Chronic Kidney Disease in Children Treatment & Management: Approach Considerations, Anemia Management, Management of Bone Disease (original) (raw)
Approach Considerations
Patients with chronic kidney disease (CKD) should be evaluated to determine the following:
- Diagnosis (type of kidney disease)
- Comorbid conditions (such as hyperlipidemia)
- Severity, which based on level of kidney function
- Complications, related to level of kidney function
- Risk for loss of kidney function
- Risk for cardiovascular disease
Treatment of chronic kidney disease should include the following:
- Specific therapy based on diagnosis
- Evaluation and management of reversible causes of renal dysfunction
- Prevention and treatment of complications of decreased kidney function (eg, anemia, bone disease, cardiovascular manifestations, hypertension, growth failure)
- Evaluation and management of comorbid conditions
- Slowing the loss of kidney function
- Preparation for kidney failure therapy
- Replacement of kidney function with dialysis and transplantation if signs and symptoms of uremia are present
- Management of complications
Evaluation of reversible causes of renal dysfunction
Every physician caring for patients with chronic kidney failure must determine the various factors or clinical states that may have aggravated or exacerbated the degree of kidney failure. Once these factors are corrected or reversed, the severity of kidney failure may improve, and kidney function may return to stable basal level of function. The common reversible causes include volume depletion, drugs (nonsteroidal anti-inflammatory drugs [NSAIDs], contrast agents), infection, and congestive heart failure.
Retarding progression of renal disease
In adults with chronic kidney disease, interventions to slow the progression of kidney disease that have been proven to be effective include strict blood pressure control and angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor–blocker (ARB) therapy, lipid lowering therapy, and correction of anemia. In these patients, aggressive goals are recommended for both proteinuria and blood pressure. In addition, antihypertensive therapy is used for both renal protection and cardiovascular protection, because chronic kidney disease is associated with a marked increase in cardiovascular risk.
Consultations
According to the recommendations of the Pediatric Work Group of Kidney Disease Outcomes Quality Initiative (KDOQI) for chronic kidney disease (CKD), all children with evidence of CKD should be referred to a pediatric nephrologist for consultation and comanagement. [7]
Transfer
Patients with any complications require transfer to a center with a pediatric nephrology unit where acute dialysis can be performed if required.

Anemia Management
The presence of anemia 1 month after dialysis initiation is associated with an increased risk of prolonged hospitalization and death in pediatric patients. The beneficial effects of treating anemia with erythropoietin in patients who are dialysis-dependent include the improvement of cardiac status, exercise capacity, cognitive function, and quality of life. Recombinant human erythropoietin (rHuEPO) has been used for chronic kidney disease (CKD)–associated anemia since 1986. Based on the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, the recommended target hemoglobin-to-hematocrit (Hgb/Hct) ratio is 11-12 g/dL / 33-36%. [25]
Iron supplementation is essential to ensure an adequate response to erythropoietin. This is targeted to maintain a transferrin saturation level of 20% or higher and serum ferritin level of 100 ng/dL or higher in children with chronic kidney disease. The pediatric dose of oral iron is 2-3 mg/kg/d divided in 2-3 doses.
Oral iron is best absorbed when ingested without food or other medications. The percentage of iron absorbed orally is affected by the iron salt form (eg, ferrous sulfate, ferrous gluconate), the amount administered, the dosing regimen, and size of iron stores. Foods that enhance iron absorption include protein from meat and vitamin C. Foods that may inhibit absorption include unrefined grains, soy, coffee, cocoa, herb teas, red wine, calcium, and some proteins (eg, soy, eggs, casein).
Intravenous iron may be necessary for maintenance treatment of anemia associated with CKD. Intravenous iron products that are FDA-approved for use in children include iron dextran (DexFerrum, InFed), iron sucrose (Venofer), and ferric gluconate (Ferrlecit).

Management of Bone Disease
Children with stage II chronic kidney disease usually have no signs or symptoms of bone abnormalities. However, these children may have evidence of abnormalities on laboratory testing (eg, decreased serum calcitriol [1,25 dihydroxyvitamin D] and elevated serum parathyroid hormone [PTH]). Counsel the child and family about chronic kidney disease and its impact on bone metabolism. The importance of laboratory monitoring should be emphasized, and future interventions to prevent renal osteodystrophy should be discussed. Subtle signs of renal osteodystrophy begin to be observed when the glomerular filtration rate (GFR) decreases to 50% of the reference range (stage III disease).
The 2 major types of bone disease commonly encountered in patients with chronic kidney disease before maintenance dialysis include enhanced bone resorption (osteitis fibrosa) and osteomalacia/rickets. As chronic kidney disease advances to end-stage renal disease (ESRD), adynamic bone disease may also be found. Mild forms of these derangements in bone metabolism may be observed in the early stages (eg, stage II) and may become more severe as kidney function deteriorates.
Serum concentrations of calcium, phosphate, and PTH should be measured on an ongoing basis in all children with chronic kidney disease, even those with mild disease who often have evidence of abnormalities in bone metabolism. Vitamin D insufficiency and deficiency are very prevalent in pediatric patients across all stages of chronic kidney disease, particularly in nonwhite and obese patients, and may contribute to growth deficits during the earliest stages of chronic kidney disease. [26]
For calcium and phosphorus measurements, the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend monthly measurements in stage V disease, whereas PTH measurements should be obtained at least every 3 months. [7, 19] Early detection of bone metabolic abnormalities ensures that therapeutic interventions can be initiated, thereby preventing or minimizing renal osteodystrophy.
According to the KDOQI clinical practice guidelines for pediatric osteodystrophy, phosphate binders are recommended if phosphorus or intact PTH levels cannot be controlled within the target range despite dietary phosphorus restriction. [7, 19] Calcium-based phosphate binders are effective in lowering serum phosphorus levels and may be used as the initial binder therapy, but total calcium uptake should be rechecked. [27] The serum levels of corrected total calcium should be maintained within the reference range for the laboratory used. The serum calcium-phosphorus product should be maintained at less than 55 mg2/dL in adolescents.
Serum PTH concentration is inversely correlated with renal function and is almost always elevated when the GFR falls below 60 mL/min per 1.73 m2. Although the optimal serum PTH values in children with chronic kidney disease are uncertain, the KDOQI guidelines recommend targeted levels of serum intact PTH in stage V disease to be 200-300 pg/mL. [7, 19]
Patients with serum levels of intact PTH of more than 300 pg/mL may be treated with active vitamin D sterols to maintain PTH levels at about 2-4 times the reference range.

Management of Cardiovascular Manifestations
Cardiovascular disease (CVD) is the major cause of mortality in both adults and children on long-term dialysis and in adults after kidney transplantation. The prevalence of coronary artery disease (CAD) and left ventricular hypertrophy (LVH), which are precursors of cardiovascular disease mortality and morbidity, is high. The prevalence of congestive heart failure (CHF), which is an independent predictor of death in chronic renal disease, is also high. Treatment strategies should include identification and treatment of modifiable risk factors for cardiovascular disease such as smoking, obesity, hypertension, hyperlipidemia, hypertriglyceridemia, anemia, hypercalcemia, and hyperphosphatemia.
Both hypertension and anemia are associated with LVH in chronic renal disease. Treatment of each condition causes regression of LVH in chronic renal disease.
Homocysteine levels are elevated in chronic kidney disease, and elevated homocysteine levels are associated with cardiovascular disease. The effect of dietary fortification with folic acid on homocysteine levels in chronic kidney disease is unknown.

Hyperlipidemia Management
The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines on dyslipidemias recommend that all children as well as adults with chronic kidney disease should be evaluated for dyslipidemia. [7, 19] The patients should be evaluated with a complete fasting lipid profile, including total cholesterol, LDL, high-density lipoprotein (HDL), and triglycerides at presentation, and should be evaluated annually thereafter or 2-3 months after a change in treatment or other conditions known to cause dyslipidemia. Elevated levels of total and low-density lipoprotein (LDL) cholesterol are associated with cardiovascular disease in chronic renal disease.
There is a lack of conclusive data, and thus controversy exists, regarding the risks and benefits of systematic treatment of dyslipidemia in children with chronic renal disease. [28] The National Cholesterol Expert Panel on Children (NCEP-C) treatment guidelines should be followed for children with chronic kidney disease (stages I-IV) and prepubertal children on dialysis. The approach for pubertal children with stage V chronic kidney disease is similar to that for adults, but higher thresholds are used for treating LDL and non-HDL cholesterol. Recommendations for adolescents are discussed in detail elsewhere.
Hepatic 3-methylglutaryl coenzyme A reductase inhibitors (statins), fibrates, plant stanols, bile acid–binding resins, and dietary manipulation are options for individualized treatment.

Hypertension Management
Hypertension is a highly significant and independent predictor for progression of chronic kidney disease (CKD) in children. [29] It has been reported that at least 38% of children with chronic kidney disease in the United States receive antihypertensive therapy. [30] Hypertension has also been found to be a predictor of mortality in children with CKD. [31]
The optimal target blood pressure for children with chronic renal failure is recommended to be below the 90th percentile for age. Treatment of even mild hypertension is important in patients with chronic renal failure to protect against both progressive renal failure and cardiovascular disease, which is markedly increased in even moderate chronic renal disease.
Treatment of hypertension in children, with and without chronic kidney disease, is based on 3 factors: (1) degree of blood pressure elevation, (2) the presence of cardiovascular risk factors, and (3) the presence of end-organ damage. Additionally, the initial antihypertensive agent may be selected based on cause of chronic kidney disease and age.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have an additional benefit in at least some patients with chronic renal disease, slowing the rate of progressive renal injury, independent of the activity of the underlying disease.

Metabolic Acidosis Management
The kidneys play a critical role in acid-base homeostasis by excreting an acid load (produced by cellular metabolism and skeletal growth in children) and preventing bicarbonate loss in the urine. An increasing tendency to retain hydrogen ions has been observed among patients with chronic kidney disease (CKD), eventually leading to a progressive metabolic acidosis. In children, overt acidosis is characteristically present when the estimated glomerular filtration rate (eGFR) is less than 30 mL/min per 1.73 m2 (stage IV).
The acidosis in chronic kidney disease in children can be associated with an increased or normal anion gap. Guidelines recommend maintaining a serum bicarbonate level of 22 mmol/L. If necessary, the authors recommend supplementation with sodium bicarbonate, started at 1-2 mEq/kg/d in 2-3 divided doses; the dose is titrated to the clinical target.

Management of Growth Disruption
Disruption of the hypothalamic-pituitary growth hormone axis contributes to the growth hormone–resistant state in uremia. Long-term growth hormone treatment in children with chronic kidney disease (CKD) induces catch-up growth, and most patients may achieve normal adult height if treatment is initiated before end-stage renal disease (ESRD). [32] A Cochrane review of 16 studies yielded similar results finding that recombinant human growth hormone (rhGH) increased height in children with CKD by about 4 cm after 1 year and by an additional 2 cm after 2 years of treatment compared with no treatment. [33]
In children who have received a kidney transplant and fulfil the above growth criteria, we recommend initiation of growth hormone (GH) therapy 1 year after transplantation if spontaneous catch-up growth does not occur and steroid-free immunosuppression is not a feasible option. GH should be given at dosages of 0.045-0.05 mg/kg per day by daily subcutaneous injections until patients have reached their final height or until renal transplantation. [34]
Based on the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, treatment with rhGH should be considered under the following conditions [7, 19, 34] :
- Children whose height for chronologic age varies by more than 2 negative standard deviation scores (SDS)
- Children whose height velocity for chronologic age varies by more than 2 negative SDS
- Children with growth potential documented by open epiphyses
- No other contraindication for recombinant hGH use
Additionally, the following nutritional and metabolic imbalances should be corrected before use of recombinant hGH:
- Insufficient intake of energy, protein, and other nutrients
- Acidosis
- Hyperphosphatemia (correct serum phosphorus level to < 1.5 times the upper limit for age)
- Secondary hyperparathyroidism

Dietary Management
Dietary management is of paramount importance in children with chronic kidney disease (CKD). These patients have an altered metabolic milieu due to deranged kidney function. The challenge for pediatricians is to optimize the growth and development of children in this setting.
Nutritional management is one of the most important components of care for children with CKD that can improve key outcomes. Aggressive control of hypertension; correction of metabolic acidosis, water, and electrolyte abnormalities; and preventing episodes of acute on chronic kidney injury appear to constitute the most promising strategies. Effective dietary support requires the coordinated team efforts of a pediatric nephrologist, a pediatric renal dietitian, a social worker, the primary care provider, and the family.
In a cross-sectional study of dietary intake assessed by food frequency questionnaire (FFQ) in the North American Chronic Kidney Disease in Children (CKiD) prospective cohort study, children in the CKiD cohort were found to consume more sodium, phosphorus, protein, and calories than recommended. The gap between actual consumption and recommendations indicates a need for improved nutritional counseling and monitoring. [35]
The challenge for both pediatricians and dietitians is to make the diet interesting and palatable in order to ensure compliance. The goal is not only to add years to life but also to add life to years.
Energy
Energy requirements should meet at least the recommended dietary allowance (RDA) for normal children of same height age.
If protein-energy malnutrition (PEM) is present, protein and energy requirements need to be increased further to improve weight gain and linear growth. Calorie intake should be enough to enhance the efficiency of protein (protein-sparing effect) and to prevent the patient from lapsing into a catabolic state. Poor intake is common in these patients due to anorexia, nausea, and dietary restrictions.
When use of chronologic age does not account for the growth disruption, height age should be the basis for energy estimation. Supplementation can be used as per requirement (enteral or parenteral nutrition as needed).
Protein
Protein is required to maintain positive nitrogen balance for growth and maintain body protein turn over. The protein intake must be carefully controlled, avoiding protein malnutrition from an excessively restricted diet while avoiding toxicity from nitrogenous waste products from an excessively generous diet.
The diet should include 1.1-1.2 g/kg/d protein, with 60-70% protein from high biologic value origin. High biologic value proteins are of utmost importance, because they are beneficial in promoting muscle anabolism and decreasing muscle wasting.
Protein restriction is not recommended in children, because it has not been shown to influence the decrease in renal function in children with chronic kidney disease.
Phosphorus and calcium
As the glomerular filtration rate (GFR) progressively declines, excretion of phosphate decreases, and, hence, serum phosphorus levels increase. Because of this process, care must be taken for the following:
- Dietary phosphorus restriction
- Regular phosphate binders with the meals
The elemental calcium intake recommended for pediatric patients with chronic kidney disease is as follows:
- Age 1-10 years: 500-600 mg/d
- Age 11-18 years: 800-1000 mg/d
High amounts of phosphorus affect growth in children and, if the levels are high over a long period, may cause renal osteodystrophy. Prolonged elevation of serum calcium and phosphorus levels leads to vascular calcification. The daily elemental calcium requirement is about 80-100 mg/kg/d.
Potassium
The potassium requirement should be individualized depending on the serum potassium levels. Approximately 1600-2400 mg of potassium can be given.
Close watch should be kept on the potassium levels, and modifications can be made accordingly. Hyperkalemia may occur due to excessive intake of high-potassium foods, catabolism, and other causes. Leaching of pulses and vegetables should be suggested if the child is hyperkalemic.
Special attention should be given if the child is anuric.
Daily bowel movements are important, because the gastrointestinal route accounts for as much as 30% of potassium excretion in patients with chronic renal failure.
Sodium and fluid
No added salt (NAS) and restriction of salty snacks is recommended. The allowance of salt depends on the presence of edema, hypertension, and administration of sodium-containing medications. Salt intake should be kept to less than 2400 mg/d.
If the child is hypertensive and edematous, further restriction of salt and fluid is emphasized. However, exceptions include diseases in which sodium is lost in the urine (salt-losing nephropathies).
Once these children progress to dialysis or opt for kidney transplantation, a dietician should be consulted again, because the dietary requirements change.

Surgical Intervention
Surgical intervention is often recommended in children with obstructive uropathy to relieve acute kidney failure due to initial or recurrent obstruction. These children should be provided follow-up because, despite surgical intervention, they have persistent underlying chronic kidney disease (CKD). In those children who opt for hemodialysis, an arteriovenous fistula needs to be created by the vascular surgery team as an access for hemodialysis.
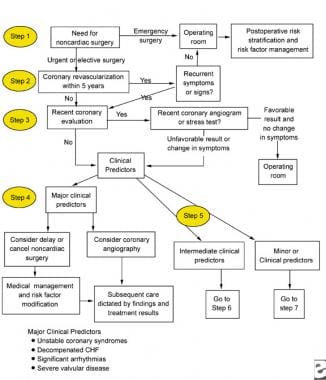
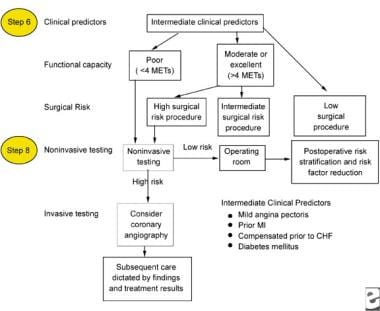
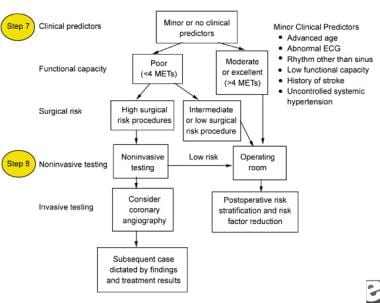
The following images depict diagrams of major, intermediate, and minor predictors that may be used in the perioperative management of patients with chronic renal failure.
Major clinical predictors to be used for the perioperative management of a patient with chronic renal failure. CHF = congestive heart failure.
Intermediate clinical predictors to be used for the perioperative management of a patient with chronic renal failure. CHF = congestive heart failure; METs = metabolic equivalents of task; MI = myocardial infarction.
Minor clinical predictors to be used for the perioperative management of a patient with chronic renal failure. ECG = electrocardiogram; METs = metabolic equivalents of task.

Long-Term Monitoring
All children with chronic kidney disease (CKD) require regular follow-up on an outpatient basis in a dedicated chronic kidney disease clinic until initiation of long-term renal replacement therapy. This involves a multidisciplinary team approach that involves the nephrologist, primary care physician, renal dietitian, nurse, and social worker. They should work in close coordination with the primary pediatrician or family physician.
Monitor calcium, phosphate, parathyroid hormone (PTH), and vitamin D levels on an ongoing basis in all children with chronic kidney disease, even those with mild disease who often have evidence of abnormalities in bone metabolism. There is a high prevalence of vitamin D insufficiency and deficiency across all stages of pediatric chronic kidney disease, particularly in nonwhite and obese patients, which may contribute to growth deficits during the earliest stages of this disease. [26]

- [Guideline] Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct. 100 (4S):S1-S276. [QxMD MEDLINE Link].
- Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015 Jul. 66 (1 Suppl 1):Svii, S1-305. [QxMD MEDLINE Link].
- United States Renal Data System. Chronic Kidney Disease: Chapter 5. Kidney and urologic disease among children and adolescents. 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2023. [Full Text].
- [Guideline] Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis. 2020 Sep. 76 (3 Suppl 1):S1-S107. [QxMD MEDLINE Link].
- [Guideline] Kopple JD. National Kidney Foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001 Jan. 37(1 Suppl 2):S66-70. [QxMD MEDLINE Link].
- [Guideline] National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb. 39(2 Suppl 1):S1-266. [QxMD MEDLINE Link].
- [Guideline] KDOQI. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009 Mar. 53(3 Suppl 2):S11-104. [QxMD MEDLINE Link].
- Beck LH Jr, Ayoub I, Caster D, et al. KDOQI US Commentary on the 2021 KDIGO Clinical Practice Guideline for the Management of Glomerular Diseases. Am J Kidney Dis. 2023 Aug. 82 (2):121-75. [QxMD MEDLINE Link].
- Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, et al. Kidney Outcomes 5 Years After Pediatric Cardiac Surgery: The TRIBE-AKI Study. JAMA Pediatr. 2016 Nov 1. 170 (11):1071-1078. [QxMD MEDLINE Link].
- Seikaly MG, Ho PL, Emmett L, et al. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatr Nephrol. 2003 Aug. 18(8):796-804. [QxMD MEDLINE Link].
- Saydah SH, Xie H, Imperatore G, Burrows NR, Pavkov ME. Trends in albuminuria and GFR among adolescents in the United States, 1988-2014. Am J Kidney Dis. 2018 Nov. 72 (5):644-52. [QxMD MEDLINE Link].
- Gulati S, Mittal S, Sharma RK, Gupta A. Etiology and outcome of chronic renal failure in Indian children. Pediatr Nephrol. 1999 Sep. 13(7):594-6. [QxMD MEDLINE Link].
- Ardissino G, Dacco V, Testa S, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003 Apr. 111(4 Pt 1):e382-7. [QxMD MEDLINE Link].
- Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O''Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009 Jul. 122(7):672-8. [QxMD MEDLINE Link]. [Full Text].
- Cañadas-Garre M, Anderson K, Cappa R, et al. Genetic susceptibility to chronic kidney disease - some more pieces for the heritability puzzle. Front Genet. 2019. 10:453. [QxMD MEDLINE Link].
- Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis. 2015 Jun. 65 (6):878-88. [QxMD MEDLINE Link].
- Craven AM, Hawley CM, McDonald SP, et al. Predictors of renal recovery in Australian and New Zealand end-stage renal failure patients treated with peritoneal dialysis. Perit Dial Int. 2007 Mar-Apr. 27(2):184-91. [QxMD MEDLINE Link].
- Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT. Prenatal risk factors for childhood CKD. J Am Soc Nephrol. 2014 Sep. 25(9):2105-11. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Hogg RJ, Furth S, Lemley KV, et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003 Jun. 111(6 Pt 1):1416-21. [QxMD MEDLINE Link].
- Eknoyan G. The importance of early treatment of the anaemia of chronic kidney disease. Nephrol Dial Transplant. 2001. 16 Suppl 5:45-9. [QxMD MEDLINE Link].
- Greenberg JH, Kakajiwala A, Parikh CR, Furth S. Emerging biomarkers of chronic kidney disease in children. Pediatr Nephrol. 2018 Jun. 33 (6):925-33. [QxMD MEDLINE Link].
- Mian AN, Schwartz GJ. Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis. 2017 Nov. 24 (6):348-56. [QxMD MEDLINE Link].
- Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021 Apr. 99 (4):948-56. [QxMD MEDLINE Link]. [Full Text].
- Sanchez CP. Secondary hyperparathyroidism in children with chronic renal failure: pathogenesis and treatment. Paediatr Drugs. 2003. 5(11):763-76. [QxMD MEDLINE Link].
- [Guideline] Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT. The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005 Nov. 46(5):925-32. [QxMD MEDLINE Link].
- Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M. Vitamin D insufficiency and deficiency in children with early chronic kidney disease. J Pediatr. 2009 Jun. 154(6):906-11.e1. [QxMD MEDLINE Link].
- Salusky IB. A new era in phosphate binder therapy: what are the options?. Kidney Int Suppl. 2006 Dec. (105):S10-5. [QxMD MEDLINE Link].
- Saland JM, Ginsberg H, Fisher EA. Dyslipidemia in pediatric renal disease: epidemiology, pathophysiology, and management. Curr Opin Pediatr. 2002 Apr. 14(2):197-204. [QxMD MEDLINE Link].
- Soergel M, Schaefer F. Effect of hypertension on the progression of chronic renal failure in children. Am J Hypertens. 2002 Feb. 15(2 Pt 2):53S-56S. [QxMD MEDLINE Link].
- Swinford RD, Portman RJ. Measurement and treatment of elevated blood pressure in the pediatric patient with chronic kidney disease. Adv Chronic Kidney Dis. 2004 Apr. 11(2):143-61. [QxMD MEDLINE Link].
- Kari JA, El Desoky SM, Farag YM, Singh AK. Predictors of renal replacement therapy and mortality in children with chronic kidney disease. Saudi Med J. 2015 Jan. 36 (1):32-9. [QxMD MEDLINE Link].
- Haffner D, Schaefer F, Nissel R, et al. Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med. 2000 Sep 28. 343(13):923-30. [QxMD MEDLINE Link].
- Hodson EM, Willis NS, Craig JC. Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev. 2012 Feb 15. CD003264. [QxMD MEDLINE Link].
- Drube J, Wan M, Bonthuis M, et al. Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat Rev Nephrol. 2019 Sep. 15 (9):577-89. [QxMD MEDLINE Link].
- Hui WF, Betoko A, Savant JD, et al. Assessment of dietary intake of children with chronic kidney disease. Pediatr Nephrol. 2017 Mar. 32 (3):485-94. [QxMD MEDLINE Link].
- Mak RH. Chronic kidney disease in children: state of the art. Pediatr Nephrol. 2007 Oct. 22(10):1687-8. [QxMD MEDLINE Link].
- Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007 Dec. 22 (12):2011-22. [QxMD MEDLINE Link].
- Muscheites J, Wigger M, Drueckler E, Fischer DC, Kundt G, Haffner D. Cinacalcet for secondary hyperparathyroidism in children with end-stage renal disease. Pediatr Nephrol. 2008 Oct. 23(10):1823-9. [QxMD MEDLINE Link].