Impetigo: Practice Essentials, Background, Pathophysiology (original) (raw)
Overview
Practice Essentials
Impetigo is the most common bacterial infection in children. This acute, highly contagious infection of the superficial layers of the epidermis is primarily caused by Streptococcus pyogenes or Staphylococcus aureus. Secondary skin infections of existing skin lesions (eg, cuts, abrasions, insect bites, chickenpox) can also occur. [1] Methicillin-resistant S aureus (MRSA) [2] and gentamicin-resistant S aureus strains have also been reported to cause impetigo. [3] Impetigo is classified as either nonbullous (impetigo contagiosa) (about 70% of cases [4] ) or bullous, as shown in the image below.
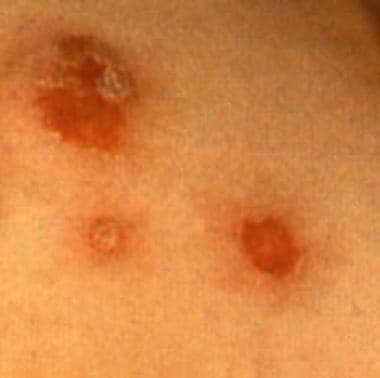
Bullous impetigo with circumscribed lesions with a thin collarette of scale.
Signs and symptoms
Children with nonbullous impetigo commonly have multiple coalescing lesions on their face (perioral, perinasal) and extremities or in areas with a break in the natural skin defense barrier. The initial lesions are small vesicles or pustules (< 2 cm) that rupture and become a honey-colored crust with a moist erythematous base. Pharyngitis is absent, but mild regional lymphadenopathy is commonly present. Nonbullous impetigo is usually a self-limited process that resolves within 2 weeks.
Bullous impetigo is considered to be less contagious than the nonbullous form. [5] It tends to affect the face, extremities, axillae, trunk, and perianal region of neonates, but older children and adults can also be infected. [4] The initial lesions are fragile thin-roofed, flaccid, and transparent bullae (< 3 cm) with a clear, yellow fluid that turns cloudy and dark yellow. Once the bullae rupture, they leave behind a rim of scale around an erythematous moist base but no crust, which is followed by a brown-lacquered or scalded-skin appearance, with a collarette of scale or a peripheral tubelike rim.
Bullous impetigo also differs from nonbullous impetigo in that bullous impetigo may involve the buccal mucous membranes, and regional adenopathy rarely occurs. However, extensive lesions in infants may be associated with systemic symptoms such as fever, malaise, generalized weakness, and diarrhea. Rarely, infants may present with signs of pneumonia, septic arthritis, or osteomyelitis.
See Clinical Presentation for more detail.
Diagnosis
The diagnosis of impetigo is usually made on the basis of the history and physical examination. However, bacterial culture and sensitivity can be used to confirm the diagnosis and are recommended in the following scenarios:
- When MRSA is suspected
- In the presence of an impetigo outbreak
Biopsy may be appropriate in doubtful or refractory cases of impetigo. [6]
See Workup for more detail.
Management
Treatment of impetigo typically involves local wound care in conjunction with either a topical antibiotic or a combination of systemic and topical agents. In general, the antibiotic selection has coverage against both S aureus and S pyogenes. In areas with a high prevalence of community-acquired MRSA with susceptible isolates, children older than 8 years may take clindamycin or doxycycline in cases. Trimethoprim-sulfamethoxazole can be used in situations in which group A streptococci are unlikely.
See Treatment and Medication for more detail.

Background
Impetigo is an acute, highly contagious gram-positive bacterial infection of the superficial layers of the epidermis. Skin lesions such as cuts, abrasions, and chickenpox can also become secondarily infected (impetiginized) with the same pathogens that produce classic impetigo.
Impetigo occurs most commonly in children, especially those who live in hot, humid climates. The name is believed to be derived from the Latin impetere (to assail).
Impetigo occurs in 2 forms: bullous and nonbullous, as shown in the photographs below. Nonbullous impetigo is the more common form, constituting approximately 70% of impetigo cases. [4] It tends to affect skin on the face or extremities that has been disrupted by bites, cuts, abrasions, other trauma, or diseases such as varicella. [1]
Bullous impetigo with circumscribed lesions with a thin collarette of scale.
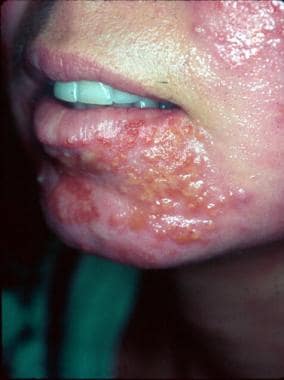
Following dermabrasion, this patient developed nonbullous impetigo in the same area as several herpes simplex lesions. Note the grouped vesicles which are classic for herpetic lesions, as well as additional crusted pink plaques. Concurrent bacterial impetigo can often be seen with herpes simplex and aerobic bacterial cultures are important to identify secondary impetigo.
Nonbullous impetigo, also known as impetigo contagiosa, is the most common skin infection in children, accounting for approximately 10% of all cutaneous problems in pediatric clinics. It is more contagious than the bullous type. [5] Common impetigo is the term applied when the infection occurs in preexisting wounds. Impetigo as a secondary infection of preexisting skin disease or traumatized skin has also been referred to as impetiginous dermatitis.
Nonbullous impetigo is caused by Staphylococcus aureus, group A beta hemolytic streptococci (GABHS, also known as Streptococcus pyogenes), or a combination of both. Most infections begin as a streptococcal infection, but staphylococci replace the streptococci over time.
Methicillin-resistant S aureus (MRSA), which can be hospital or community acquired, is an increasingly common cause of impetigo [2] ; this pathogen is observed more often with the nonbullous form of impetigo than the bullous form. Over the last decade, an increasing number of community-acquired MRSA and gentamicin-resistant S aureus strains have been reported as a cause of impetigo. [3]
Bullous impetigo may affect intact skin and is caused almost exclusively by S aureus. Bullous impetigo is a toxin-mediated erythroderma in which the epidermal layer of the skin sloughs, resulting in large areas of skin loss. Ecthyma is a deeper, ulcerated infection, often occurring with lymphadenitis that may be a complication of impetigo.
Impetigo seldom progresses to systemic infection, although poststreptococcal glomerulonephritis is a rare complication with GABHS infection only. Certain serotypes of GABHS (eg, types 49, 55, 57, 59) are associated with impetigo and acute glomerulonephritis.
Impetigo can also present as folliculitis, which is considered to be impetigo of the hair follicles caused by S aureus. Chronic recalcitrant impetigo/folliculitis can result in sycosis barbae (similar to lupoid sycosis) with scarring and a presentation similar to that of discoid lupus. Tinea may also cause this presentation.
Diagnosis of impetigo is usually based solely on the history and clinical appearance. Treatment typically involves local wound care, along with antibiotic therapy, either topical or systemic plus topical.

Pathophysiology
Intact skin is usually resistant to colonization or infection by S aureus or GABHS. These bacteria can be introduced from the environment and only transiently colonize the cutaneous surface. Experimental studies have shown that inoculation of multiple strains of GABHS on to the surface of subjects did not produce cutaneous disease unless skin disruption had occurred.
The teichoic acid adhesions for GABHS and S aureus require the epithelial cell receptor component, fibronectin, for colonization. These fibronectin receptors are unavailable on intact skin; however, skin disruption may reveal fibronectin receptors and allow for colonization or invasion in these disrupted surfaces. Factors that can modify the usual skin flora and facilitate transient colonization by GABHS and S aureus include high temperature or humidity, preexisting cutaneous disease, young age, or recent antibiotic treatment.
Common mechanisms for disruption of skin that can facilitate bacterial colonization or infection include the following:
- Scratching
- Atopic dermatitis
- Dermatophytosis
- Varicella
- Herpes simplex
- Scabies
- Pediculosis [7]
- Thermal burns
- Surgery
- Trauma
- Radiation therapy
- Insect bites
Immunosuppression by medications (eg, systemic corticosteroids, oral retinoids, chemotherapy), systemic diseases (eg, HIV infection, diabetes mellitus), intravenous drug abuse, and dialysis encourages bacterial growth.
After initial infection, new lesions may be seen in areas with no apparent break in the skin. Frequently, however, upon close examination, these lesions will demonstrate some underlying physical damage.
GABHS colonization
If an individual is in close contact with others (eg, household members, classmates, teammates) who have GABHS skin infection or who are carriers of the organism, the normal skin of that individual may be colonized. Once the healthy skin is colonized, minor trauma, such as abrasions or insect bites, may result in the development of impetigo lesions within 1-2 weeks.
GABHS can be detected in the nose and throat of some individuals 2-3 weeks after lesions develop, although they do not have symptoms of streptococcal pharyngitis. This is because impetigo and pharyngitis are caused by different strains of the bacteria. Impetigo is usually due to pattern D strains, whereas pharyngitis is due to pattern A, B, and C strains.
Staphylococcus aureus colonization
Approximately 30% of the population is colonized in the anterior nares by S aureus. Some individuals colonized by S aureus experience recurrent episodes of impetigo on the nose and lip. Bacteria can spread from the nose to healthy skin within 7-14 days, with impetigo lesions appearing 7-14 days later.
Approximately 10% of individuals are colonized with S aureus in the perineum and, more uncommonly, in the axillae, pharynx, and hands. Individuals who are permanent carriers serve as reservoirs of the infection for other people. Most healthy persons transiently harbor S aureus as part of their microbial flora. S aureus often passes from one individual to another through direct hand contact, entering through broken skin created by cutaneous diseases.
Patients with atopic dermatitis or other inflammatory skin conditions more commonly have skin colonized by S aureus. Studies have shown a 60-90% S aureus colonization rate in patients with atopic dermatitis. Patients with atopic dermatitis, particularly those with a history of eczema herpeticum, are at higher risk of developing an infection caused by MRSA.
One study found significantly lower expression of proteins related to the skin barrier and generation of natural moisturizing factor in lesional versus nonlesional sites in patients with atopic dermatitis. In addition, epidermal fatty acid–binding protein was expressed at significantly higher levels in patients colonized with MRSA, and this might perpetuate the inflammatory response through eicosanoid signaling. [8]
Bullous impetigo
Bullous impetigo (see the image below) is commonly due to exfoliative toxins of S aureus termed exfoliatins A and B. In 2006, exfoliative toxin D (ETD) was identified in 10% of S aureus isolates. [9] These exotoxins cause a loss of cell adhesion in the superficial dermis, which, in turn, causes blisters and skin sloughing by cleaving of the granular cell layer of the epidermis.
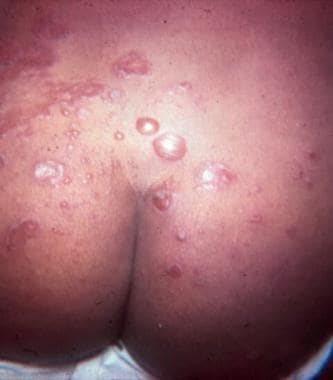
Bullous impetigo on the buttocks. Courtesy of Medical University of South Carolina, Department of Dermatology.
One of the target proteins for exotoxin A is desmoglein I, which maintains cell adhesion. These molecules are also superantigens that act locally and activate T lymphocytes. Coagulase may cause these toxins to remain localized within the upper epidermis by producing fibrin thrombi. Unlike nonbullous impetigo, the lesions of bullous impetigo occur on intact skin.
While the number of isolates of community- and hospital-acquired MRSA in lesions of impetigo remains low, this incidence has been increasing. Community-acquired MRSA can be differentiated from hospital-acquired MRSA. Most strains of community-acquired MRSA contain Panton-Valentine leucocidin (P-VL), a highly virulent, pore-forming exotoxin that causes dermal necrosis and has cytolytic activity against neutrophils and monocytes. Destruction of leukocytes by P-VL is one of the reasons that MRSA is more likely to produce clinical infection.
P-VL-positive S aureus strains are more frequently associated with cellulitis (38%) and abscesses (75%). [6, 10, 11, 12] In immunodeficient or immunocompromised patients, the toxin may disseminate hematogenously and lead to generalized staphylococcal scalded skin syndrome.

Etiology
Impetigo is caused by bacterial infection. Both S aureus and GABHS cause nonbullous impetigo; S aureus accounts for approximately 80% of cases, GABHS accounts for 10% of cases, and both organisms are recovered in 10% of cases. Bullous impetigo is caused almost exclusively by S aureus.
Nonbullous impetigo
Nonbullous impetigo can be caused by GABHS (types 49, 52, 53, 55-57, 59, 61) or by S aureus; in approximately 20-45% of cases, both agents are present. S aureus produces bacteriotoxins toxic to streptococci. These bacteriotoxins may be the reason that only S aureus is isolated in lesions that are caused predominantly by streptococci.
While in the past, GABHS and S aureus were equally frequent causative agents for nonbullous impetigo, currently S aureus accounts for 50-60% of cases. In developing nations and warm climates, however, GABHS is still the more common cause. [6]
Groups B, C, and G streptococci are rare causes of nonbullous impetigo. Group B streptococci are associated with impetigo in the newborn.
Bullous impetigo
Coagulase-positive group II S aureus, most often phage type 71, is the predominant causative organism. This strain of bacteria produces an exfoliating toxin that causes subcorneal epidermal cleavage and the condition known as Staphylococcal scalded skin syndrome (SSSS).
MRSA has been isolated in as many as 20% of bullous impetigo cases. [6] Methicillin resistance is found on the mecA gene, which has 4 elements, I-IV. Element IV is associated with community-acquired MRSA, and elements I-III are associated with hospital-acquired MRSA.
Among the risk factors for hospital-acquired MRSA are the following:
- Working in a health care center
- Hospitalization within the past year
- Residence in a long-term facility
- Having a chronic indwelling catheter or medical device
Community-acquired MRSA is a growing problem. Community-acquired MRSA is seen in greater frequency in closed populations in prisons, day care centers, and athletic teams, as well as in patients with diabetes or an underlying skin condition. The prevalence in these communities has been reported to be as high as 50%.

Epidemiology
United States statistics
Impetigo accounts for approximately 10% of skin problems observed in pediatric clinics. It is the most common bacterial skin infection and the third most common skin disease among children. [4]
Because it occurs more frequently in a warm, humid environment, impetigo is more common in the southeastern United States than in the cooler northern states. The prevalence of impetigo varies seasonally, with peak incidence during summer and fall [13] ; however, in regions that remain warm and humid throughout the year, seasonality may not occur.
International statistics
Impetigo occurs more frequently in tropical climates and at lower altitudes. Warm, humid conditions combined with frequent cutaneous disruption via biting insects favor its development throughout the year in tropical climates. Crowded conditions or poor hygiene also promote impetigo.
British statistics published in 1995 show an annual incidence of impetigo of 2.8% in children aged 4 years and younger and 1.69% in children aged 5-15 years. Impetigo has been reported as the third most frequent skin condition in children seen by general practitioners in the Netherlands, with a mean incidence of 10.8-22.2 cases per 1000 children per year, depending on the geographic region in the country. [14, 15]
A Dutch study reported an increase in the annual incidence in children younger than 18 years from 1.65% in 1987 to 2.06% in 2001. [7] In an observational study from Queensland, Australia, impetigo was diagnosed in 22 of 60 patients (37%) who presented with purulent skin infections; the median age was 19 years (range, 2 months to 91 years), and 38% of patients were male. [16]
A systematic review looking at the worldwide prevalence of scabies found that impetigo as a secondary bacterial skin infection to scabies was common, particularly in children, with the highest prevalence in Australian Aboriginal communities. [17, 18]
Race-, sex-, and age-related demographics
Impetigo can affect people of all races. Overall, the incidence in males and that in females are equal; in adults, however, impetigo is more common in men. Impetigo occurs in individuals of all ages but is most common in children 2-5 years of age. Rapid dissemination can occur through day care centers, nurseries, and grade schools.
Bullous impetigo is most common in neonates and infants; 90% of cases occur in children younger than 2 years. If premature rupture of membranes occurs during labor, lesions of impetigo may be present at birth. However, some authors suggest that statistics on bullous impetigo may be skewed because adult cases often go underreported.

Prognosis
Even without treatment, impetigo usually heals within 2-3 weeks. [19] Randomized placebo arms in prospective clinical trials have noted a 13-52% spontaneous resolution rate. [20] However, treatment produces a higher cure rate and reduces the spread of infection to other parts of the body (via inoculation) or to other people. [21, 22] Scarring is unusual, but postinflammatory hyperpigmentation or hypopigmentation may occur. Untreated lesions of nonbullous impetigo may rarely progress to ecthyma, a deep dermal infection, after which subsequent scarring can occur.
With appropriate treatment, lesions usually resolve after 7-10 days. If lesions persist beyond that point, cultures should be performed to look for resistant organisms. However, patients with eczema or an underlying parasitic infection may have a protracted course.
Complications
Beyond the neonatal period, patients who receive early and appropriate therapy have an excellent chance of recovery without complications. Neonates have a much higher incidence of developing a more generalized infection and meningitis.
Cellulitis, lymphangitis, and suppurative lymphadenitis may occur in as many as 10% of patients with impetigo. No correlation between the amount of impetiginous lesions and the involvement of the surrounding soft tissues, lymphatics, or regional lymph nodes has been observed. Cellulitis rarely follows bullous impetigo.
If the exfoliative toxins are absorbed into the bloodstream, staphylococcal scalded skin syndrome can result. This occurs more commonly in younger children, who have not developed antibodies against this toxin.
Acute poststreptococcal glomerulonephritis (APSGN) is a rare complication of nonbullous impetigo from nephritogenic strains of GABHS, with an annual incidence of less than 1 case per 1,000,000 population in developed countries. [22, 23, 24] The frequency of APSGN varies widely, depending on the strain of GABHS. Many GABHS strains have no nephritogenic potential, but types M-60 and M-49 cause APSGN in 70% and 25% of cases, respectively.
APSGN appears 18-21 days after infection. No difference between the clinical appearance of impetigo due to nephritogenic and impetigo due to nonnephritogenic strains has been observed. Children aged 3-7 years are most commonly affected. Treatment of impetigo with systemic antibiotics does not prevent the development of APSGN, most likely because activation of the immune response precedes antibiotic treatment.
Interestingly, in certain tropical and subtropical climates, skin infection is the most common predecessor of APSGN. Data from Pacific communities where rheumatic fever is endemic demonstrate increasing evidence that skin-associated strains of group A streptococcal organisms are being linked to cases of rheumatic fever. [25] Uncommon complications include the following [4, 26] :
- Scarlet fever
- Erysipelas
- Guttate psoriasis
- Pneumonia
- Osteomyelitis
- Septic arthritis
- Bacterial endocarditis
Rarely, pedal edema and hypertension may be noted in a patient with nonbullous impetigo. These are signs of renal dysfunction most likely resulting from poststreptococcal glomerulonephritis. Most often, these are children aged 2-4 years. The onset is usually 10 days after impetigo lesions first appear, but it can occur from 1-5 weeks later.
The onset of poststreptococcal glomerulonephritis is usually 10 days after impetigo lesions first appear, but it can occur from 1-5 weeks later. Transient proteinuria and hematuria may occur during impetigo and resolve before renal involvement develops. Antibiotic treatment does not prevent the development of glomerulonephritis, but it limits the spread of the disease to other individuals.
Other complications may include the following:
- Staphylococcal scalded skin syndrome (SSSS)
- Septicemia
- Cellulitis
- Erysipelas
- Erythema multiforme
- Urticaria

Patient Education
Discourage touching the lesions. Inform patients about early and proper care of predisposing factors (eg, insect bites, minor trauma). Recommend that patients properly cleanse and apply a topical antibiotic to minor skin traumas.
Because of the contagious nature, children should not return to daycare or school until 24 hours after the initiation of appropriate antimicrobial therapy. [27] Caretakers should be instructed about hygienic issues and prevention. This infection is transmitted by direct contact and by fomites, including hygiene items, clothing, and toys.
For patient education information, see the Infections Center and Skin Conditions & Beauty Center, as well as Impetigo, Skin Rashes in Children, and Antibiotics.

- Moulin F, Quinet B, Raymond J, Gillet Y, Cohen R. [Managing children skin and soft tissue infections]. Arch Pediatr. 2008 Oct. 15 Suppl 2:S62-7. [QxMD MEDLINE Link].
- Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infect Dis. 2005 Jun. 11(6):928-30. [QxMD MEDLINE Link].
- Kuniyuki S, Nakano K, Maekawa N, Suzuki S. Topical antibiotic treatment of impetigo with tetracycline. J Dermatol. 2005 Oct. 32(10):788-92. [QxMD MEDLINE Link].
- Cole C, Gazewood J. Diagnosis and treatment of impetigo. Am Fam Physician. 2007 Mar 15. 75(6):859-64. [QxMD MEDLINE Link].
- Hirschmann JV. Impetigo: etiology and therapy. Curr Clin Top Infect Dis. 2002. 22:42-51. [QxMD MEDLINE Link].
- Geria AN, Schuartz RA. Impetigo Update: New Challenges in the Era of Methicillin Resistance. Cutis. 2010. 85(2):65-70.
- Treating impetigo in primary care. Drug Ther Bull. 2007 Jan. 45(1):2-4. [QxMD MEDLINE Link].
- Broccardo CJ, Mahaffey S, Schwarz J, et al. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J Allergy Clin Immunol. 2011 Jan. 127(1):186-93, 193.e1-11. [QxMD MEDLINE Link]. [Full Text].
- Yamasaki O, Tristan A, Yamaguchi T, et al. Distribution of the exfoliative toxin D gene in clinical Staphylococcus aureus isolates in France. Clin Microbiol Infect. 2006 Jun. 12(6):585-8. [QxMD MEDLINE Link].
- Daskalaki M, Rojo P, Marin-Ferrer M, Barrios M, Otero JR, Chaves F. Panton-Valentine leukocidin-positive Staphylococcus aureus skin and soft tissue infections among children in an emergency department in Madrid, Spain. Clin Microbiol Infect. 2010 Jan. 16(1):74-7. [QxMD MEDLINE Link].
- Geng W, Yang Y, Wu D, et al. Molecular characteristics of community-acquired, methicillin-resistant Staphylococcus aureus isolated from Chinese children. FEMS Immunol Med Microbiol. 2010 Apr. 58(3):356-62. [QxMD MEDLINE Link].
- Liu Y, Kong F, Zhang X, Brown M, Ma L, Yang Y. Antimicrobial susceptibility of Staphylococcus aureus isolated from children with impetigo in China from 2003 to 2007 shows community-associated methicillin-resistant Staphylococcus aureus to be uncommon and heterogeneous. Br J Dermatol. 2009 Dec. 161(6):1347-50. [QxMD MEDLINE Link].
- Loffeld A, Davies P, Lewis A, Moss C. Seasonal occurrence of impetigo: a retrospective 8-year review (1996-2003). Clin Exp Dermatol. 2005 Sep. 30(5):512-4. [QxMD MEDLINE Link].
- Koning S, Verhagen AP, van Suijlekom-Smit LW, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2004. CD003261. [QxMD MEDLINE Link].
- Razmjou RG, Willemsen SP, Koning S, et al. Determinants of regional differences in the incidence of impetigo. Environ Res. 2009 Jul. 109(5):590-3. [QxMD MEDLINE Link].
- Spurling G, Askew D, King D, Mitchell GK. Bacterial skin infections--an observational study. Aust Fam Physician. 2009 Jul. 38(7):547-51. [QxMD MEDLINE Link].
- Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015 Aug. 15 (8):960-7. [QxMD MEDLINE Link].
- Tasani M, Tong SY, Andrews RM, Holt DC, Currie BJ, Carapetis JR, et al. The Importance of Scabies Coinfection in the Treatment Considerations for Impetigo. Pediatr Infect Dis J. 2016 Apr. 35 (4):374-8. [QxMD MEDLINE Link].
- Patrizi A, Raone B, Savoia F, Ricci G, Neri I. Recurrent toxin-mediated perineal erythema: eleven pediatric cases. Arch Dermatol. 2008 Feb. 144(2):239-43. [QxMD MEDLINE Link].
- Bangert S, Levy M, Hebert AA. Bacterial resistance and impetigo treatment trends: a review. Pediatr Dermatol. 2012 May-Jun. 29(3):243-8. [QxMD MEDLINE Link].
- George A, Rubin G. A systematic review and meta-analysis of treatments for impetigo. Br J Gen Pract. 2003 Jun. 53(491):480-7. [QxMD MEDLINE Link]. [Full Text].
- Koning S, van der Sande R, Verhagen AP, van Suijlekom-Smit LW, Morris AD, Butler CC, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012 Jan 18. 1:CD003261. [QxMD MEDLINE Link].
- George A, Rubin G. A systematic review and meta-analysis of treatments for impetigo. Br J Gen Pract. 2003 Jun. 53(491):480-7. [QxMD MEDLINE Link]. [Full Text].
- Brown J, Shriner DL, Schwartz RA, Janniger CK. Impetigo: an update. Int J Dermatol. 2003 Apr. 42(4):251-5. [QxMD MEDLINE Link].
- Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis. 2012 Apr. 25(2):145-53. [QxMD MEDLINE Link].
- Mancini AJ. Bacterial skin infections in children: the common and the not so common. Pediatr Ann. 2000 Jan. 29(1):26-35. [QxMD MEDLINE Link].
- American Academy of Pediatrics. Group A Streptococcal infections. Pickering LK, ed. Red Book: 2012 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2015. 732-744.
- Ludlam H, Cookson B. Scrum kidney: epidemic pyoderma caused by a nephritogenic Streptococcus pyogenes in a rugby team. Lancet. 1986 Aug 9. 2(8502):331-3. [QxMD MEDLINE Link].
- Belgemen T, Suskan E, Dogu F, Ikinciogullari A. Selective immunoglobulin M deficiency presenting with recurrent impetigo: a case report and review of the literature. Int Arch Allergy Immunol. 2009. 149(3):283-8. [QxMD MEDLINE Link].
- Sarabi K, Khachemoune A. Tinea capitis: a review. Dermatol Nurs. 2007 Dec. 19(6):525-9; quiz 530. [QxMD MEDLINE Link].
- Popovich D, McAlhany A. Accurately diagnosing commonly misdiagnosed circular rashes. Dermatol Nurs. 2008 Aug. 20(4):294-300. [QxMD MEDLINE Link].
- Gorani A, Oriani A, Cambiaghi S. Seborrheic dermatitis-like tinea faciei. Pediatr Dermatol. 2005 May-Jun. 22(3):243-4. [QxMD MEDLINE Link].
- Hayakawa K, Hirahara K, Fukuda T, Okazaki M, Shiohara T. Risk factors for severe impetiginized atopic dermatitis in Japan and assessment of its microbiological features. Clin Exp Dermatol. 2009 Jul. 34(5):e63-5. [QxMD MEDLINE Link].
- Rashid R, Hymes S. Folliculitis, follicular mucinosis, and papular mucinosis as a presentation of chronic myelomonocytic leukemia. Dermatol Online J. 2009 May 15. 15(5):16. [QxMD MEDLINE Link].
- Gorwitz RJ. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and update. Pediatr Infect Dis J. 2008 Oct. 27(10):925-6. [QxMD MEDLINE Link].
- [Guideline] Rush, J and Dinulos JG. Childhood skin and soft tissue infections: new discoveries and guidelines regarding the management of bacterial soft tissue infections, molluscum contagiousum, and warts. Current Opinion in Pediatrics. April 2016. 28(2):250-257.
- [Guideline] Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014 Jul 15. 59 (2):147-59. [QxMD MEDLINE Link].
- American Academy of Pediatrics. Staphylococcal infections. Pickering LK, ed. Red Book: 2012 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015. 715-732.
- Scheinfeld N. A Primer In Topical Antibiotics For The Skin And Eyes. J Drugs Dermatol. 2008. 7(4):409-415.
- Wilkinson RD, Carey WD. Topical mupirocin versus topical neosporin in the treatment of cutaneous infections. Int J Dermatol. 1988 Sep. 27(7):514-5. [QxMD MEDLINE Link].
- Bass JW, Chan DS, Creamer KM, et al. Comparison of oral cephalexin, topical mupirocin and topical bacitracin for treatment of impetigo. Pediatr Infect Dis J. 1997 Jul. 16(7):708-10. [QxMD MEDLINE Link].
- Silverberg N, Block S. Uncomplicated skin and skin structure infections in children: diagnosis and current treatment options in the United States. Clin Pediatr (Phila). 2008 Apr. 47(3):211-9. [QxMD MEDLINE Link].
- Koning S, van der Wouden JC, Chosidow O, et al. Efficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre placebo-controlled trial. Br J Dermatol. 2008 May. 158(5):1077-82. [QxMD MEDLINE Link].
- Jacobs MR. Retapamulin: a semisynthetic pleuromutilin compound for topical treatment of skin infections in adults and children. Future Microbiol. 2007 Dec. 2(6):591-600. [QxMD MEDLINE Link].
- Jones RS. Expert advice on erasing the MRSA threat. Pract Dermatol. 2005. 34-7.
- Drucker CR. Update on topical antibiotics in dermatology. Dermatol Ther. 2012 Jan-Feb. 25(1):6-11. [QxMD MEDLINE Link].
- Woodford N, Afzal-Shah M, Warner M, Livermore DM. In vitro activity of retapamulin against Staphylococcus aureus isolates resistant to fusidic acid and mupirocin. J Antimicrob Chemother. 2008 Oct. 62(4):766-8. [QxMD MEDLINE Link].
- Boyd B, Castañar J. Retapamulin. Drugs Future. 2006. 31:107.
- Oranje AP, Chosidow O, Sacchidanand S, et al. Topical retapamulin ointment, 1%, versus sodium fusidate ointment, 2%, for impetigo: a randomized, observer-blinded, noninferiority study. Dermatology. 2007. 215(4):331-40. [QxMD MEDLINE Link].
- Drug and Therapeutics Bulletin. Retapamulin for impetigo and other infections. Drug Ther Bull. 2008 Oct. 46(10):76-9. [QxMD MEDLINE Link].
- Denton M, O'Connell B, Bernard P, Jarlier V, Williams Z, Henriksen AS. The EPISA study: antimicrobial susceptibility of Staphylococcus aureus causing primary or secondary skin and soft tissue infections in the community in France, the UK and Ireland. J Antimicrob Chemother. 2008 Mar. 61(3):586-8. [QxMD MEDLINE Link].
- O'Neill AJ, Larsen AR, Skov R, Henriksen AS, Chopra I. Characterization of the epidemic European fusidic acid-resistant impetigo clone of Staphylococcus aureus. J Clin Microbiol. 2007 May. 45(5):1505-10. [QxMD MEDLINE Link]. [Full Text].
- Laurent F, Tristan A, Croze M, et al. Presence of the epidemic European fusidic acid-resistant impetigo clone (EEFIC) of Staphylococcus aureus in France. J Antimicrob Chemother. 2009 Feb. 63(2):420-1; author reply 421. [QxMD MEDLINE Link].
- Alsterholm M, Flytström I, Bergbrant IM, Faergemann J. Fusidic acid-resistant Staphylococcus aureus in impetigo contagiosa and secondarily infected atopic dermatitis. Acta Derm Venereol. 2010. 90(1):52-7. [QxMD MEDLINE Link].
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther. 2008 May-Jun. 21(3):187-95. [QxMD MEDLINE Link].
- Langner A, Chu A, Goulden V, Ambroziak M. A randomized, single-blind comparison of topical clindamycin + benzoyl peroxide and adapalene in the treatment of mild to moderate facial acne vulgaris. Br J Dermatol. 2008 Jan. 158(1):122-9. [QxMD MEDLINE Link].
- Capizzi R, Landi F, Milani M, Amerio P. Skin tolerability and efficacy of combination therapy with hydrogen peroxide stabilized cream and adapalene gel in comparison with benzoyl peroxide cream and adapalene gel in common acne. A randomized, investigator-masked, controlled trial. Br J Dermatol. 2004 Aug. 151(2):481-4. [QxMD MEDLINE Link].
- Albareda, N, Rosenberg, N., Roth, S, et al. Ozenoxacin, a Novel, Topical Antibacterial Agent for Treatment of Adult and Pediatric Patients with Impetigo: Phase III Clinical Trials Pooled Analysis Results. SKIN The Journal of Cutaneous Medicine. 2017. 1(3.1):s102. [Full Text].
- Gropper S, Albareda N, Chelius K, Kruger D, Mitha I, Vahed Y, et al. Ozenoxacin 1% cream in the treatment of impetigo: a multicenter, randomized, placebo- and retapamulin-controlled clinical trial. Future Microbiol. 2014. 9 (9):1013-23. [QxMD MEDLINE Link].
- Chamny S, Miron D, Lumelsky N, Shalev H, Gazal E, Keynan R, et al. Topical Minocycline Foam for the Treatment of Impetigo in Children: Results of a Randomized, Double-Blind, Phase 2 Study. J Drugs Dermatol. 2016 Oct 1. 15 (10):1238-1243. [QxMD MEDLINE Link].
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008 Feb. 81(2):115-22. [QxMD MEDLINE Link].
- Bernard P. Management of common bacterial infections of the skin. Curr Opin Infect Dis. 2008 Apr. 21(2):122-8. [QxMD MEDLINE Link].
- Romani L, Whitfeld MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. Mass Drug Administration for Scabies Control in a Population with Endemic Disease. N Engl J Med. 2015 Dec 10. 373 (24):2305-13. [QxMD MEDLINE Link].
- Garcia J. Mass Treatment With Ivermectin Decreases Scabies Prevalence. Medscape Medical News. Available at https://www.medscape.com/viewarticle/856375. December 22, 2015; Accessed: April 1, 2016.
- Yang LP, Keam SJ. Retapamulin: a review of its use in the management of impetigo and other uncomplicated superficial skin infections. Drugs. 2008. 68(6):855-73. [QxMD MEDLINE Link].
- Rist T, Parish LC, Capin LR, Sulica V, Bushnell WD, Cupo MA. A comparison of the efficacy and safety of mupirocin cream and cephalexin in the treatment of secondarily infected eczema. Clin Exp Dermatol. 2002 Jan. 27(1):14-20. [QxMD MEDLINE Link].
- Bullous impetigo with circumscribed lesions with a thin collarette of scale.
- Bullous impetigo on the buttocks. Courtesy of Medical University of South Carolina, Department of Dermatology.
- Following dermabrasion, this patient developed nonbullous impetigo in the same area as several herpes simplex lesions. Note the grouped vesicles which are classic for herpetic lesions, as well as additional crusted pink plaques. Concurrent bacterial impetigo can often be seen with herpes simplex and aerobic bacterial cultures are important to identify secondary impetigo.
- A nummular eczema lesion on the knee, impetiginized with Staphylococcus aureus.


Author
Coauthor(s)
Sahithi Talasila, BS MD Candidate, Sidney Kimmel Medical College of Thomas Jefferson University
Sahithi Talasila, BS is a member of the following medical societies: American Medical Student Association/Foundation
Disclosure: Nothing to disclose.
Chief Editor
Russell W Steele, MD Clinical Professor, Tulane University School of Medicine; Staff Physician, Ochsner Clinic Foundation
Russell W Steele, MD is a member of the following medical societies: American Academy of Pediatrics, American Association of Immunologists, American Pediatric Society, American Society for Microbiology, Infectious Diseases Society of America, Louisiana State Medical Society, Pediatric Infectious Diseases Society, Society for Pediatric Research, Southern Medical Association
Disclosure: Nothing to disclose.
Additional Contributors
Lisa S Lewis, MD Attending Physician, Division of Emergency Medicine, Cincinnati Children's Hospital Medical Center
Lisa S Lewis, MD is a member of the following medical societies: American Academy of Pediatrics
Disclosure: Nothing to disclose.
Acknowledgements
Sadegh Amini, MD Senior Clinical Research Fellow, Skin Research Group, Department of Dermatology and Cutaneous Surgery, Miller School of Medicine, University of Miami
Sadegh Amini, MD is a member of the following medical societies: American Society for Dermatologic Surgery, International Society for Dermatologic Surgery, and International Society of Dermatology
Disclosure: Nothing to disclose.
Anne E Burdick, MD, MPH Professor of Dermatology, Director of Leprosy Program, Associate Dean for TeleHealth and Clinical Outreach, University of Miami Miller School of Medicine
Anne E Burdick, MD, MPH is a member of the following medical societies: Women's Dermatologic Society
Disclosure: Nothing to disclose.
Ivan D Camacho, MD, Assistant Professor of Clinical Dermatology, Department of Dermatology and Cutaneous Surgery, University of Miami, Leonard M Miller School of Medicine; Medical Director of Dermatology Clinic, Jackson Memorial
Ivan D Camacho, MD is a member of American Academy of Dermatology, American Medical Association, American Society for Dermatologic Surgery, American Society for MOHS Surgery, Florida Medical Association, International Society of Dermatology, and the Women's Dermatologic Society.
Disclosure: Nothing to disclose.
Burke A Cunha, MD Professor of Medicine, State University of New York School of Medicine at Stony Brook; Chief, Infectious Disease Division, Winthrop-University Hospital
Burke A Cunha, MD is a member of the following medical societies: American College of Chest Physicians, American College of Physicians, and Infectious Diseases Society of America
Disclosure: Nothing to disclose.
Joseph Domachowske, MD Professor of Pediatrics, Microbiology and Immunology, Department of Pediatrics, Division of Infectious Diseases, State University of New York Upstate Medical University
Joseph Domachowske, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Pediatrics, American Society for Microbiology, Infectious Diseases Society of America, Pediatric Infectious Diseases Society, and Phi Beta Kappa
Disclosure: Nothing to disclose.
Pamela L Dyne, MD Professor of Clinical Medicine/Emergency Medicine, David Geffen School of Medicine at UCLA; Attending Physician, Department of Emergency Medicine, Olive View-UCLA Medical Center
Pamela L Dyne, MD is a member of the following medical societies: American Academy of Emergency Medicine, American College of Emergency Physicians, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Dirk M Elston, MD Director, Ackerman Academy of Dermatopathology, New York
Dirk M Elston, MD is a member of the following medical societies: American Academy of Dermatology
Disclosure: Nothing to disclose.
Glenn J Fennelly, MD, MPH Director, Division of Pediatric Infectious Diseases, Jacobi Medical Center; Associate Professor, Department of Pediatrics, Albert Einstein College of Medicine
Glenn J Fennelly, MD, MPH is a member of the following medical societies: Pediatric Infectious Diseases Society
Disclosure: Nothing to disclose.
Allan D Friedman, MD, MPH (Retired) Chairman, Division of General Pediatrics, VCUH Health System; Professor of Pediatrics, Virginia Commonwealth University School of Medicine
Allan D Friedman, MD, MPH is a member of the following medical societies: American Academy of Pediatrics
Disclosure: Nothing to disclose.
Eric M Kardon, MD, FACEP Attending Emergency Physician, Georgia Emergency Medicine Specialists; Physician, Division of Emergency Medicine, Athens Regional Medical Center
Eric M Kardon, MD, FACEP is a member of the following medical societies: American College of Emergency Physicians
Disclosure: Nothing to disclose.
Paul Krusinski, MD Director of Dermatology, Fletcher Allen Health Care; Professor, Department of Internal Medicine, University of Vermont College of Medicine
Paul Krusinski, MD is a member of the following medical societies: American Academy of Dermatology, American College of Physicians, and Society for Investigative Dermatology
Disclosure: Nothing to disclose.
Andrew C Miller, MD Fellow, Department of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh Medical Center; Attending Physician, Department of Emergency Medicine, University of Pittsburgh Medical Center
Andrew C Miller, MD is a member of the following medical societies: American College of Emergency Physicians, American Medical Association, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
James J Nordlund, MD Professor Emeritus, Department of Dermatology, University of Cincinnati College of Medicine
James J Nordlund, MD is a member of the following medical societies: American Academy of Dermatology, Sigma Xi, and Society for Investigative Dermatology
Disclosure: Nothing to disclose.
Rashid M Rashid, MD, PhD Resident Physician, Department of Dermatology, University of Texas, Houston, MD Anderson Cancer Center, and Morzak Research Initiative
Rashid M Rashid, MD, PhD is a member of the following medical societies: American Academy of Dermatology, Council for Nail Disorders, Houston Dermatological Society, Texas Dermatological Society, and Texas Medical Association
Disclosure: Nothing to disclose.
John Ratz, MD, MBA Staff Dermatologist, Mohs Surgeon, Center for Dermatology and Skin Surgery, Inc
John Ratz, MD, MBA is a member of the following medical societies: American Academy of Dermatology, American College of Mohs Micrographic Surgery and Cutaneous Oncology, American College of Physicians, American Society for Dermatologic Surgery, American Society for Laser Medicine and Surgery, International Society for Dermatologic Surgery, and Southern Medical Association
Disclosure: Nothing to disclose.
Gregory William Rutecki, MD Associate Professor, Program Director, Department of Internal Medicine, Feinberg School of Medicine, Northwestern University
Gregory William Rutecki, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Physicians, American Society of Nephrology, National Kidney Foundation, and Society of General Internal Medicine
Disclosure: Nothing to disclose.
Mark A Silverberg, MD, FACEP, MMB Assistant Professor, Assistant Residency Director, Department of Emergency Medicine, State University of New York Downstate College of Medicine; Consulting Staff, Department of Emergency Medicine, Staten Island University Hospital, Kings County Hospital, University Hospital, State University of New York Downstate at Brooklyn
Mark A Silverberg, MD, FACEP, MMB is a member of the following medical societies: American College of Emergency Physicians, American Medical Association, Council of Emergency Medicine Residency Directors, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Daniel B Ward Jr, MD Clinical Assistant Professor, Department of Dermatology and Dermatologic Surgery, Medical University of South Carolina
Daniel B Ward Jr, MD is a member of the following medical societies: American Academy of Dermatology, American Medical Association, and South Carolina Medical Association
Disclosure: Nothing to disclose.
Eric L Weiss, MD, DTM&H Director of Stanford Travel Medicine, Medical Director of Stanford Lifeflight, Assistant Professor, Departments of Emergency Medicine and Infectious Diseases, Stanford University School of Medicine
Eric L Weiss, MD, DTM&H is a member of the following medical societies: American College of Emergency Physicians, American College of Occupational and Environmental Medicine, American Medical Association, American Society of Tropical Medicine and Hygiene, Physicians for Social Responsibility, Southeastern Surgical Congress, Southern Association for Oncology, Southern Clinical Neurological Society, and Wilderness Medical Society
Disclosure: Nothing to disclose.
Michael J Wells, MD Associate Professor, Department of Dermatology, Texas Tech University Health Sciences Center, Paul L Foster School of Medicine
Michael J Wells, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Dermatology, American Medical Association, and Texas Medical Association
Disclosure: Nothing to disclose.
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Pharmacy Editor, Medscape Reference
Disclosure: Nothing to disclose.