Analysis of novel risk loci for type 2 diabetes in a general French population: the D.E.S.I.R. study (original) (raw)
Abstract
Recently, Genome Wide Association (GWA) studies identified novel single nucleotide polymorphisms (SNPs), highly associated with type 2 diabetes (T2D) in several case-control studies of European descent. However, the impact of these markers on glucose homeostasis in a population-based study remains to be clarified.
The French prospective D.E.S.I.R. study (N = 4,707) was genotyped for 22 polymorphisms within 14 loci showing nominal to strong association with T2D in recently published GWA analyses (CDKAL1, IGFBP2, CDKN2A/2B, EXT2, HHEX, LOC646279, SLC30A8, MMP26, KCTD12, LDLR, CAMTA1, LOC38776, NGN3 and CXCR4). We assessed their effects on quantitative traits related to glucose homeostasis in 4,283 normoglycemic middle-aged participants at baseline and their contribution to T2D incidence during 9 years of follow-up.
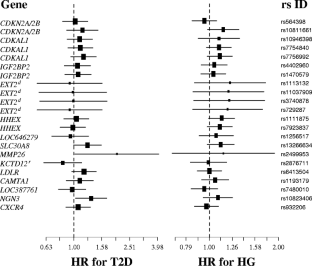
Individuals carrying T2D risk alleles of CDKAL1 or SLC30A8 had lower fasting plasma insulin level (rs7756992 P = 0.003) or lower basal insulin secretion (rs13266634 P = 0.0005), respectively, than non-carriers. Furthermore, NGN3 and MMP26 risk alleles associated with higher fasting plasma glucose levels (rs10823406 P = 0.01 and rs2499953 P = 0.04, respectively). However, for these SNPs, only modest associations were found with a higher incidence of T2D: hazard ratios of 2.03 [1.00–4.11] for MMP26 (rs2499953 P = 0.05) and 1.33 [1.02–1.73] for NGN3 (rs10823406 P = 0.03).
We confirmed deleterious effects of SLC30A8, CDKAL1, NGN3 and MMP26 risk alleles on glucose homeostasis in the D.E.S.I.R. prospective cohort. However, in contrast to TCF7L2, the contribution of novel loci to T2D incidence seems only modest in the general middle-aged French population and should be replicated in larger cohorts.
Access this article
Subscribe and save
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime Subscribe now
Buy Now
Price excludes VAT (USA)
Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Fig. 1
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.
References
- Sladek R, Rocheleau G, Rung J et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885
Article PubMed CAS Google Scholar - Zeggini E, Weedon MN, Lindgren CM et al (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341
Article PubMed CAS Google Scholar - Scott LJ, Mohlke KL, Bonnycastle LL et al (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345
Article PubMed CAS Google Scholar - Saxena R, Voight BF, Lyssenko V et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336
Article PubMed CAS Google Scholar - Steinthorsdottir V, Thorleifsson G, Reynisdottir I et al (2007) A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39:770–775
Article PubMed CAS Google Scholar - Hattersley AT, McCarthy MI (2005) What makes a good genetic association study. Lancet 366:1315–1323
Article PubMed Google Scholar - Balkau B (1996) An epidemiologic survey from a network of French Health Examination Centres, (D.E.S.I.R.): epidemiologic data on the insulin resistance syndrome. Rev Epidemiol Sante Publique 44:373–375
PubMed CAS Google Scholar - Balkau B, Eschwege E, Tichet J et al (1997) Proposed criteria for the diagnosis of diabetes: evidence from a French epidemiological study (D.E.S.I.R.). Diabetes Metab 23:428–434
PubMed CAS Google Scholar - Cauchi S, Meyre D, Choquet H et al (2006) TCF7L2 variation predicts hyperglycemia incidence in a French general population: the data from an epidemiological study on the Insulin Resistance Syndrome (DESIR) study. Diabetes 55:3189–3192
Article PubMed CAS Google Scholar - Meyre D, Bouatia-Naji N, Vatin V et al (2007) ENPP1 K121Q polymorphism and obesity, hyperglycaemia and type 2 diabetes in the prospective DESIR Study. Diabetologia 50:2090–2096
Article PubMed CAS Google Scholar - Pascoe L, Tura A, Patel SK et al (2007) Common variants of the novel type 2 diabetes genes, CDKAL1 and HHEX/IDE, are associated with decreased pancreatic {beta}-cell function. Diabetes 56(12):3101–4
Article PubMed Google Scholar - Staiger H, Machicao F, Stefan N et al (2007) Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS ONE 2:e832
Article PubMed Google Scholar - Chimienti F, Favier A, Seve M (2005) ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals 18:313–317
Article PubMed CAS Google Scholar - Chimienti F, Devergnas S, Pattou F et al (2006) In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119:4199–4206
Article PubMed CAS Google Scholar - Wei FY, Nagashima K, Ohshima T et al (2005) Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med 11:1104–1108
Article PubMed CAS Google Scholar - Hayes MG, Pluzhnikov A, Miyake K et al (2007) Identification of type 2 diabetes genes in Mexican Americans through genome-wide association studies. Diabetes 56(12):3033–44
Article PubMed CAS Google Scholar - Hanson RL, Bogardus C, Duggan D et al (2007) A search for variants associated with young-onset type 2 diabetes in American Indians in a 100 k genotyping array. Diabetes 56(12):3045–52
Article PubMed CAS Google Scholar - Rampersaud E, Damcott CM, Fu M et al (2007) Identification of novel candidate genes for type 2 diabetes from a genome-wide association scan in the Old Order Amish: evidence for replication from diabetes-related quantitative traits and from independent populations. Diabetes 56(12):3053–62
Article PubMed Google Scholar - Florez JC, Manning AK, Dupuis J et al (2007) A 100 k genome-wide association scan for diabetes and related traits in the Framingham heart study: replication and integration with other genome-wide datasets. Diabetes 56(12):3063–74
Article PubMed CAS Google Scholar - Mellitzer G, Bonne S, Luco RF et al (2006) IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. Embo J 25:1344–1352
Article PubMed CAS Google Scholar - Marchenko ND, Marchenko GN, Weinreb RN et al (2004) Beta-catenin regulates the gene of MMP-26, a novel metalloproteinase expressed both in carcinomas and normal epithelial cells. Int J Biochem Cell Biol 36:942–956
Article PubMed CAS Google Scholar - Grarup N, Rose CS, Andersson EA et al (2007) Studies of association of variants near the HHEX, CDKN2A/B and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects validation and extension of genome-wide association studies. Diabetes 56(12):3105–11
Article PubMed CAS Google Scholar - Schulze MB, Al-Hasani H, Boeing H et al (2007) Variation in the HHEX-IDE gene region predisposes to type 2 diabetes in the prospective, population-based EPIC-Potsdam cohort. Diabetologia 50:2405–2407
Article PubMed CAS Google Scholar - Bort R, Martinez-Barbera JP, Beddington RS et al (2004) Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development 131:797–806
Article PubMed CAS Google Scholar - Frayling TM (2007) Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 8:657–662
Article PubMed CAS Google Scholar - Krishnamurthy J, Ramsey MR, Ligon KL et al (2006) p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443:453–457
Article PubMed CAS Google Scholar - Pasmant E, Laurendeau I, Heron D et al (2007) Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res 67:3963–3969
Article PubMed CAS Google Scholar - Alvarsson M, Wajngot A, Cerasi E et al (2005) K-value and low insulin secretion in a non-obese white population: predicted glucose tolerance after 25 years. Diabetologia 48:2262–2268
Article PubMed CAS Google Scholar - Hinds DA, Stuve LL, Nilsen GB et al (2005) Whole-genome patterns of common DNA variation in three human populations. Science 307:1072–1079
Article PubMed CAS Google Scholar - American Diabetes Association (1997) Clinical practice recommendations 1997. Diabetes Care 20(Suppl 1):S1–70
Google Scholar
Acknowledgements
This work was partly supported by the French Government “Agence Nationale de la Recherche_”, and the charities: “_Association Française des Diabétiques” and “_Programme national de recherche sur le diabète_”. We thank Marianne Deweider and Frederic Allegaert for the DNA bank management. We are indebted to all subjects who participated to this study.
The D.E.S.I.R. study has been supported by CNAMTS, Lilly, Novartis Pharma and Sanofi-Aventis, by INSERM (Réseaux en Santé Publique, Interactions entre les determinants de la santé), by the Association Diabète Risque Vasculaire, the Fédération Française de Cardiologie, La Fondation de France, ALFEDIAM, ONIVINS, Ardix Medical, Bayer Diagnostics, Becton Dickinson, Cardionics, Merck Santé, Novo Nordisk, Pierre Fabre, Roche, Topcon.
The D.E.S.I.R. Study Group: INSERM U780: B. Balkau, P. Ducimetière, E. Eschwège; INSERM U367: F. Alhenc-Gelas; CHU D'Angers: Y. Gallois, A. Girault; Bichat Hospital: F. Fumeron, M. Marre; Medical Examination Services: Alençon, Angers, Caen, Chateauroux, Cholet, Le Mans, and Tours; Research Institute for General Medicine: J. Cogneau; General practitioners of the region; Cross-Regional Institute for Health: C. Born, E. Caces, M. Cailleau, J.G. Moreau, F. Rakotozafy, J. Tichet, S. Vol.
Duality of interest
The authors declare that there is no duality of interest.
Author information
Authors and Affiliations
- CNRS 8090-Institute of Biology, Pasteur Institute, Lille, France
Stéphane Cauchi, Christine Proença, Hélène Choquet, Stefan Gaget, Franck De Graeve, David Meyre, Martine Vaxillaire & Philippe Froguel - René Diderot-Paris 7 University, Paris, France
Michel Marre - Department of Endocrinology-Diabetology and Nutrition, Bichat Claude Bernard Hospital, INSERM U695, Paris, France
Michel Marre - INSERM U780-IFR69, Villejuif, France
Beverley Balkau - University of Paris-Sud, Paris, France
Beverley Balkau - Regional Institute for Health, La Riche, France
Jean Tichet - Imperial College, Section of Genomic Medicine, Imperial College London, Hammersmith Hospital, Du Cane Road, London, W12 0NN, UK
Philippe Froguel
Authors
- Stéphane Cauchi
- Christine Proença
- Hélène Choquet
- Stefan Gaget
- Franck De Graeve
- Michel Marre
- Beverley Balkau
- Jean Tichet
- David Meyre
- Martine Vaxillaire
- Philippe Froguel
Consortia
D.E.S.I.R. Study Group
Corresponding author
Correspondence toPhilippe Froguel.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Clinical characteristics of the D.E.S.I.R. population at baseline (DOC 36.0 KB)
Rights and permissions
About this article
Cite this article
Cauchi, S., Proença, C., Choquet, H. et al. Analysis of novel risk loci for type 2 diabetes in a general French population: the D.E.S.I.R. study.J Mol Med 86, 341–348 (2008). https://doi.org/10.1007/s00109-007-0295-x
- Received: 25 October 2007
- Revised: 26 November 2007
- Accepted: 28 November 2007
- Published: 22 January 2008
- Issue Date: March 2008
- DOI: https://doi.org/10.1007/s00109-007-0295-x