The Pro12Ala and C–681G variants of the PPARG locus are associated with opposing growth phenotypes in young schoolchildren (original) (raw)
Abstract
Aims/hypothesis
Peroxisome proliferator-activated receptor γ is an important regulator of adiposity in mouse and man, and common variation in the PPARG gene has been associated with birthweight, adult obesity, insulin sensitivity and type 2 diabetes. We hypothesised that these variants may be associated with childhood obesity.
Methods
Height and weight were recorded for 2454 prepubertal children aged between 4 and 10 years, who were then genotyped for three common variants of the PPARG locus: C–681G, Pro12Ala and C1431T.
Results
No single variant of PPARG was significantly associated with height, weight or BMI. However, when modelling the variants together we detected an opposing interaction between the −681G and the Ala12 variants in height and weight, but not BMI (_p_=0.018, 0.013 and 0.119 respectively). The data were consistent with the Ala12 carriers being deficient in energy storage/utilisation, leading to reduced growth. In contrast, the −681G variant, which has been associated with increased adult height, was associated with accelerated growth. The two variants were in strong linkage disequilibrium. However, rare individuals bearing the isolated variants demonstrated the greatest variation from the mean, the most contrasting genotypes being associated with a variation of 7 kg in weight and 6 cm in height, standardised to 7.4-year-olds (_p_=0.006 and _p_=0.02 respectively).
Conclusions/interpretation
This study demonstrates that quantitative trait analysis of energy balance/growth and the PPARG locus is complex and requires the use of multiple genetic markers.
Similar content being viewed by others
Introduction
The epidemic of obesity that is occurring throughout the world is reflected in an increasing prevalence of childhood obesity [1–3]. Dietary factors and sedentary lifestyles combine to promote positive energy balance, resulting in excess energy intake relative to requirements and the deposition of fat in adipose stores. It has been proposed that there is a strong genetic component in the tendency to increased adiposity in the face of caloric excess. This is known as the ‘thrifty genotype’ [4]. A strong candidate gene for a component of this thrifty genotype is PPARG [5]. PPARG encodes peroxisome proliferator-activated receptor γ (PPARγ), a nuclear receptor that senses fatty acids and controls the programme of gene expression required for the formation of new adipocytes. Studies in mice deleted for the PPARG gene have shown that PPARG is essential for the formation of adipose tissue [6], and humans who are heterozygous for dominant-negative variants of PPARG display partial peripheral lipodystrophy [7–9]. Common variants of this gene have been described and have been associated with various traits related to obesity. The most studied of these is the substitution of alanine for proline at codon 12 of PPARγ2 [10]. The proline form is the most common and is associated with susceptibility to the development of type 2 diabetes [11, 12]. This variant is associated with higher levels of insulin and a corresponding increase in weight, although reports of the associations with altered BMI have been conflicting. This may be due to gene–gene and gene–environment interactions, in addition to the action of additional variants in the PPARG gene itself [13, 14]. The penetrance of the Ala12 variant appears to interact with diet, in the form of both fat [15–17] and carbohydrate intake [18]. The proline variant also appears to interact with the response to exercise, with increased body weight in response to training, whereas individuals with the alanine variant respond poorly to training, with a marked loss of weight [14]. This is supported by a recent study that demonstrated an interaction between Ala12, diet and exercise in the control of insulin secretion [19].
The effect of the variation at codon 12 may be modulated by its chromosomal context. Other variants have been found to be in linkage disequilibrium with this variant, and together these have been associated with diabetes-related traits, which modulate the associations observed with the codon 12 substitution. In particular, the C1431T variant, which is a synonymous codon change in exon 6, has been associated with increased body mass [20], altered BMI/leptin ratios [21] and susceptibility to cardiovascular disease [22, 23]. We have previously presented data indicating that the association of the codon 12 substitution with body weight and susceptibility to type 2 diabetes is modulated by its context with respect to this C1431T variant [13, 14]. Recently a third polymorphism in strong linkage disequilibrium with the codon 12 variant has been described (C–681G) [24]. This variant resides in the PPARγ3 promoter region and is used in a range of non-adipose cells, including bone marrow-derived leucocytes. The C–681G variant affects the binding of the transcription factor STAT5 (signal transducer and activator of transcription 5) and has been proposed to modulate the regulation of PPARγ expression by growth hormone, which may in turn regulate bone growth. The −681G variant has been associated with increased height in an adult population, providing support for this hypothesis. However, detailed mechanistic studies regarding PPARγ and growth have not been performed. The role of PPARγ as a regulator of growth has been established recently in pparg+/− mice, in which lean mass growth was reduced, and IGF-1 production in white adipose tissue was attenuated [25]. However, this study did not examine the possibility of altered growth hormone signalling in muscle or bone.
Most work on genetic associations with body weight and variation at the PPARG locus has focused on adults. However, recent studies have presented evidence that PPARG is involved in birth size [26], and therefore PPARG will have a role in energy balance throughout life. In this study we explore the role of the PPARG locus in the regulation of growth in a large cohort of young primary school children (_n_=2454), and we find that the PPARG locus has a role in modulating prepubescent growth. This modulation is dependent on the haplotype structure of the PPARG locus and is consistent with the emerging hypotheses regarding the role of specific variants in adult energy balance.
Subjects and methods
Subject recruitment
Subjects were healthy prepubertal schoolchildren, recruited through 47 primary schools in the Tayside region of East Scotland (_n_=2454). The sample was largely Caucasian (98%). All children aged between 4 and 10 years were invited to participate in the study, through letters and information sheets distributed in the schools to parents and guardians. Parents provided written informed consent for their child’s participation. Researchers visited each school and genomic DNA isolated from saliva was taken from the consenting sample. Standing height, without shoes, was measured (to the nearest 0.1 cm) using a stadiometer (Seca Leicester height measure). Body weight was measured (to the nearest 0.1 kg) using a mechanical scale (Seca mechanical floor scale) with subjects wearing light clothing. BMI was calculated as weight/height2 (kg/m2). Date of birth was also obtained. Ethical approval was granted from the Tayside Committee on Medical Research Ethics and the Fife Local Research Ethics Committee. In addition, the Education Department of each school authority approved the study.
DNA preparation and genotyping
DNA was prepared from mouthwash pellets as previously described [27]. The failure rate for DNA preparation was less than 0.1%, and failure was associated with small cellular pellets from younger children (4 to 5-year-olds), who had difficulty spitting. Genotyping was performed using Taqman-based allelic discrimination assays, analysed on an ABI 7700 (Applied Biosystems, Foster City, CA, USA). The Pro12Ala and C1431T variants were genotyped as described previously [14]. The C–681G polymorphism was genotyped using the following probes and primers: primer 1, CTGATGATAAGGCTTTTGGCATT; primer 2, CTCTTATGAAAGGCTCAAGGATCC; probe 1 (Fam-labelled), ATGCTGTTTTGTCTTCATGGAAAATACAGCTATTCT; probe 2, (Tet-labelled), ATGCTGTTTTGTCTTGATGGAAAATACAGCTATTCT.
All three genotyping assays provided genotypes for more than 98% of the total population, and intra-individual variation of replicate genotypes was greater than 99%. Three sample replicates of known genotype were included on each plate to monitor genotyping accuracy and to confirm cluster analysis.
Statistical analysis
Haplotype frequencies were determined statistically using PHASE version 1.0.1 under Mac OS X [28]. D′ was calculated using the 2-LD program [29].
Quantitative traits (height, weight and BMI) were analysed using univariate general linear modelling with SPSS version 11.5 for Windows (SPSS, Chicago, IL, USA). Age and sex were included in all models. The role of the individual variants was assessed by step-wise removal. This resulted in a final model containing only the −681G and Ala12 variants. Both recessive and co-dominant genetic models were tested. Standard deviation scores for height, weight and BMI were generated relative to the 1990 British growth reference charts [30, 31]. All p values are two-way. A p value of <0.05 was considered significant. Correction for multiple testing was not applied, as only primary hypotheses were tested and the PPARG locus has been associated with energy utilisation and obesity in many studies. The graphs shown in Figs. 1, 2, 3, 4 were prepared using Graphpad Prism for Macintosh, version 4 (GraphPad, San Diego, CA, USA).
Results
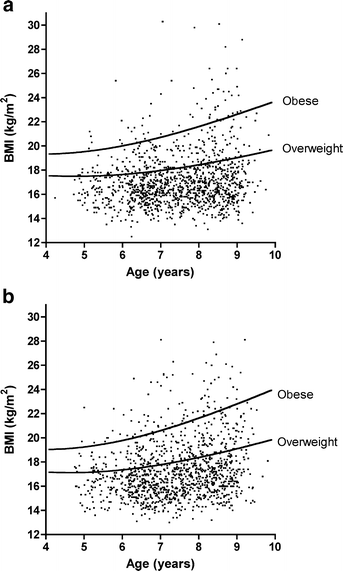
The height, weight and BMI was determined in 2454 children (Table 1) and compared with an international standard for overweight and obesity [32]. In this cohort there were 5.0% obese boys and 7.2% obese girls. In addition, 21.1% of the boys and 28.1% of the girls were overweight or obese (Fig. 1). This represents a markedly higher prevalence of obesity and overweight in both sexes compared with a 1994 survey of Scottish children of similar age range [3].
Table 1 Summary of population characteristics
Fig. 1
Prevalence of overweight and obese children in the cohort. Distribution plots of BMI vs age for males (a) and females (b). The standard curves for obesity and overweight specific to each sex are shown. These curves were derived from a summary of international cohorts
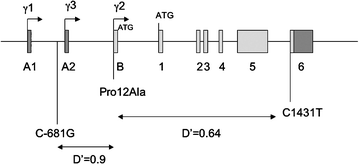
The allele frequencies of all three PPARG variants were similar to those reported for other Caucasian populations (Table 2). The strong linkage disequilibrium between the −681G and the Ala12 variants (Fig. 2, Table 3) results in very few Ala12 carriers that do not carry the −681G; however, the greater frequency of the −681G variant means that a substantial number of individuals carry the −681G without Ala12 (Table 3). This allows us to examine the role of this variant in isolation.
Table 2 Genotypes of study population
Fig. 2
A schematic illustration of the genomic context of the PPARG variants. The transcribed regions are shown as boxes, coding sequences in light grey and non-coding sequences in dark grey. γ1,γ2 and γ3 represent alternative 5′ exons derived from different promoters
Table 3 Haplotype frequencies and linkage disequilibrium
We initially examined the role of the individual variants in determining height, weight and BMI in the children. We used univariate general linear modelling and found that sex and age were major determinants of all three parameters, as would be expected (Table 4). Therefore sex and age were retained in the model. All three variants were included in the model and interactions between these variants were tested. Using this model, none of the variants associated individually with height, weight or BMI. However, a gene dose-dependent trend was observed with Pro12Ala and C–681G on height and weight, Ala12 being associated with reduced height and weight and −681G being associated with increased height and weight (Table 4). The tests for interactions revealed a consistent and statistically significant interaction with Pro12Ala and C–681G for all heights and weights, with the strongest interaction being for weight.
Table 4 Association of PPARG variants with height, weight and BMI
As the C1431T polymorphism had very little effect in this model, we repeated the test with this variant removed. This resulted in the Pro12Ala polymorphism becoming significantly associated with reduced weight and height (p 2, Table 4); C–681G alone remained insignificant but the interaction between C–681G and Pro12Ala was significant. Examination of the individual variants in isolation did not provide any significant associations with height, weight or BMI (data not shown). The relationships between height, weight, BMI and age are highly non-linear up to the age of 10 years; therefore, there was a possibility that the observed genotypic effects were subject to error in the adjustment for age and sex. We therefore analysed the same model for C–681G and Pro12Ala using standard deviation scores of height, weight and BMI, based on the UK reference growth charts (Table 5), which resulted in outcomes similar to those obtained using age as a covariate in the analysis of the raw measures.
Table 5 Association of PPARG variants with deviation from UK standard growth charts
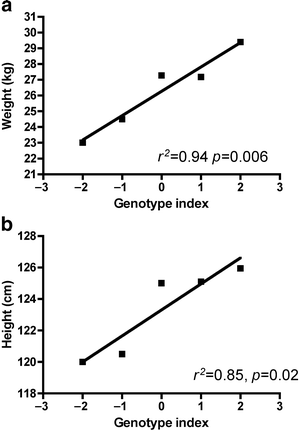
As the Pro12Ala and C–681G variants appeared to oppose each other and an interaction was indicated by the general linear modelling, we visualised the relationship of the two variants based on an allele scoring scheme (genotype index). As the Ala12 variant was associated with reduced weight, a chromosomal copy of the Ala12 was scored as −1; and as the −681G was associated with increased height and weight, a single copy was scored as +1. Therefore homozygote Ala12 individuals with no −681G scored as −2, homozygote −681G individuals with no Ala12 scored as +2, and dual homozygote Ala12/−681G individuals scored as 0. The weights and heights of all children were adjusted to a mean age of 7.4 years and for sex, and were plotted against the genotype index as an ordinal value (Fig. 3). This characterises the dose–response of the genetic interaction between these two variants, with an almost 7 kg difference in weight and a 6 cm difference in height between the most contrasting genotypes (_p_=0.006 and _p_=0.02 respectively).
Fig. 3
Opposing associations of Pro12Ala and C–681G with height and weight. Individuals were scored with −1 per copy of the Ala12 variant and +1 per copy of the −681G variant to derive a genotype index. This genotype index was used as an ordinal to plot against estimated means of weight (a) and height (b). The resulting plots were analysed by linear regression and the resulting r 2 and p values are shown. Numbers of individuals in each group: genotype index −2, _n_=1; index −1, _n_=12; index 0, _n_=1888; index +1, _n_=481; index+2, _n_=31
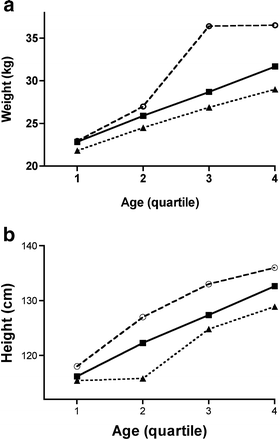
As the data reflect a growing population, with children at various ages, we explored the hypothesis that the variants affect the growth of children. This was characterised by plotting the height and weights by quartile of age (Fig. 4). This analysis demonstrates that the genotype effect was consistent across the age groups, the −681G being associated with accelerated growth and the Ala12 being associated with reduced growth compared with individuals bearing the common Pro12/−681C variants, who were intermediate in growth. Both height and weight were associated with both polymorphisms in a concerted fashion, and this was consistent with an effect on growth rather than adiposity, indicating that the polymorphisms are associated with body size.
Fig. 4
Opposing associations of Pro12Ala and C–681G with growth. The population was categorised for quartiles of age and the resulting quartile value was used as an ordinal value. Estimated means of weight (a) and height (b) were plotted against the resulting ordinal values. Individuals with only the Ala12 variant (one or two copies, _n_=13) are represented by triangles, individuals carrying only the common variants Pro12 and −681C (_n_=1410) are represented by squares, and individuals carrying only the −681G variant (one or two copies, _n_=442) are represented by open circles. The quartiles of age were: 1, 4.23–6.59 years; 2, 6.59–7.50 years; 3, 7.50–8.36; 4, 8.36–9.83 years
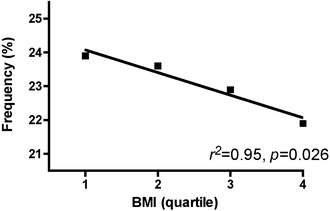
As our study included a substantial number of obese and overweight children, we also explored the relationship between these alleles and susceptibility to obesity. The frequencies of the T1431 and −681G variants were not altered in any of the quartiles of BMI (data not shown). However, the Ala12 carrier frequency showed a significant trend in depletion towards the higher quartiles of BMI (Fig. 5, _p_=0.026), supporting the hypothesis that this variant protects from childhood obesity.
Fig. 5
The Ala12 variant is depleted in obesity. The population was characterised for quartiles of BMI and the resulting quartile values were used as an ordinal value. The Ala12 carrier frequency was plotted against this ordinal value of BMI and analysed by linear regression. The resulting r 2 and p values are shown. The quartiles of BMI were: 1, 10.8–15.6 kg/m2; 2, 15.7–16.7 kg/m2; 3, 16.8–18.1 kg/m2; 4, 18.2–30.3 kg/m2
Discussion
The PPARG locus has been studied extensively for traits associated with diabetes and obesity in adults. The emerging consensus is that the Ala12 variant confers a subtle protection from obesity, insulin resistance and type 2 diabetes. The Ala12 variant also appears to be pleiotropic and is associated with protection from other obesity-associated diseases, such as hypertension and myocardial infarction [33–35]. It is therefore important to note that we can observe significant differences in susceptibility to obesity associated with this variant even at this early age. The magnitude of the effects on BMI and obesity are much smaller than we have observed in three adult populations [14], and this may reflect the fact that PPARγ is mainly affecting growth in this population. The main site of PPARγ action in growth is unclear; however, it is possible that the Ala12 variant affects insulin secretion and may therefore indirectly affect muscle and bone growth. The −681G polymorphism, on the other hand, has been postulated to interfere directly with growth factor signalling in the bone [24]; however, exact mechanistic details of this have not been demonstrated in vivo. This study represents an important extension of the initial report of this variant, where it was associated with adult height and not BMI. The lack of effect of the C1431T variant does not appear to reflect the inclusion of the −681G variant in the model, as the C1431T did not associate with any parameters on its own or in any combination of the other variants. This is in contrast to our previous reports on several populations of adults [13, 14]. It is possible that the impact of this variant on BMI may become apparent later in life. As yet no functional mechanism has been given to this variant, although we consistently find diabetes-related traits associating in adults with this variant in opposition to the Ala12 variant [13, 36].
This investigation forms part of a large population-based study in which we have looked at the interactions of haplotypes of three variants in one locus, and it is clear that most of the signal obtained is from rare haplotypes in which the variants are dissociated. This is problematic for smaller studies, in which these rare haplotypes will be extremely sparse and may be completely absent. In addition, as the main signal is obtained from rare haplotypes, it is possible that population stratification may be responsible for the observed result. Examination of the non-Caucasian subgroup (_n_=50) in this study revealed that the allele frequency, linkage disequilibrium, height for age and weight for age were all similar between both cohorts, and that the rare haplotypes were only present in the Caucasian sample; therefore, gross ethnic differences were not responsible for the observed genetic effect. In fact, similar trends by genotype in both cohorts were apparent, the interactions in the Caucasian cohort remaining significant. The allele frequencies did not differ in age group or by sex, and therefore these parameters did not confound this study.
Finally, although we have found an association with apparent growth, this is based on single measures of individuals within a population. It will be important to follow a genetically enriched subpopulation and to examine the energy balance/growth characteristics of these individuals throughout their entire growing period.
Abbreviations
PPAR:
peroxisome proliferator-activated receptor
References
- Lobstein T, Frelut ML (2003) Prevalence of overweight among children in Europe. Obes Rev 4:195–200
PubMed Google Scholar - Lobstein TJ, James WP, Cole TJ (2003) Increasing levels of excess weight among children in England. Int J Obes Relat Metab Disord 27:1136–1138
PubMed Google Scholar - Chinn S, Rona RJ (2001) Prevalence and trends in overweight and obesity in three cross sectional studies of British Children, 1974–94. BMJ 322:24–26
PubMed Google Scholar - Neel JV (1962) Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am J Hum Genet 14:353–362
PubMed Google Scholar - Auwerx J (1999) PPARgamma, the ultimate thrifty gene. Diabetologia 42:1033–1049
PubMed Google Scholar - Rosen ED, Hsu CH, Wang X et al (2002) C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 16:22–26
PubMed Google Scholar - Agarwal AK, Garg A (2002) A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab 87:408–411
PubMed Google Scholar - Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T (2002) PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes 51:3586–3590
PubMed Google Scholar - Savage DB, Tan GD, Acerini CL et al (2003) Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes 52:910–917
PubMed Google Scholar - Yen CJ, Beamer BA, Negri C et al (1997) Molecular scanning of the human peroxisome proliferator activated receptor gamma (hPPAR gamma) gene in diabetic Caucasians: identification of a Pro12Ala PPAR gamma 2 missense mutation. Biochem Biophys Res Commun 241:270–274
PubMed Google Scholar - Deeb SS, Fajas L, Nemoto M et al (1998) A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 20:284–287
PubMed Google Scholar - Altshuler D, Hirschhorn JN, Klannemark M et al (2000) The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 26:76–80
PubMed Google Scholar - Doney AS, Fischer B, Cecil JE et al (2004) Association of the Pro12Ala and C1431T variants of PPARG and their haplotypes with susceptibility to type 2 diabetes. Diabetologia 47:555–558
PubMed Google Scholar - Doney A, Fischer B, Frew D et al (2002) Haplotype analysis of the PPARgamma Pro12Ala and C1431T variants reveals opposing associations with body weight. BMC Genet 3:21
PubMed Google Scholar - Robitaille J, Despres JP, Perusse L, Vohl MC (2003) The PPAR-gamma P12A polymorphism modulates the relationship between dietary fat intake and components of the metabolic syndrome: results from the Quebec Family Study. Clin Genet 63:109–116
PubMed Google Scholar - Memisoglu A, Hu FB, Hankinson SE et al (2003) Interaction between a peroxisome proliferator-activated receptor gamma gene polymorphism and dietary fat intake in relation to body mass. Hum Mol Genet 12:2923–2929
PubMed Google Scholar - Luan J, Browne PO, Harding AH et al (2001) Evidence for gene–nutrient interaction at the PPARgamma locus. Diabetes 50:686–689
PubMed Google Scholar - Marti A, Corbalan MS, Martinez-Gonzalez MA, Forga L, Martinez JA (2002) CHO intake alters obesity risk associated with Pro12Ala polymorphism of PPARgamma gene. J Physiol Biochem 58:219–220
PubMed Google Scholar - Franks PW, Luan J, Browne PO et al (2004) Does peroxisome proliferator-activated receptor gamma genotype (Pro12ala) modify the association of physical activity and dietary fat with fasting insulin level? Metabolism 53:11–16
PubMed Google Scholar - Valve R, Sivenius K, Miettinen R et al (1999) Two polymorphisms in the peroxisome proliferator-activated receptor-gamma gene are associated with severe overweight among obese women. J Clin Endocrinol Metab 84:3708–3712
PubMed Google Scholar - Meirhaeghe A, Fajas L, Helbecque N et al (1998) A genetic polymorphism of the peroxisome proliferator-activated receptor gamma gene influences plasma leptin levels in obese humans. Hum Mol Genet 7:435–440
PubMed Google Scholar - Chao TH, Li YH, Chen JH et al (2004) The 161TT genotype in the exon 6 of peroxisome proliferator-activated receptor gamma gene is associated with premature acute myocardial infarction and increased lipid peroxidation in habitual heavy smokers. Clin Sci (Lond) 107:461–466
Google Scholar - Wang XL, Oosterhof J, Duarte N (1999) Peroxisome proliferator-activated receptor gamma C161→T polymorphism and coronary artery disease. Cardiovasc Res 44:588–594
PubMed Google Scholar - Meirhaeghe A, Fajas L, Gouilleux F et al (2003) A functional polymorphism in a STAT5B site of the human PPAR gamma 3 gene promoter affects height and lipid metabolism in a French population. Arterioscler Thromb Vasc Biol 23:289–294
PubMed Google Scholar - Rieusset J, Seydoux J, Anghel SI et al (2004) Altered growth in male peroxisome proliferator-activated receptor gamma (PPARgamma) heterozygous mice: involvement of PPARgamma in a negative feed-back regulation of growth hormone action. Mol Endocrinol 18:2363–2377
PubMed Google Scholar - Pihlajamaki J, Vanhala M, Vanhala P, Laakso M (2004) The Pro12Ala polymorphism of the PPAR gamma 2 gene regulates weight from birth to adulthood. Obes Res 12:187–190
PubMed Google Scholar - Doney AS, Fischer B, Cecil JE et al (2003) Male preponderance in early diagnosed type 2 diabetes is associated with the ARE insertion/deletion polymorphism in the PPP1R3A locus. BMC Genet 4:11
PubMed Google Scholar - Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
PubMed Google Scholar - Zhao JH (2004) 2LD, GENECOUNTING and HAP: Computer programs for linkage disequilibrium analysis. Bioinformatics 20:1325–1326
PubMed Google Scholar - Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA (1995) Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 73:17–24
PubMed Google Scholar - Cole TJ, Freeman JV, Preece MA (1995) Body mass index reference curves for the UK, 1990. Arch Dis Child 73:25–29
PubMed Google Scholar - Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
PubMed Google Scholar - Rodriguez-Esparragon FJ, Rodriguez-Perez JC, Macias-Reyes A, Alamo-Santana F (2003) Peroxisome proliferator-activated receptor-gamma2-Pro12Ala and endothelial nitric oxide synthase-4a/b gene polymorphisms are associated with essential hypertension. J Hypertens 21:1649–1655
PubMed Google Scholar - Ostgren CJ, Lindblad U, Melander O, Melander A, Groop L, Rastam L (2003) Peroxisome proliferator-activated receptor-gammaPro12Ala polymorphism and the association with blood pressure in type 2 diabetes: Skaraborg Hypertension and Diabetes Project. J Hypertens 21:1657–1662
PubMed Google Scholar - Ridker PM, Cook NR, Cheng S et al (2003) Alanine for proline substitution in the peroxisome proliferator-activated receptor gamma-2 (PPARG2) gene and the risk of incident myocardial infarction. Arterioscler Thromb Vasc Biol 23:859–863
PubMed Google Scholar - Doney AS, Fischer B, Leese G, Morris AD, Palmer CN (2004) Cardiovascular risk in type 2 diabetes is associated with variation at the PPARG locus. A Go-DARTS study. Arterioscler Thromb Vasc Biol 24:2403–2407
PubMed Google Scholar
Acknowledgements
We thank Ms I. Murrie and Ms D. Wallis for their help in the sample collection. This study was funded by the Biotechnology and Biological Sciences Research Council project grant D13460. C.N.A. Palmer is supported by the Scottish Executive Genetic Health Initiative.
Author information
Authors and Affiliations
- The Bute Medical School, University of St Andrews, St Andrews, Scotland, UK
J. E. Cecil - Biomedical Research Centre, Ninewells Hospital and Medical School, University of Dundee, Dundee, Scotland, UK
B. Fischer, A. S. F. Doney & C. N. A. Palmer - Department of Psychology, University of Liverpool, Liverpool, UK
M. Hetherington - Department of Sport and Exercise Science, Chelsea School, University of Brighton, Brighton, UK
P. Watt - Centre for Public Health Nutrition Research, Ninewells Hospital and Medical School, Dundee, Scotland, UK
W. Wrieden - MRC Human Nutrition Unit, Cambridge, UK
C. Bolton-Smith
Authors
- J. E. Cecil
You can also search for this author inPubMed Google Scholar - B. Fischer
You can also search for this author inPubMed Google Scholar - A. S. F. Doney
You can also search for this author inPubMed Google Scholar - M. Hetherington
You can also search for this author inPubMed Google Scholar - P. Watt
You can also search for this author inPubMed Google Scholar - W. Wrieden
You can also search for this author inPubMed Google Scholar - C. Bolton-Smith
You can also search for this author inPubMed Google Scholar - C. N. A. Palmer
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toC. N. A. Palmer.
Rights and permissions
About this article
Cite this article
Cecil, J.E., Fischer, B., Doney, A.S.F. et al. The Pro12Ala and C–681G variants of the PPARG locus are associated with opposing growth phenotypes in young schoolchildren.Diabetologia 48, 1496–1502 (2005). https://doi.org/10.1007/s00125-005-1817-0
- Received: 30 November 2004
- Accepted: 04 April 2005
- Published: 09 July 2005
- Issue Date: August 2005
- DOI: https://doi.org/10.1007/s00125-005-1817-0