE Link].
- Holcomb GW 3rd, Rothenberg SS, Bax KM, Martinez-Ferro M, Albanese CT, Ostlie DJ, et al. Thoracoscopic repair of esophageal atresia and tracheoesophageal fistula: a multi-institutional analysis. Ann Surg. 2005 Sep. 242 (3):422-8; discussion 428-30. [QxMD MEDLINE Link].
- Rothenberg SS. Thoracoscopic repair of tracheoesophageal fistula in newborns. J Pediatr Surg. 2002 Jun. 37 (6):869-72. [QxMD MEDLINE Link].
- Chiarenza SF, Bleve C, Zolpi E, Costa L, Mazzotta MR, Novek S, et al. The Use of Endoclips in Thoracoscopic Correction of Esophageal Atresia: Advantages or Complications?. J Laparoendosc Adv Surg Tech A. 2019 Jul. 29 (7):976-980. [QxMD MEDLINE Link].
- van Tuyll van Serooskerken ES, Lindeboom MYA, Verweij JW, van der Zee DC, Tytgat SHAJ. Childhood outcome after correction of long-gap esophageal atresia by thoracoscopic external traction technique. J Pediatr Surg. 2021 Oct. 56 (10):1745-1751. [QxMD MEDLINE Link]. [Full Text].
- van der Zee DC, Tytgat SHA, van Herwaarden MYA. Esophageal atresia and tracheo-esophageal fistula. Semin Pediatr Surg. 2017 Apr. 26 (2):67-71. [QxMD MEDLINE Link].
- Wu Y, Kuang H, Lv T, Wu C. Comparison of clinical outcomes between open and thoracoscopic repair for esophageal atresia with tracheoesophageal fistula: a systematic review and meta-analysis. Pediatr Surg Int. 2017 Nov. 33 (11):1147-1157. [QxMD MEDLINE Link].
- Okuyama H, Tazuke Y, Ueno T, Yamanaka H, Takama Y, Saka R, et al. Learning curve for the thoracoscopic repair of esophageal atresia with tracheoesophageal fistula. Asian J Endosc Surg. 2018 Feb. 11 (1):30-34. [QxMD MEDLINE Link].
- Tanaka Y, Tainaka T, Sumida W, Shirota C, Murase N, Oshima K, et al. Comparison of outcomes of thoracoscopic primary repair of gross type C esophageal atresia performed by qualified and non-qualified surgeons. Pediatr Surg Int. 2017 Oct. 33 (10):1081-1086. [QxMD MEDLINE Link].
- Marinho AS, Saxena AK. Thoracoscopic Esophageal Atresia Repair: Outcomes Analysis Between Primary and Staged Procedures. Surg Laparosc Endosc Percutan Tech. 2020 Dec 23. 31 (3):363-367. [QxMD MEDLINE Link].
- Li S, Cao G, Zhou R, Zhang X, Zhou Y, Tang ST. Feasible techniques in robotic thoracoscopic repair of congenital esophageal atresia: case report and literature review. Surg Case Rep. 2021 Jun 15. 7 (1):142. [QxMD MEDLINE Link].
- Evans LL, Chen CS, Muensterer OJ, Sahlabadi M, Lovvorn HN, Novotny NM, et al. The novel application of an emerging device for salvage of primary repair in high-risk complex esophageal atresia. J Pediatr Surg. 2022 Dec. 57 (12):810-818. [QxMD MEDLINE Link].
- Conforti A, Pellegrino C, Valfré L, Iacusso C, Schingo PMS, Capolupo I, et al. Magnamosis for long gap esophageal atresia: Minimally invasive "fatal attraction". J Pediatr Surg. 2023 Mar. 58 (3):405-411. [QxMD MEDLINE Link].
- Lal DR, Gadepalli SK, Downard CD, Ostlie DJ, Minneci PC, Swedler RM, et al. Challenging surgical dogma in the management of proximal esophageal atresia with distal tracheoesophageal fistula: Outcomes from the Midwest Pediatric Surgery Consortium. J Pediatr Surg. 2018 Jul. 53 (7):1267-1272. [QxMD MEDLINE Link].
- Yasuda JL, Clark SJ, Staffa SJ, Blansky B, Ngo PD, Hamilton TE, et al. Esophagitis in Pediatric Esophageal Atresia: Acid May Not Always Be the Issue. J Pediatr Gastroenterol Nutr. 2019 Aug. 69 (2):163-170. [QxMD MEDLINE Link].
- Chittmittrapap S, Spitz L, Kiely EM, Brereton RJ. Anastomotic leakage following surgery for esophageal atresia. J Pediatr Surg. 1992 Jan. 27 (1):29-32. [QxMD MEDLINE Link].
- Mathur S, Vasudevan SA, Patterson DM, Hassan SF, Kim ES. Novel use of glycopyrrolate (Robinul) in the treatment of anastomotic leak after repair of esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2011 Mar. 46 (3):e29-32. [QxMD MEDLINE Link].
- Yang S, Li S, Yang Z, Liao J, Hua K, Zhang Y, et al. Risk Factors for Recurrent Tracheoesophageal Fistula After Gross Type C Esophageal Atresia Repair. Front Pediatr. 2021. 9:645511. [QxMD MEDLINE Link]. [Full Text].
- Lakoma A, Fallon SC, Mathur S, Kim ES. Use of Mitomycin C for Refractory Esophageal Stricture following Tracheoesophageal Fistula Repair. European J Pediatr Surg Rep. 2013 Jun. 1 (1):24-6. [QxMD MEDLINE Link].
- Pederiva F, Burgos E, Francica I, Zuccarello B, Martinez L, Tovar JA. Intrinsic esophageal innervation in esophageal atresia without fistula. Pediatr Surg Int. 2008 Jan. 24 (1):95-100. [QxMD MEDLINE Link].
- Blair GK, Cohen R, Filler RM. Treatment of tracheomalacia: eight years' experience. J Pediatr Surg. 1986 Sep. 21 (9):781-5. [QxMD MEDLINE Link].
- Deurloo JA, Ekkelkamp S, Taminiau JA, Kneepkens CM, ten Kate FW, Bartelsman JF, et al. Esophagitis and Barrett esophagus after correction of esophageal atresia. J Pediatr Surg. 2005 Aug. 40 (8):1227-31. [QxMD MEDLINE Link].
- Deurloo JA, Ekkelkamp S, Hartman EE, Sprangers MA, Aronson DC. Quality of life in adult survivors of correction of esophageal atresia. Arch Surg. 2005 Oct. 140 (10):976-80. [QxMD MEDLINE Link].
Author
Amulya K Saxena, MD, PhD, DSc, FRCS(Glasg) Consultant Pediatric Surgeon, Department of Pediatric Surgery, Chelsea Children's Hospital, Chelsea and Westminster Healthcare NHS Fdn Trust, Imperial College London, UK
Amulya K Saxena, MD, PhD, DSc, FRCS(Glasg) is a member of the following medical societies: Austrian Society for Pediatric and Adolescent Surgery, British Association of Paediatric Surgeons, European Paediatric Surgeons' Association, German Association of Pediatric Surgeons, German Society of Surgery, International Pediatric Endosurgery Group, Tissue Engineering and Regenerative Medicine International Society
Disclosure: Nothing to disclose.
Coauthor(s)
Geoffrey Blair, MD Clinical Professor of Pediatric General Surgery, University of British Columbia; Head, Department of Pediatric Surgery, British Columbia's Children's Hospital
Geoffrey Blair, MD is a member of the following medical societies: American Pediatric Surgical Association
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Michael G Caty, MD Professor of Surgery and Pediatrics, State University of New York at Buffalo; Consulting Staff, Department of Pediatric Surgery, Children's Hospital of Buffalo
Michael G Caty, MD is a member of the following medical societies: American Academy of Pediatrics, American Association for Physician Leadership, American College of Surgeons, American Medical Association, American Pediatric Surgical Association, Association for Academic Surgery, Association for Surgical Education
Disclosure: Nothing to disclose.
Chief Editor
Eugene S Kim, MD, FACS, FAAP Director, Division of Pediatric Surgery, Vice Chair, Department of Surgery, Cedars Sinai Medical Center; Professor of Surgery and Pediatrics, Division of Pediatric Surgery, Division of Hematology, Oncology, Blood and Marrow Transplantation, Keck School of Medicine of the University of Southern California
Eugene S Kim, MD, FACS, FAAP is a member of the following medical societies: American Academy of Pediatrics, American Association for Cancer Research, American College of Surgeons, American Medical Association, American Pediatric Surgical Association, American Surgical Association, Association for Academic Surgery, Children's Oncology Group, Society of Laparoscopic and Robotic Surgeons, Society of University Surgeons, Texas Medical Association
Disclosure: Nothing to disclose.