Halite Mineral | Uses and Properties (original) (raw)
The mineral that everyone knows as "salt"
Article by: , PhD, RPG

Halite: Halite from Retsof, New York. Specimen is approximately 3 inches (7.6 centimeters) across.
What is Halite?
Halite is the mineral name for the substance that everyone knows as "salt." Its chemical name is sodium chloride, and a rock composed primarily of halite is known as "rock salt."
How Does Halite Form?
Halite is mainly a sedimentary mineral that usually forms in arid climates where ocean water evaporates. However, many inland lakes such as the Great Salt Lake of North America and the Dead Sea between Jordan and Israel are also locations where halite is forming today. Over geologic time, several enormous salt deposits have been formed when repeated episodes of seawater evaporation occurred in restricted basins. Some of these deposits are thousands of feet thick. When buried deeply they can erupt to form salt domes.

Salton Sea Halite: Halite from the Salton Sea, California. Specimen is approximately 4 inches (10 centimeters) across.
How is Halite Used?
Salt has many uses. Most of the salt produced is crushed and used in the winter on roads to control the accumulation of snow and ice. Significant amounts of salt are also used by the chemical industry. Salt is an essential nutrient for humans and most animals, and it is also a favorite seasoning for many types of food. Salt is a mineral that everyone knows.
| Physical Properties of Halite | |
|---|---|
| Chemical Classification | Halide |
| Color | Colorless or white when pure; impurities produce any color but usually yellow, gray, black, brown, red |
| Streak | White |
| Luster | Vitreous |
| Diaphaneity | Transparent to translucent |
| Cleavage | Perfect, cubic, three directions at right angles |
| Mohs Hardness | 2.5 |
| Specific Gravity | 2 |
| Diagnostic Properties | Cleavage, solubility, salty taste (The taste test is discouraged. Some minerals are toxic or contaminated by other people tasting them.) |
| Chemical Composition | NaCl |
| Crystal System | Isometric |
| Uses | Winter road treatment, a source of sodium and chlorine for chemical processes, food preservation, seasoning |
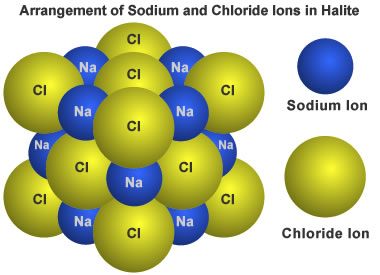
Halite structure: This diagram shows the arrangement of sodium and chloride ions in a crystal of halite.
The best way to learn about minerals is to study with a collection of small specimens that you can handle, examine, and observe their properties. Inexpensive mineral collections are available in the Geology.com Store. Image copyright iStockphoto / Anna Usova.
Find Other Topics on Geology.com:
 Rocks: Galleries of igneous, sedimentary and metamorphic rock photos with descriptions. Rocks: Galleries of igneous, sedimentary and metamorphic rock photos with descriptions. |
 Minerals: Information about ore minerals, gem materials and rock-forming minerals. Minerals: Information about ore minerals, gem materials and rock-forming minerals. |
|---|---|
 Volcanoes: Articles about volcanoes, volcanic hazards and eruptions past and present. Volcanoes: Articles about volcanoes, volcanic hazards and eruptions past and present. |
 Gemstones: Colorful images and articles about diamonds and colored stones. Gemstones: Colorful images and articles about diamonds and colored stones. |
 General Geology: Articles about geysers, maars, deltas, rifts, salt domes, water, and much more! General Geology: Articles about geysers, maars, deltas, rifts, salt domes, water, and much more! |
 Geology Store: Hammers, field bags, hand lenses, maps, books, hardness picks, gold pans. Geology Store: Hammers, field bags, hand lenses, maps, books, hardness picks, gold pans. |
 |
 Diamonds: Learn about the properties of diamond, its many uses, and diamond discoveries. Diamonds: Learn about the properties of diamond, its many uses, and diamond discoveries. |
