Pediatric HIV Infection: Practice Essentials, Background, Pathophysiology (original) (raw)
Practice Essentials
Since the first cases of human immunodeficiency virus (HIV) infection were identified, the number of children infected with HIV has risen dramatically in developing countries, the result of an increased number of HIV-infected women of childbearing age in these areas. HIV is a retrovirus and can be transmitted vertically, sexually, or via contaminated blood products or intravenous (IV) drug abuse. Vertical HIV infection occurs before birth, during delivery, or after birth.
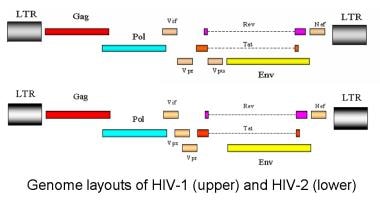
The genome layouts of HIV-1 and HIV type 2 (HIV-2) are shown in the image below.
Genome layout of human immunodeficiency virus (HIV)–1 and HIV-2.
Essential update: Study suggests benefits to starting HAART earlier in HIV-infected children
In a study of HIV-1-infected, highly active antiretroviral therapy (HAART)-naive children, Yin et al found that beginning HAART at younger ages and healthier CD4 levels results in better immune recovery. [1, 2] In all, 72% of children who were immunosuppressed at baseline recovered to normal within 4 years after initiating HAART therapy. Compared with children with severe immunosuppression, more children with mild immunosuppression (+36%) or advanced immunosuppression (+20.8%) recovered a normal CD4 percentage.
For every 5-year increase in baseline age, the proportion of children who achieved a normal CD4 percentage fell by 19%. [2] Combining age effects and baseline CD4 percentage resulted in more than 90% recovery when HAART was initiated in children with mild immunosuppression at any age or advanced immunosuppression at an age younger than 3 years. Most of the immunologic benefits of HAART remained significant at 4 years.
Signs and symptoms
History
Signs and symptoms of pediatric HIV infection include the following:
- Unusually frequent and severe occurrences of common childhood bacterial infections, such as otitis media, sinusitis, and pneumonia
- Recurrent fungal infections, such as candidiasis (thrush), that do not respond to standard antifungal agents: Suggests lymphocytic dysfunction
- Recurrent or unusually severe viral infections, such as recurrent or disseminated herpes simplex or zoster infection or cytomegalovirus (CMV) retinitis; seen with moderate to severe cellular immune deficiency
- Growth failure
- Failure to thrive
- Wasting
- Failure to attain typical milestones: Suggests a developmental delay; such delays, particularly impairment in the development of expressive language, may indicate HIV encephalopathy
- Behavioral abnormalities (in older children), such as loss of concentration and memory, may also indicate HIV encephalopathy
Physical examination
Signs and symptoms of pediatric HIV infection found during physical examination include the following:
- Candidiasis: Most common oral and mucocutaneous presentation of HIV infection
- Thrush in the oral cavity and posterior pharynx: Observed in approximately 30% of HIV-infected children
- Linear gingival erythema and median rhomboid glossitis
- Oral hairy leukoplakia
- Parotid enlargement and recurrent aphthous ulcers
- Herpetic infection with herpes simplex virus (HSV): May manifest as herpes labialis, gingivostomatitis, esophagitis, or chronic erosive, vesicular, and vegetating skin lesions; the involved areas of the lips, mouth, tongue, and esophagus are ulcerated
- HIV dermatitis: An erythematous, papular rash; observed in about 25% of children with HIV infection
- Dermatophytosis: Manifesting as an aggressive tinea capitis, corporis, versicolor, or onychomycosis
- Pneumocystis jiroveci (formerly P carinii) pneumonia (PCP): Most commonly manifests as cough, dyspnea, tachypnea, and fever
- Lipodystrophy: Presentations include peripheral lipoatrophy, truncal lipohypertrophy, and combined versions of these presentations; a more severe presentation occurs at puberty
- Digital clubbing: As a result of chronic lung disease
- Pitting or nonpitting edema in the extremities
- Generalized cervical, axillary, or inguinal lymphadenopathy
See Clinical Presentation for more detail.
Diagnosis
Detection of antibody to HIV is the usual first step in diagnosing HIV infection. The 2010 Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children [3] recommendations for diagnosing infants include the following:
- Because of the persistence of the maternal HIV antibody, infants younger than 18 months require virologic assays that directly detect HIV in order to diagnose HIV infection
- Preferred virologic assays include HIV bDNA polymerase chain reaction (PCR) and HIV RNA assays. The HIV PCR DNA qualitative test is usually less expensive.
- Further virologic testing in infants with known perinatal HIV exposure is recommended at 2 weeks, 4 weeks, and 4 months
An antibody test to document seroreversion to HIV antibody–negative status in uninfected infants is no longer recommended.
In older children and adults, an enzyme-linked immunosorbent assay (ELISA) to detect HIV antibody, followed by a confirmatory Western blot (which has increased specificity), should be used to diagnose HIV infection.
Rapid HIV tests, which provide results in minutes, simplify and expand the availability of HIV testing. Their sensitivity is as high as 100%, but they must be followed with confirmatory Western blotting or immunofluorescence antibody testing, as with conventional HIV antibody tests.
See Workup for more detail.
Management
Appropriate ART and therapy for specific infections and malignancies are critical in treating patients who are HIV positive. Classes of antiretroviral agents include the following:
- Nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs)
- Protease inhibitors (PIs)
- Nonnucleoside reverse transcriptase inhibitors (NNRTIs)
- CCR5 coreceptor antagonists (entry inhibitors)
- HIV integrase strand transfer inhibitors
Combination ART with at least 3 drugs from at least 2 classes of drugs is recommended for initial treatment of infected infants, children, and adolescents because it provides the best opportunity to preserve immune function and delay disease progression. Drug combinations for initial therapy in ART-naive children include a backbone of 2 NRTIs plus 1 NNRTI or 1 PI.
Pediatric HIV experts agree that infected infants who have clinical symptoms of HIV disease or evidence of immune compromise should be treated. [3] Patients aged 1 year or older with acquired immunodeficiency syndrome (AIDS) or significant symptoms should be aggressively treated regardless of CD4+ percentage and count or plasma HIV RNA level.
In addition to antiretroviral drugs (ARDs), other types of medication are required as appropriate for specific infections or malignancies. For example, P jiroveci pneumonia prophylaxis is recommended in patients who are HIV positive and younger than 1 year and in older children based on CD4+ counts.
See Treatment and Medication for more detail.

Background
Over the past 30 years, since the first cases of what is now recognized as human immunodeficiency virus (HIV) infection were identified in 1981, the number of children infected with HIV has increased dramatically in developing countries because the number of HIV-infected women of childbearing age has risen. However, great advances have been made in the United States and in other industrialized nations to control transmission of the virus from mother to infant.
In the United States, universal prenatal HIV testing has been recommended to obstetricians since 1995. However, this testing was not mandatory in all states. Before prenatal testing was common, diagnosing HIV infections in a woman after diagnosing it in her child was not unusual, and the diagnosis of acquired immunodeficiency syndrome (AIDS) in a previously healthy child was not rare.
Before 1985, one way in which children were infected was the transfusion of blood products. Improved screening tests have essentially eliminated such transmission. A common way adolescents become infected is by engaging in high-risk behaviors such as unprotected sexual intercourse and injection drug abuse.
Surveillance data now show that the only group with increasing HIV incidence is men who have sex with men. The proportion of this population who are unaware of their infection is high, with unawareness among young men (18-29 y) reaching 63%. [4]
In the United States, youths aged 13-24 years accounted for 25.7% of new HIV infections in 2010. [5]
In pediatric patients, HIV infection progresses as it does in adults, although surveillance data from the Centers for Disease Control and Prevention (CDC) suggest that patients who are aged 13-24 years when diagnosed with AIDS survive longer than older individuals do. Vertically transmitted HIV can cause rapidly progressive, chronically progressive, or adultlike disease in which a significant clinical latency period occurs before symptoms appear.
The World Health Organization (WHO) [6] estimates that approximately 2.5 million children were living with HIV infection as of 2009. In 2009 alone, 370,000 children were newly infected. [7] This is a drop of 24% from 5 years earlier. [8]
Not only are the children themselves ravaged by disease, but their primary caregivers have also often succumbed to AIDS. This is most prevalent in sub-Saharan Africa, where an estimated 11.6 million children had been orphaned by AIDS as of 2007.
Although 2 strains of HIV have currently been identified, most patients who have AIDS are positive for HIV type 1 (HIV-1) or are positive for both HIV-1 and HIV type 2 (HIV-2). HIV-2 infection is most commonly observed in West Africa.
Vertical transmission of HIV from mother to child is the main route by which childhood HIV infection is acquired; the risk of perinatal acquisition is 25-40% without intervention. [9] Perinatal transmission of infection by the mother accounts for 80% of pediatric HIV disease cases in the United States. Perinatal transmission can occur in utero, during the peripartum period, and from breastfeeding.
Other routes of transmission, such as transfusion of blood and blood components, are rare in the United States but still exist in developing countries. Sexual abuse of children and high-risk behaviors in adolescents also contribute to youth HIV infection.
A variety of signs and symptoms should alert the clinician to the possibility of HIV infection in a child. The presentations include recurrent bacterial infections, unrelenting fever, unrelenting diarrhea, unrelenting thrush, recurrent pneumonia, chronic parotitis, generalized lymphadenopathy, delay in development with failure to thrive, and significant pruritic dermatoses. Mucocutaneous eruptions may be the first sign of HIV infection and may vary in presentation, depending on the child's immune status.
For information on HIV infection in adults and adolescents, see HIV Disease.

Pathophysiology
HIV can be transmitted vertically, sexually, or via contaminated blood products or IV drug abuse. Vertical HIV infection occurs before birth, during delivery, or after birth. With infection before birth (period 1), the fetus can be hematologically infected by means of transmission across the placenta or across the amniotic membranes, especially if the membranes are inflamed or infected.
Most vertical infections occur during delivery (period 2), and many factors affect the risk of infection during this period (see Deterrence/Prevention). In general, the longer and the greater amount of contact the neonate has with infected maternal blood and cervicovaginal secretions, the greater the risk of vertical transmission. Premature and low-birthweight neonates appear to have an increased risk of infection during delivery because of their reduced skin barrier and immunologic defenses.
Postnatal vertical transmission (period 3) occurs with the ingestion of HIV in the breast milk.
HIV virology
HIV is a retrovirus. Structurally, a lipid bilayer envelope surrounds the cylindrical core of HIV, which contains the RNA genetic information and the machinery that promotes viral replication and integration during initial cellular infection. From the outside, the virion appears spherical, with a diameter of 110 nm.
HIV has a variety of structural and nonstructural proteins that determine the interaction of the virus with the host's immune system and cellular components. The genome layouts of HIV-1 and HIV type 2 (HIV-2) are shown in the image below.
Genome layout of human immunodeficiency virus (HIV)–1 and HIV-2.
The HIV virus attaches to the host cell by the association of a surface glycoprotein to the CD4 molecule; therefore, it primarily infects CD4+ lymphocytes and macrophages.
After HIV enters a host, trimeric gp120 glycoproteins that protrude from its lipoprotein bilayer envelope bind to CD4 cell-surface receptors and CCR5 or CXCR4 chemokine co-receptors. Juxtapositioned co-receptors are needed for viral infection. The V3 region of the gp120 glycoprotein determines cellular tropism, and tropism is involved in syncytial formation. M-tropic (nonsyncytial) strains prefer the CCR5 co-receptor and are the primary causes of infection.
Deficiency of CCR5 chemokine co-receptors is present in as many as 10% of Europeans and 20% of Ashkenazi Jews, and it appears to confer some protection against infection. After gp120 binds to the receptors, an associated gp41 transmembrane glycoprotein is inserted into the cell membrane and initiates cell-membrane fusion.
Upon entering the cell, the protease enzyme produces the reverse transcriptase and ribonuclease (RNAse) H enzymes responsible for synthesizing the single-stranded DNA (ssDNA) molecules and primers necessary to produce the complementary DNA strand. Because reverse transcriptase lacks proofreading capacity, considerable base-to-base variability results. The high mutation rate, combined with the high reproductive rate, results in substantial evolution and subsequent resistance to treatment.
Once the virus core enters the cell cytoplasm of the host, viral reverse transcriptase copies viral RNA to the DNA of the host. The viral DNA is then transported into the nucleus and incorporated into the DNA of that cell. If activated, viral expression can result in new viral RNA and proteins. New viral core proteins, enzymes, and viral RNA molecules can induce budding, with additional cell infection.
Immune response
Acute infection rapidly increases the viral load and causes a mild-to-moderate viremia. Although viral loads tend to diminish rapidly after acute infection in adults, they decrease slowly in vertically infected children and may not reach baseline levels until age 4-5 years. Although infants possess numerous antigen-presenting and effector cells compared with adults, their cytokine production, proliferation, and cytotoxicity are reduced.
Envelope-specific cytotoxic T-lymphocytes are less common in children who vertically acquire the disease than in children who acquire HIV by means of blood transfusion. Among those with vertically acquired disease, such lymphocytes are least common in those with rapidly progressing disease. Precursors of cytotoxic T-lymphocyte that are specific to HIV type 1 (HIV-1) do not develop in significant number until the child is aged 1 year.
In adults, antibodies to gp120 develop several months after the initial viremia occurs. The development of broadly neutralizing antibodies is associated with slowed disease progression in adults, children, and infants.
The reduction in cell-mediated immunity and secondary B-cell dysfunction result in the immunocompromised state and in the proliferation of opportunistic infections and malignancies. An elevated level of activation-induced cell death resulting from apoptosis of T cells occurs in patients who are HIV positive.
The CD95/Fas receptor/ligand system is necessary for the apoptosis of T cells, and abnormalities in this system are linked with increased T-cell death in patients who are HIV positive. As the immune status deteriorates, an increase in CD95+ T cells is found; conversely, a low CD95+ T-cell count is found in asymptomatic patients who are HIV positive.
Hematopoietic effects
Although HIV infects hematopoietic stem cells, the importance is minor. Hematopoietic disturbances are believed to occur as a consequence of changes in the microenvironment of the marrow and of deficiencies in local and systemic growth factors.
In typical conditions, the stroma of the marrow promotes stem cell proliferation and differentiation by producing granulocyte colony-stimulating factor (G-CSF) and interleukin (IL)-3. HIV-infected stroma produces less G-CSF and IL-3 than normal and produces excessive tumor necrosis factor (TNF)-alpha and IFN-gamma. This cytokine dysregulation halts the production of badly needed hematopoietic cell lines and causes apoptosis of committed progenitor cells.
HIV also appears to retard the production of thrombopoietin in the liver and erythropoietin in the kidney. In addition to a low serum erythropoietin level, HIV-induced anemia is also a result of a blunted response to erythropoietin.
Thrombocytopenia occurs in 40% of patients with HIV infection during the course of the disease. It is most common in people with advanced disease, those who use IV drugs, African Americans, and those with a history of anemia or lymphoma. The presence of thrombocytopenia suggests a shortened survival time.
Immune thrombocytopenia may occur in half of the cases and appears to be the result of molecular mimicry of the platelet glycoprotein (GP)-IIb/IIIa receptor by the HIV-GP 160/120 antigen. Decreased platelet production is common in HIV infection regardless of the platelet count, and it may be associated with the ultrastructural damage in HIV-infected megakaryocytes.
Anemia may be present in as many as 20% of patients at the time of diagnosis, and it occurs in as many as 80% of patients at some point. Patients with clinical AIDS are more likely than others to have anemia, as are patients with low CD4+ counts.
The etiology is probably multifactorial in most patients. Common contributing factors are bone marrow suppression, iatrogenic causes, vitamin deficiencies, suppressed erythropoietin production, and a blunted erythropoietin response. Bone marrow infiltration with lymphoma or Kaposi sarcoma may be noted. Bone marrow suppression may be due to pathogens such as MAC, parvovirus B19, or CMV. Disseminated fungemia can cause anemia.
Neutropenia is observed in 10% of patients with early asymptomatic HIV infections and in 50% of patients with AIDS. Neutropenia results from the aforementioned mechanisms, as well as from medication. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF deficiencies not only reduce neutrophil production but also reduce granulocytic and monocytic function. GM-CSF and G-CSF promote increased neutrophilic function, including superoxide production, phagocytosis, intracellular killing, and antibody-dependent cellular cytotoxicity.
Neurologic effects
HIV exhibits tropism for the CNS, especially the microglia. As many as 10% of children with AIDS have progressive encephalopathy. Progressive white matter degeneration and brain atrophy may develop. Neurologic symptoms develop along with developmental delay.
Viral resistance
In terms of the mechanisms of resistance development, the rapid turnover rate and high error rate of reverse transcriptase induces 3300 new single mutations per day. When a mutation improves the survival of the virus in an existing drug environment, that quasispecies is selected to reproduce. The higher the viral load and the higher the rate of replication, the greater the number of resistant quasispecies. Quasispecies can be transmitted to a fetus or neonate.
HIV resistance develops because of low antiretroviral drug (ARD) levels due to several factors including variations in drug absorption and metabolism and noncompliance because of adverse effects or a poor understanding of the importance of the medication. Viral sanctuary sites may be exposed to low levels of ARDs, and resistant quasispecies may develop.

Etiology
Infection is due to HIV, a complex member of the Lentivirus genus of the Retroviridae family. HIV-1 is the most common cause of HIV infection in the Americas, in Europe, in Asia, and in Africa. HIV-2 has caused epidemics in West Africa, although this virus is also found in European countries. HIV-2 disease progresses more slowly than HIV-1 disease, and HIV-2 is less transmissible than HIV-1.
HIV-1 subtypes differ by geographic region. HIV-1 subtype B is predominant in the United States. Non-B subtypes are particularly prevalent in Africa and Asia. The high transmission rate from Africa to Europe has increased the diversity of subtypes in Europe. Non-B subtype HIV-1 infections are increasing in the United States.
Vertical transmission of HIV from mother to child is the main route by which childhood HIV infection is acquired; the risk of perinatal acquisition is 25%. African epidemiologic data of almost 2000 infants indicate that female infants may be more susceptible to HIV infection before birth and continuing after birth compared with male infants.

Epidemiology
United States statistics
The HIV seroprevalence rate in pregnant women is as high as 0.3%. The seroprevalence of women infected with HIV is highest in the Northeast, followed by the South. Perinatal HIV transmission rates are 25% but as low as 2% in untreated women with viral loads of less than 100 copies/mL.
Although prophylactic interventions have reduced vertical transmissions, cases of perinatal HIV transmission continue to occur. [10] This is largely because of missed opportunities for prevention, particularly among women who lack prenatal care or who are not being offered voluntary HIV counseling and testing during pregnancy. In many as 40% of the mothers of infants with perinatally acquired HIV infection, the HIV infection was not known before delivery.
The CDC estimates that in 2009, in the 40 states with confidential name-based HIV infection reporting, an estimated 131 infants acquired HIV infection by means of vertical transmission. [11] Estimates place the peak of perinatally transmitted HIV in the United States at 1651 cases in 1991.
According to the CDC, the number of US infants with perinatal HIV infection declined from 74 in 2010 to 32 in 2019. Perinatal HIV diagnosis rates decreased from 1.9 to 0.9 per 100,000 live births during the same period. Racial and ethnic differences persisted, however. Although rates of perinatal HIV infection for infants of Black mothers declined over the decade-long period, the diagnosis rate was 3.1 per 100,000 live births in 2019. [12, 13]
The CDC estimates that in 2009, in those 40 states, the number of pediatric HIV infections diagnosed was as follows [14] :
- Under age 13 years: 166
- Ages 13-14 years: 21
- Ages 15-19 years: 2036
In 2009, 12 cases of perinatally transmitted late HIV disease (AIDS) were diagnosed. The estimated cumulative number of perinatally transmitted AIDS cases diagnosed through 2009 is 8640.
At the end of 2008, 3022 children younger than 13 years were living with HIV infection in the 40 states with confidential name-based HIV infection reporting.
In the entire United States in 2009, an estimated 13 cases of AIDS were diagnosed in children younger than 13 years. The cumulative estimated number of diagnoses of AIDS in children younger than 13 years through 2009 in the United States is 9448.
In the United States, the number of new cases of pediatric AIDS is decreasing, mostly because of public health initiatives regarding universal HIV testing for pregnant women and use of zidovudine and other antiretroviral therapies in infected pregnant women and their newborn infants.
In 2007, 19 US children younger than 15 years died of HIV disease. [14] These numbers are in stark contrast to what is occurring internationally.
Adolescents and young adults
CDC HIV surveillance statistics from 2010 report that 25.7% (approximately 12,200 individuals) of new cases of HIV infection in the United States are in adolescents and young adults aged 13-24 years. Males accounted for 82.8% of new cases of HIV infection among this age group. Of these, 7000 (57.4%) were African Americans, 2390 (19.6%) were Latino, and 2380 (19.5%) were White. Male-to-male sexual contact accounted for 72.1% (8800 individuals). The percentage of youths tested for HIV infection was 12.9% in high-school students and 34.5% in individuals aged 18-24 years. Testing rates were lower in males than in females. More than half (59.5%) of youths with HIV infection are unaware of their infection. [5]
International statistics
The WHO estimates that over 33 million individuals are infected with HIV worldwide, and 90% of them are in developing countries. HIV has infected 4.4 million children and has resulted in the deaths of 3.2 million. Each day, 1800 children—the vast majority newborns—are infected with HIV. Approximately 7% of the population in sub-Saharan Africa is infected with HIV; these individuals represent 64% of the world's HIV-infected population. Furthermore, 76% of all women infected with HIV live in this region.
HIV-1 is the most common cause of HIV infection in the Americas, in Europe, in Asia, and in Africa. HIV-1 subtypes differ by geographic region. HIV-1 subtype B is predominant in the United States, though non-B subtype HIV-1 infections are increasing.
The HIV seroprevalence rate among pregnant women in South America is 0.3-5%; in sub-Saharan Africa, the range is 13-45%. In Europe, the HIV seroprevalence is greatest in western countries; France, Spain, and Italy have the highest incidences. Pregnant women in urban areas of these countries have a seroprevalence rate as high as 1%.
Although the annual number of new HIV infections has been steadily declining since the late 1990s, the epidemics in Eastern Europe and in Central Asia continue to grow; the number of people living with HIV in these regions reached an estimated 1.6 million in 2005—an increase of almost 20-fold in less than 10 years. [8] The overwhelming majority of these people living with HIV are young; 75% of infections reported between 2000 and 2004 were in people younger than 30 years. In Western Europe, the corresponding percentage was 33%.
The magnitude of the AIDS epidemic in Asia is significant. Although national HIV infection prevalence rates are low in Asia compared with other continents (notably Africa), the populations of many Asian nations are so large that even low prevalence rates reflect large numbers of people are living with HIV. The seroprevalence rate in pregnant women is already 2%, and the vertical transmission rate is 24% without breastfeeding. Indian mothers infected with HIV routinely breastfeed and have transmission rates as high as 48%.
Perinatal transmission rates are relatively low in Europe and high in Africa, independent of treatment. Untreated women infect 13% and 40% of children in Europe and Africa, respectively. The rate of postnatal transmission in Africa and other developing countries is elevated because of the need for breastfeeding.
HIV-1 is the most common cause of HIV infection in the Americas, Europe, Asia, and Africa. HIV type 2 (HIV-2) has caused epidemics in West Africa, though this virus is also found in European countries. HIV-1 subtypes differ by geographic region. Non-B subtypes are particularly prevalent in Africa and in Asia. The high transmission rate from Africa to Europe has increased the diversity of subtypes in Europe.
Globally, children outside the United States are not faring as well. Every day, 1400 children become HIV positive and 1000 children die of HIV-related causes. An estimated 2.5 million children worldwide younger than 15 years are living with HIV/AIDS. In sub-Saharan Africa alone, 1.9 million children are living with HIV/AIDS and more than 60% of all new HIV infections occur in women, infants, or young children. As of 2007, 90% of the newly infected children are infants who acquire HIV from their infected mothers. Alarmingly, 90% of babies who acquire the disease from infected mothers are found in sub-Saharan Africa. The prevalence of HIV infection among undernourished children has been estimated to be as high as 25%.
The prevalence of HIV infection in Asia and Europe varies considerably because of varied cultural practices and lack of a national reporting system in many areas. The commercial sex worker industry in countries such as Thailand and in the Caribbean Islands is responsible for increased HIV transmission to young girls and, vertically, to infants.
In 2004, more than half a million children younger than 15 years died from HIV/AIDS. In 2006, this number decreased to 380,000. In 2002, HIV/AIDS was the seventh leading cause of mortality in children in developing countries. The disease progresses rapidly in approximately 10-20% of children who are infected, and they die of AIDS by age 4 years, whereas 80-90% survive to a mean age of 9-10 years.
In affected regions of sub-Saharan Africa, the infant mortality rate has increased by 75% due, in part, to the orphaned status of most children. In contrast to much of the developed world, the mortality rates for children younger than 5 years are higher today than in 1990 in many African countries, mostly because of the devastating effects of HIV/AIDS.
A 2006 South African study estimated that HIV/AIDS is the single largest cause of infant and childhood deaths in rural South Africa. [15] HIV/AIDS is now responsible for 332,000 child deaths in sub-Saharan Africa, almost 8% of all child deaths in the region.
A systematic analysis of global data from 1990 to 2019 showed an increase in mortality in older children and adolescents with HIV infection. In 2019, HIV and AIDS caused 138,949 deaths among children and adolescents worldwide, primarily in areas of lower socioeconomic development. [16]
The results of one study noted that pneumonia and malnutrition are highly prevalent and are significantly associated with high rates of mortality among hospitalized, HIV-infected or HIV-exposed children in sub-Saharan Africa. Other independent predictors of death were septicemia, Kaposi sarcoma, meningitis, and esophageal candidiasis for HIV-infected children; and meningitis and severe anemia for inpatients exposed to HIV. These results stress the importance of expediently establishing therapeutic strategies in African pediatric hospitals. [17]
Racial differences in incidence
Black and Hispanic children are disproportionately infected in the United States. As of 2002, HIV infection was the 7th and 10th leading cause of death in Black children and in Hispanic teens, respectively. [18] Approximately 62% of children with AIDS are Black.
In the United States, children from minority communities have been most affected by AIDS. More than 50% of infected children are Black, and slightly less than 25% are Hispanic. Of the new childhood HIV cases in 2003, 68% occurred in African Americans. The number of pediatric AIDS cases reported in Black non-Hispanic children is 3.4 times higher than in White non-Hispanic children and is 2.6 times higher than that of Hispanic children.
Sexual differences in incidence
Women of childbearing age are one of the fastest growing groups with AIDS; 20% of AIDS cases in adults in the United States occur in this group.
Young people (aged 15-44 y) account for one of the fastest growing infected groups and account for almost half of all infections. Among young people, young women are more likely to become infected. In sub-Saharan Africa, more than two thirds of all youth infected are young girls. Variations in frequencies in the sexes in other regions of the world depend on the predominance of commercial sex workers and the proportion of a transient and mobile workforce more likely to be separated from family.
Age-related differences in incidence
Because vertical transmission from mother to child is the main route by which pediatric HIV infection is acquired, most children who are HIV positive should be identified in infancy. Although current treatment strategies can prevent vertical transmission, the drugs are simply not available in many places, especially in Africa.
Nevertheless, the age of presentation can be highly variable in a high-risk child who was previously unidentified. Children can be asymptomatic for many years, and the appearance of an opportunistic infection in a 10-year-old child or in an adolescent in whom AIDS is subsequently diagnosed is not rare. Children who acquire HIV by means of nonvertical transmission may have an illness during the acute phase of the retroviral syndrome, or they may present many years later with opportunistic or recurrent infections.
The CDC estimates that 50% of all new HIV infections in the United States occur among individuals aged 13-24 years. This is an important statistic that influences the mortality rates in young adults. For example, HIV is the 5th leading cause of death among Black women aged 20-24 years, and it is the principal cause of mortality in Black women aged 25-34 years.

Prognosis
Although HIV infection is usually deadly in children, especially in developing countries, the development of new antiretroviral drugs is promising. The lack of access to antiretroviral agents by children in developing countries is of particular concern.
The nutritional status of the child and the diligence with which viral replication is controlled are paramount in determining the outcome of most children with HIV disease.
Aggressive treatment of opportunistic infections prevents the more deleterious effects of secondary disease from progressing and further weakening the patient. The social setting and the stressors to which children are exposed have also been linked to the progression of the disease.
Hematologic disturbances, such as anemia, thrombocytopenia, and neutropenia, increase the risk of complications and death. Resolution of anemia improves the prognosis, and treatment of anemia with erythropoietin improves survival. Neutropenia significantly increases the risk of bacterial infection, and treatment of neutropenia with granulocyte colony-stimulating factor substantially decreases the risk of bacteremia and death.
Infection with Mycobacterium avium complex (MAC) hastens death, especially in patients with coexisting anemia (defined as a hematocrit < 25%).
The following factors are associated with rapidly progressive disease in infants:
- Advanced maternal disease
- High maternal viral load
- Low maternal CD4+ count
- Prematurity
- In utero transmission
- High viral load in the first 2 months of life
- Lack of neutralizing antibodies
- Presence of p24 antigen
- AIDS-defining illnesses
- Early cytomegalovirus (CMV) infection
- Early neurologic disease
- Failure to thrive
- Early-onset diarrhea
Each logarithmic decrease in the viral load after the start of therapy decreases the risk of progression by 54%.
Baseline CD4+ T-lymphocyte percentage and associated intermediate-term risk of death in HIV-infected children is as follows [19] :
- < 5%: 97%
- 5-9%: 76%
- 10-14%: 43%
- 15-19%: 44%
- 20-24%: 25%
- 25-29%: 31%
- 30-34%: 10%
- ≥35%: 33%
Baseline HIV RNA copy number (copies/mL) and associated intermediate-term risk for death in HIV-infected children is as follows [19] :
- Undetectable (ie, ≤4,000): 24%
- 4,001-50,000: 28%
- 50,001- 100,000: 15%
- 100,001- 500,000: 40%
- 500,001-1,000,00: 40%
- 1,000,000: 71%
The natural progression of vertically acquired HIV infection appears to have a trimodal distribution. Approximately 15% of children have rapidly progressive disease, and the remainder has either a chronic progressive course or an infection pattern typical of that observed in adults. Mean survival is about 10 years.
In resource-poor nations, the progression to death is accelerated. In some instances, close to 45-90% of HIV-infected children died by the age of 3 years. However, among children and adolescents, the start of combination therapy including protease inhibitors reduces the intermediate-term risk of death by an estimated 67%. Also, host genetics play an important role in HIV-1–related disease progression and neurologic impairment.
The patient's overall progression and prognosis is followed up by using the CDC classification system for children infected with HIV (see Staging).
A CDC study found that mortality rates for children aged younger than 5 years who are receiving antiretroviral therapy (ART) are higher than the rates for older children, adolescents, and adults living with HIV who are receiving ART. For example, the proportion of reported deaths among infants aged younger than 12 months is about 4-9 times higher than that among older children, adolescents, and adults. [20]

Patient Education
Educating parents regarding the importance of compliance with prescribed medications and health care visits is a major challenge because of many factors. See Deterrence/Prevention for further discussion about this topic.
Patients should be educated regarding the transmission of HIV. Increasing their awareness of the mechanism and consequences of HIV transmission is important. Safe social interactions that do not expose people to an increased risk for HIV transmission should also be emphasized.
A single-blinded, randomized controlled trial reported that adolescent family-centered pediatric advance care planning on HIV-specific symptoms was worthwhile in increasing and maintaining agreement about goals and length of care, which resulted in lowering physical symptoms and pain. [21]

- Brooks M. Study supports earlier initiation of HAART in HIV-infected children. Medscape Medical News. October 2, 2014. [Full Text].
- Yin DE, Warshaw MG, Miller WC, Castro H, Fiscus SA, Harper LM, et al. Using CD4 percentage and age to optimize pediatric antiretroviral therapy initiation. Pediatrics. 2014 Oct. 134(4):e1104-16. [QxMD MEDLINE Link].
- [Guideline] Guidelines for the use of antiretroviral agents in pediatric HIV infection. HIV.gov. Available at https://clinicalinfo.hiv.gov/en/guidelines/pediatric-arv/whats-new. 2024 Jun 27; Accessed: August 29, 2024.
- Prevalence and awareness of HIV infection among men who have sex with men --- 21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2010 Sep 24. 59(37):1201-7. [QxMD MEDLINE Link].
- Vital Signs: HIV Infection, Testing, and Risk Behaviors Among Youths - United States. MMWR Morb Mortal Wkly Rep. 2012 Nov 30. 61(47):971-6. [QxMD MEDLINE Link]. [Full Text].
- World Health Organization. Paediatric HIV and treatment of children living with HIV. Available at https://www.who.int/hiv/paediatric/en/index.html. Accessed: June 22, 2011.
- World Health Organization. Global summary of the AIDS epidemic: 2009. Available at https://www.who.int/hiv/data/2009_global_summary.png. Accessed: June 21, 2011.
- UNAIDS Report on the Global AIDS Epidemic 2010. Available at https://www.unaids.org/globalreport/Global_report.htm. Accessed: June 21, 2011.
- World Health Organization. Strategic Vision. World Health Organization. Available at https://www.who.int/hiv/pub/mtct/strategic_vision.pdf. Accessed: June 21, 2011.
- Centers for Disease Control and Prevention. Achievements in public health. Reduction in perinatal transmission of HIV infection--United States, 1985-2005. MMWR Morb Mortal Wkly Rep. 55(21):592-7. [QxMD MEDLINE Link]. [Full Text].
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report 2004. US Department of Health and Human Services, Centers for Disease Control and Prevention. 2005. Available at https://www.cdc.gov/hiv/topics/surveillance/resources/reports/2004report/pdf/2004SurveillanceReport.p.
- Lampe MA, Nesheim SR, Oladapo KL, Ewing AC, Wiener J, Kourtis AP. Achieving Elimination of Perinatal HIV in the United States. Pediatrics. 2023 May 1. 151 (5):[QxMD MEDLINE Link]. [Full Text].
- Schmidt A. Perinatal HIV Nearly Eradicated in US. Medscape Medical News. April 18, 2023. Available at https://www.medscape.com/viewarticle/990889.
- Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final data for 2007. National vital statistics reports; vol 58 no 19. Hyattsville, MD: National Center for Health Statistics. 2010. Available at https://www.cdc.gov/NCHS/data/nvsr/nvsr58/nvsr58_19.pdf. Accessed: June 21, 2011.
- Garrib A, Jaffar S, Knight S, Bradshaw D, Bennish ML. Rates and causes of child mortality in an area of high HIV prevalence in rural South Africa. Trop Med Int Health. 2006 Dec. 11(12):1841-8. [QxMD MEDLINE Link].
- GBD 2019 Child and Adolescent Communicable Disease Collaborators. The unfinished agenda of communicable diseases among children and adolescents before the COVID-19 pandemic, 1990-2019: a systematic analysis of the Global Burden of Disease Study 2019. Lancet. 2023 Jul 22. 402 (10398):313-35. [QxMD MEDLINE Link]. [Full Text].
- Preidis GA, McCollum ED, Mwansambo C, Kazembe PN, Schutze GE, Kline MW. Pneumonia and malnutrition are highly predictive of mortality among African children hospitalized with human immunodeficiency virus infection or exposure in the era of antiretroviral therapy. J Pediatr. 2011 Sep. 159(3):484-9. [QxMD MEDLINE Link].
- Kochanek KD, Xu JQ, Murphy SL, Miniño AM, Kung HC. Deaths: Preliminary Data for 2009. National Vital Statistics Reports. DHHS, National Center for Health Statistics. 2011. Available at https://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf.
- Guidelines for the use of antiretroviral agents in pediatric HIV infection. Center for Disease Control and Prevention. MMWR Recomm Rep. 1998 Apr 17. 47:1-43. [QxMD MEDLINE Link].
- Agathis NT, Faturiyele I, Agaba P, et al. Mortality Among Children Aged MMWR Morb Mortal Wkly Rep. 2023 Dec 1. 72 (48):1293-9. [QxMD MEDLINE Link]. [Full Text].
- Lyon ME, Garvie PA, D'Angelo LJ, Dallas RH, Briggs L, Flynn PM, et al. Advance Care Planning and HIV Symptoms in Adolescence. Pediatrics. 2018 Nov. 142 (5):[QxMD MEDLINE Link].
- Chiou CC, Groll AH, Gonzalez CE, Callender D, Venzon D, Pizzo PA, et al. Esophageal candidiasis in pediatric acquired immunodeficiency syndrome: clinical manifestations and risk factors. Pediatr Infect Dis J. 2000 Aug. 19(8):729-34. [QxMD MEDLINE Link].
- Brown DM, Jabra-Rizk MA, Falkler WA Jr, Baqui AA, Meiller TF. Identification of Candida dubliniensis in a study of HIV-seropositive pediatric dental patients. Pediatr Dent. 2000 May-Jun. 22(3):234-8. [QxMD MEDLINE Link].
- Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010 Jun 17. 362(24):2282-94. [QxMD MEDLINE Link].
- Lipshultz SE, Shearer WT, Thompson B, et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study). J Am Coll Cardiol. 2010 Dec 28. 57(1):76-85. [QxMD MEDLINE Link].
- Dias EP, Israel MS, Silva Junior A, Maciel VA, Gagliardi JP, Oliveira RH. Prevalence of oral hairy leukoplakia in 120 pediatric patients infected with HIV-1. Braz Oral Res. 2006 Apr-Jun. 20(2):103-7. [QxMD MEDLINE Link].
- Mohle-Boetani JC, Koehler JE, Berger TG, LeBoit PE, Kemper CA, Reingold AL, et al. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus: clinical characteristics in a case-control study. Clin Infect Dis. 1996 May. 22(5):794-800. [QxMD MEDLINE Link].
- Perry RT, Mmiro F, Ndugwa C, Semba RD. Measles infection in HIV-infected African infants. Ann N Y Acad Sci. 2000 Nov. 918:377-80. [QxMD MEDLINE Link].
- Enwonwu CO, Falkler WA Jr, Idigbe EO, Savage KO. Noma (cancrum oris): questions and answers. Oral Dis. 1999 Apr. 5(2):144-9. [QxMD MEDLINE Link].
- Jaquet D, Lévine M, Ortega-Rodriguez E, Faye A, Polak M, Vilmer E, et al. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000 Sep 29. 14(14):2123-8. [QxMD MEDLINE Link].
- Chiarelli F, Galli L, Verrotti A, di Ricco L, Vierucci A, de Martino M. Thyroid function in children with perinatal human immunodeficiency virus type 1 infection. Thyroid. 2000 Jun. 10(6):499-505. [QxMD MEDLINE Link].
- Smith KJ, Skelton HG 3rd, Vogel P, Yeager J, Baxter D, Wagner KF. Exaggerated insect bite reactions in patients positive for HIV. Military Medical Consortium for the Advancement of Retroviral Research. J Am Acad Dermatol. 1993 Aug. 29(2 Pt 1):269-72. [QxMD MEDLINE Link].
- Kest H, Brogly S, McSherry G, Dashefsky B, Oleske J, Seage GR 3rd. Malignancy in perinatally human immunodeficiency virus-infected children in the United States. Pediatr Infect Dis J. 2005 Mar. 24(3):237-42. [QxMD MEDLINE Link].
- Pongsiriwet S, Iamaroon A, Kanjanavanit S, Pattanaporn K, Krisanaprakornkit S. Oral lesions and dental caries status in perinatally HIV-infected children in Northern Thailand. Int J Paediatr Dent. 2003 May. 13(3):180-5. [QxMD MEDLINE Link].
- Ziegler JL, Katongole-Mbidde E. Kaposi's sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer. 1996 Jan 17. 65(2):200-3. [QxMD MEDLINE Link].
- Tofsky N, Nelson EM, Lopez RN, Catalanotto FA, Fine DH, Katz RV. Dental caries in HIV-infected children versus household peers: two-year findings. Pediatr Dent. 2000 May-Jun. 22(3):207-14. [QxMD MEDLINE Link].
- [Guideline] Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006 Sep 22. 55:1-17; quiz CE1-4. [QxMD MEDLINE Link]. [Full Text].
- Committee opinion no: 635: prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Obstet Gynecol. 2015 Jun. 125 (6):1544-7. [QxMD MEDLINE Link].
- Skwarecki B. ACOG updates recommendations for prenatal HIV testing. Medscape Medical News. Available at https://www.medscape.com/viewarticle/845416. May 27, 2015; Accessed: June 25, 2015.
- Adolescents and HIV Infection: The Pediatrician's Role in Promoting Routine Testing. Pediatrics. 2011 Nov. 128(5):1023-9. [QxMD MEDLINE Link].
- [Guideline] Guidelines for the Prevention and Treatment of Opportunistic Infections in Children With and Exposed to HIV. HIV.gov. Available at https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-pediatric-opportunistic-infections/whats-new. 2024 Jul 03; Accessed: August 29, 2024.
- [Guideline] Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009 Sep 4. 58:1-166. [QxMD MEDLINE Link]. [Full Text].
- Mirani G, Williams PL, Chernoff M, Abzug MJ, Levin MJ, Seage GR 3rd, et al. Changing Trends in Complications and Mortality Rates Among US Youth and Young Adults With HIV Infection in the Era of Combination Antiretroviral Therapy. Clin Infect Dis. 2015 Dec 15. 61 (12):1850-61. [QxMD MEDLINE Link]. [Full Text].
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008 Nov 20. 359(21):2233-44. [QxMD MEDLINE Link]. [Full Text].
- Boerner H. New HIV Remissions Capture the Imagination of Specialists. Medscape Medical News. Available at https://www.medscape.com/viewarticle/883386#vp_1. July 25, 2017; Accessed: July 28, 2017.
- Cotton M, Cassim H, Pavía-Ruz N, Garges HP, Perger T, Ford SL, et al. Pharmacokinetics, safety and antiviral activity of fosamprenavir/ritonavir-containing regimens in HIV-infected children aged 4 weeks to 2 years- 48 week study data. Pediatr Infect Dis J. 2013 Jul 9. [QxMD MEDLINE Link].
- Frange P, Briand N, Avettand-Fenoel V, et al. Lopinavir/Ritonavir-based Antiretroviral Therapy in Human Immunodeficiency Virus Type 1-infected Naive Children: Rare Protease Inhibitor Resistance Mutations But High Lamivudine/Emtricitabine Resistance at the Time of Virologic Failure. Pediatr Infect Dis J. 2011 Aug. 30(8):684-8. [QxMD MEDLINE Link].
- Lowes R. Tivicay Approved to Treat HIV-1 Infection. Medscape Medical News. Aug 12 2013. [Full Text].
- FDA. FDA approves new drug to treat HIV infection. Aug 12 2013. [Full Text].
- US Food and Drug Administration. New Isentress (raltegravir) dosage form: oral suspension. December 20, 2013. Available at https://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm379632.htm. Accessed: January 13, 2014.
- Merck & Co, Inc. Merck receives FDA Approval for Isentress (raltegravir) for pediatric oral suspension. January 8, 2014. Available at https://www.mercknewsroom.com/news-release/prescription-medicine-news/merck-receives-fda-approval-isentress-raltegravir-pediatric-. Accessed: January 13, 2014.
- Brooks M. FDA clears new formulation of raltegravir for infants. Medscape Medical News. January 9, 2014. [Full Text].
- Genvoya (elvitegravir/cobicistat/emtricitabine/tenofovir AF) [package insert]. Foster City, CA: Gilead Sciences, Inc. November 2015. Available at [Full Text].
- Complera (emtricitabine/rilpivirine/tenofovir DF) [package insert]. Foster City, CA: Gilead Sciences, Inc. February 2016. Available at [Full Text].
- Biktarvy (bictegravir/emtricitabine/tenofovir AF) [package insert]. Foster City, CA: Gilead Sciences, Inc. June 2019. Available at [Full Text].
- Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) [package insert]. Titusville, NJ: Janssen Therapeutics. March 2020. Available at [Full Text].
- Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010 Jun 17. 362(24):2271-81. [QxMD MEDLINE Link].
- Coovadia HM, Brown ER, Fowler MG, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012 Jan 21. 379(9812):221-8. [QxMD MEDLINE Link].
- van Dijk JH, Sutcliffe CG, Hamangaba F, Bositis C, Watson DC, Moss WJ. Effectiveness of Efavirenz-Based Regimens in Young HIV-Infected Children Treated for Tuberculosis: A Treatment Option for Resource-Limited Settings. PLoS One. 2013. 8(1):e55111. [QxMD MEDLINE Link]. [Full Text].
- Treating HIV-infected People with Antiretrovirals Protects Partners from Infection: Findings Result from NIH-funded International Study. News release May 12, 2011. Available at https://www.niaid.nih.gov/news/newsreleases/2011/pages/hptn052.aspx.
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3. 361(23):2209-20. [QxMD MEDLINE Link].
- Descovy (emtricitabine/tenofovir AF) [package insert]. Foster City, CA: Gilead Sciences. October, 2019. Available at [Full Text].
- MacNeil JR, Rubin LG, Patton M, Ortega-Sanchez IR, Martin SW. Recommendations for Use of Meningococcal Conjugate Vaccines in HIV-Infected Persons - Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016 Nov 4. 65 (43):1189-1194. [QxMD MEDLINE Link]. [Full Text].
- Benjamin DK Jr, Miller WC, Benjamin DK, Ryder RW, Weber DJ, Walter E, et al. A comparison of height and weight velocity as a part of the composite endpoint in pediatric HIV. AIDS. 2003 Nov 7. 17(16):2331-6. [QxMD MEDLINE Link].
- Brooks M. Efavirenz Gets Expanded Indication for HIV. Medscape Medical News. Available at https://www.medscape.com/viewarticle/803701. Accessed: May 15, 2013.
- [Guideline] World Health Organization. HIV and Infant Feeding. Revised Principles and Recommendations. World Health Organization. Available at https://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf. Accessed: June 29, 2011.
Author
Coauthor(s)
Richard E Frye, MD, PhD Professor of Child Health, University of Arizona College of Medicine at Phoenix; Chief of Neurodevelopmental Disorders, Director of Autism and Down Syndrome and Fragile X Programs, Division of Neurodevelopmental Disorders, Department of Neurology, Barrow Neurological Institute at Phoenix Children's Hospital
Richard E Frye, MD, PhD is a member of the following medical societies: American Academy of Neurology, American Academy of Pediatrics, Child Neurology Society
Disclosure: Nothing to disclose.
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.
Chief Editor
Russell W Steele, MD Clinical Professor, Tulane University School of Medicine; Staff Physician, Ochsner Clinic Foundation
Russell W Steele, MD is a member of the following medical societies: American Academy of Pediatrics, American Association of Immunologists, American Pediatric Society, American Society for Microbiology, Infectious Diseases Society of America, Louisiana State Medical Society, Pediatric Infectious Diseases Society, Society for Pediatric Research, Southern Medical Association
Disclosure: Nothing to disclose.
Acknowledgements
Mark Abdelmalek, MD Chief, Division of Laser and Dermatologic Surgery, Assistant Professor, Department of Dermatology, Drexel University College of Medicine
Mark Abdelmalek, MD is a member of the following medical societies: American Academy of Dermatology, American College of Mohs Micrographic Surgery and Cutaneous Oncology, American Medical Association, American Society for Dermatologic Surgery, Pennsylvania Academy of Dermatology, and Pennsylvania Medical Society
Disclosure: Nothing to disclose.
David F Butler, MD Professor of Dermatology, Texas A&M University College of Medicine; Chair, Department of Dermatology, Director, Dermatology Residency Training Program, Scott and White Clinic, Northside Clinic
David F Butler, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, American Medical Association, American Society for Dermatologic Surgery, American Society for MOHS Surgery, Association of Military Dermatologists, and Phi Beta Kappa
Disclosure: Nothing to disclose.
Joseph Domachowske, MD Professor of Pediatrics, Microbiology and Immunology, Department of Pediatrics, Division of Infectious Diseases, State University of New York Upstate Medical University
Joseph Domachowske, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Pediatrics, American Society for Microbiology, Infectious Diseases Society of America, Pediatric Infectious Diseases Society, and Phi Beta Kappa
Disclosure: Nothing to disclose.
Kathleen B Elmer, MD Consulting Staff, Department of Dermatology, First Medical Group, Langley Air Force Base
Disclosure: Nothing to disclose.
Dirk M Elston, MD Director, Ackerman Academy of Dermatopathology, New York
Dirk M Elston, MD is a member of the following medical societies: American Academy of Dermatology
Disclosure: Nothing to disclose.
Warren R Heymann, MD Head, Division of Dermatology, Professor, Department of Internal Medicine, University of Medicine and Dentistry of New Jersey-New Jersey Medical School
Warren R Heymann, MD is a member of the following medical societies: American Academy of Dermatology, American Society of Dermatopathology, and Society for Investigative Dermatology
Disclosure: Nothing to disclose.
William D James, MD Paul R Gross Professor of Dermatology, Vice-Chairman, Residency Program Director, Department of Dermatology, University of Pennsylvania School of Medicine
William D James, MD is a member of the following medical societies: American Academy of Dermatology and Society for Investigative Dermatology
Disclosure: Elsevier Royalty Other
Michael Loosemore, MD Fellow in Dermatological Surgery, The Methodist Hospital
Michael Loosemore, MD is a member of the following medical societies: American Academy of Dermatology, Massachusetts Medical Society, and Pennsylvania Academy of Dermatology
Disclosure: Nothing to disclose.
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Nothing to disclose.