Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis (original) (raw)
Abstract
Aims/hypothesis
We examined the association between serum C-reactive protein (CRP) and incident diabetes in a prospective study, and added these data to a literature-based meta-analysis to explore potential sources of heterogeneity between studies.
Methods
We analysed a case–control study nested within the European Prospective Investigation of Cancer (EPIC)-Norfolk cohort, including 293 incident diabetes cases and 708 controls. We combined 16 published studies on CRP and incident diabetes in a random-effect meta-analysis.
Results
In the EPIC-Norfolk cohort, serum CRP was associated with a higher risk of diabetes after adjusting for age, sex, BMI, family history of diabetes, smoking and physical activity (OR 1.49, comparing the extreme thirds of CRP distribution [95% CI 1.03–2.15], p = 0.03). However, the association was completely attenuated after further adjustment for WHR, serum γ-glutamyltransferase and serum adiponectin (OR 1.00; 95% CI 0.66–1.51, p = 1.0). In a meta-analysis of 16 published studies with 3,920 incident diabetes cases and 24,914 controls, the RR was 1.72 (95% CI 1.54–1.92), comparing the extreme thirds of CRP distribution, with substantial heterogeneity between studies (I 2 = 52.8%, p = 0.007).
Conclusions/interpretation
Initial evidence of association between CRP and incident diabetes was confounded by central adiposity, markers of liver dysfunction and adiponectin in the primary analysis. Despite an overall positive association in the meta-analysis, considerable heterogeneity existed between studies. The degree of adjustment for central adiposity and baseline glycaemia explained some of this heterogeneity and suggests that CRP may not be an independent risk factor for type 2 diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is associated with chronic low-grade inflammation, possibly through a pathway involving a cytokine-mediated acute-phase response to infection and other inflammatory processes [1]. C-reactive protein (CRP) is an acute-phase reactant produced primarily in the liver under the stimulation of adipocyte-derived pro-inflammatory cytokines, including IL-6 and TNF-α. CRP is the most commonly measured circulating marker for subclinical inflammation, with widely available, stable and standardised assays for its measurement [2].
A number of prospective studies have described the association between circulating CRP levels and risk of incident type 2 diabetes. There is heterogeneity between studies, with some demonstrating an independently positive association of CRP with incident diabetes [3–11], while others show no association after adjustment for adiposity and insulin resistance [12–15]. Differences in the association between CRP and diabetes by sex have also been reported [16–18].
A recent meta-analysis synthesised available data from ten prospective studies [3] and showed a positive association between serum CRP and incident diabetes independently of obesity. The review combined effect estimates, e.g. ORs, based on different distributions of CRP (e.g. tertiles, quartiles and quintiles), and used overlapping categories of CRP levels. However, this does not allow appropriate comparison of the association between CRP and diabetes across different studies. In addition, potential sources of heterogeneity were not fully explored in this report.
The major challenge in elucidating the aetiological role of CRP in the development of type 2 diabetes is confounding, i.e. the presence of other factors which might explain the association between CRP and diabetes risk. Adjustment for different potentially confounding factors in regression models and imprecise measurement of the confounders that are included (residual confounding) could at least partly account for the inconsistencies in observed associations. Two potentially confounding factors are adiponectin concentrations and markers of liver dysfunction. Emerging evidence suggests that plasma concentrations of the adipocytokine adiponectin [19–21], and markers of liver injury, such as the hepatic enzyme γ-glutamyltransferase (GGT) [22–24] predict the development of type 2 diabetes, independently of traditional risk factors. Adiponectin and GGT are also associated with CRP levels [15, 25, 26]. To date, no published studies of the association between CRP and incident diabetes have considered these potentially important confounders.
In this study, we examined the impact of confounding on the association between serum CRP and incident diabetes using two approaches. First, we used data relating to a range of potentially confounding factors in a case–control study nested within a prospective cohort. Second, we examined factors that might account for the heterogeneity of associations and the effects of the degree of adjustment for confounding on the size of the observed effect estimates in a meta-analysis of published prospective analyses on serum CRP and incident diabetes. Since CRP, GGT and adiponectin may share common biological pathways, we also sought to assess whether CRP mediates the effect of adiposity on the risk of type 2 diabetes, and whether adjusting for GGT and adiponectin, compared with traditional measures of adiposity, i.e. BMI and WHR, would alter the association between CRP and incident diabetes.
Methods
Primary analysis
Study design and population
The current case–control study was nested within the European Prospective Investigation of Cancer (EPIC)-Norfolk population-based cohort study, which recruited 25,631 men and women aged 40–74 years residing in the city of Norwich and its surrounding towns between 1993 and 1997. Approval was obtained from the Norfolk Local Research Ethics Committee. At the baseline study visit (1993–1997), after written informed consent was obtained, participants completed a health and lifestyle questionnaire, had non-fasting blood samples drawn and anthropometric measures taken. Then, 18 months later, they responded to a mailed follow-up questionnaire. Finally, they attended a second study visit for repeat anthropometric assessment and blood testing, 3–5 years after the first visit (1998–2000) [27].
People with prevalent diabetes at baseline, defined by self-report only (n = 845), were excluded from this analysis. Incident diabetes that developed after baseline and prior to 2000, or previously undiagnosed at baseline, was ascertained using multiple sources of information. These included self-reported diabetes in follow-up questionnaires (18 month follow-up and at the health check in 1998–2000), self-reported use of diabetes-specific medication on follow-up lifestyle questionnaires or brought in to follow-up health checks, or HbA1c >7% at either baseline or follow-up health checks. The sources of information external to the EPIC-Norfolk study included documentation of diabetes in the general practitioner’s register, in the local hospital diabetes register, in hospital records, or on a death certificate. A validation study estimated that three sources, general practice registers, HbA1c level and self-report, captured 96% of all known cases of diabetes [28].
After a mean follow-up of 3.7 years, 417 incident cases of diabetes had developed. Each case was matched with two controls; the first control was matched for age, sex, general practice and recruitment date, and the second control was additionally frequency matched for BMI. Of the 1,251 participants, those who had CRP levels >10 mg/l (n = 90) were excluded to avoid individuals with acute infection [2]. After exclusion of individuals with missing values of CRP (n = 146) and baseline variables (n = 14), the study population of this analysis consisted of 293 cases and 708 control participants.
Health and lifestyle questionnaire
Information on demography, socioeconomic characteristics, lifestyle factors (including physical activity, cigarette smoking and alcohol consumption), medications and personal and family histories of diabetes, was collected in the questionnaire at baseline, at the 18 month follow-up and at the second health check.
Anthropometric measurement and biochemical analysis
Anthropometric measurements including height, weight, and waist and hip circumferences were taken in light indoor clothing, without shoes. BMI was calculated as weight (kg)/height (m)2. WHR was calculated as waist (cm)/hip (cm). Non-fasting blood samples were obtained during baseline health checks and were stored frozen. The frozen serum samples were thawed in 2005 and were used to assay serum levels of CRP, GGT and adiponectin. Serum high-sensitivity CRP (mg/l) and GGT (U/l) were measured using a Dade Behring Dimension Arx automated clinical chemistry system (Deerfield, IL, USA), with between-batch CV values of 5.0% at 14 mg/l and 2.8% at 120 mg/l for CRP, and 2.9% at 80 U/l and 1.7% at 147 U/l for GGT. Serum adiponectin (μg/l) was measured by fluoroimmunoassay using an AutoDELFIA kit (Wallac Oy, Finland). The between-batch CV values were 5.2% at 3.49 μg/l, 6.95% at 8.85 μg/l and 11.9% at 15.8 μg/l for adiponectin [29].
Statistical analysis
Baseline metabolic variables and risk factors for diabetes were described separately for cases and controls, using means and SD for continuous variables, and counts and percentages for categorical variables. Differences between cases and controls were tested using t tests for the continuous variables and χ 2 tests for the categorical variables.
We used unconditional logistic regression to explore the association between CRP levels and odds of developing diabetes. CRP levels were categorised into thirds based on the baseline distribution in controls. All analyses were adjusted for age, sex and BMI to enable the use of all control participants (two controls for every case), as BMI was only frequency matched for one set of controls [29]. Established diabetes risk factors (positive family history of diabetes, higher WHR and physical inactivity) and potential confounders (cigarette smoking, serum levels of GGT and adiponectin) were included in the regression models. Fasting or random blood glucose levels at baseline were not available. HbA1c at baseline was available in approximately half of the study population. We examined potential interactions between CRP levels and baseline variables using a variable representing the product of both factors.
Meta-analysis
Literature search
We identified studies with a MEDLINE search to the end of December 2007, using the terms ‘C-reactive protein’ and ‘type 2 diabetes’ and by scanning relevant reference lists. Studies were included if they used a prospective cohort design or if they were case–control studies nested within a cohort, if CRP level was measured at baseline, and if incident diabetes was the outcome. Sixteen studies met the inclusion criteria. One study was excluded because of overlapping study populations [12] with an updated analysis of the same cohort [18]. Therefore, a total of 16 independent studies (including the present study) were included in the meta-analysis.
Data extraction
We extracted information on the characteristics of the 16 published studies including demographic data, study design, duration of follow-up, assay method for CRP, ascertainment of diabetes, adjustment for potential confounders and effect estimates (risk ratio or OR, and the 95% CI) for the association between CRP and incident diabetes. We used the effect estimates from the models with the greatest degree of covariate adjustment in the meta-analysis. From our primary analysis (the EPIC-Norfolk study), we used the ORs adjusted for age, sex, BMI, cigarette smoking, physical activity, family history of diabetes and WHR in the meta-analysis.
Data synthesis
We converted the published effect estimates (i.e. HR, OR or RR), if reported per SD, quartiles and quintiles, into common metrics of thirds of the CRP distribution. This assumed a normal distribution of the log e of CRP concentration in the general population and a log–linear association between CRP and risk of diabetes [30]. Where studies reported men and women separately, we combined the effect estimates prior to conversion. After appropriate conversion of the effect estimates, studies were combined using a random-effects meta-analysis. We performed two types of subgroup analyses to explore possible sources of heterogeneity, i.e. differences in effect measures by subgroup. First, we grouped studies according to study-level characteristics including sex, geographical location (Asia, North America, Europe), study design and number of incident diabetes cases (<250 vs ≥250). Second, we defined subgroups by whether or not a study adjusted for baseline glycaemia (fasting blood glucose or HbA1c) or central adiposity (WHR or waist circumference). For both types of analyses, we used the Cochran Q test and I 2 statistics [31] to assess heterogeneity. A p value <0.05 suggested a significant difference between effect estimates by subgroup. All statistical analyses were performed using STATA 9.1 (Stata Corporation, College Station, TX, USA).
Results
Primary analysis
The baseline characteristics of cases and controls are shown in Table 1. The mean ages of cases and controls were similar. Sixty-one per cent of cases were men, compared with 58% male control participants. Cases had significantly higher levels of CRP, GGT and adiponectin at baseline, compared with controls, as well as higher measures of adiposity, including BMI and WHR. Participants who developed diabetes reported less physical activity and were more likely to have a family history of diabetes.
Table 1 Baseline characteristics of cases and controls: the EPIC-Norfolk population-based cohort study 1993–2000
Participants with CRP levels in the top third of the baseline distribution had an increased risk of developing diabetes, compared with those in the bottom third, after adjusting for age, sex and BMI (OR 1.55; 95% CI 1.08–2.21, p for trend across thirds = 0.02). The association remained significant after adjusting for family history of diabetes, physical activity and cigarette smoking (OR 1.49; 95% CI 1.03–2.15, p for trend across thirds = 0.03). The association was no longer statistically significant after adjusting for WHR. Regardless of whether WHR was retained in the model, additional adjustment for GGT and adiponectin further attenuated the association between CRP and incident diabetes, as shown in Table 2. Sensitivity analysis of participants with HbA1c measures showed that the association was attenuated after adding HbA1c to the multivariate model (OR 0.72; 95% CI 0.17–3.07, p for trend across thirds = 0.4).
Table 2 Logistic regression models for the association between CRP and incident type 2 diabetes: the EPIC-Norfolk population-based cohort study 1993–2000
In multivariate models adjusted for age, sex, cigarette smoking, family history of diabetes and exercise, the ORs for BMI and WHR associated with the risk of diabetes were 1.06 (95% CI 1.02–1.11, p = 0.002) and 2.15 (95% CI 1.64–2.81, p < 0.001), respectively. After adjusting for CRP, we observed no change in the magnitude of the association (OR 1.06; 95% CI 1.01–1.10, p = 0.008 for BMI and OR 2.12; 95% CI 1.62–2.77, p < 0.001 for WHR). However, when CRP was replaced by GGT and adiponectin, the association was attenuated for WHR (OR 1.62; 95% CI 1.21–2.18, p < 0.001), but not for BMI (OR 1.04; 95% CI 0.99–1.09, p = 0.08).
Meta-analysis
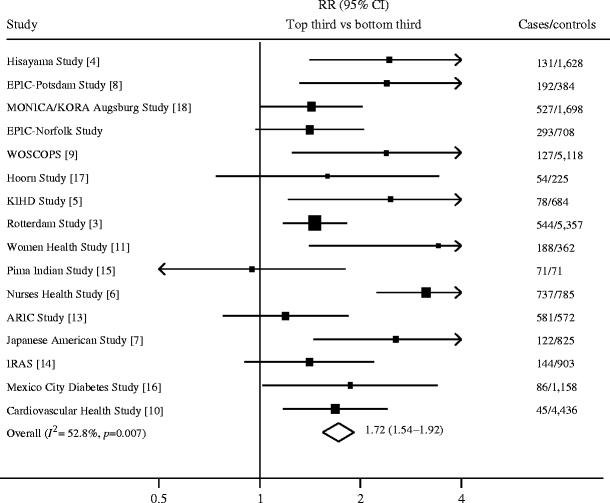
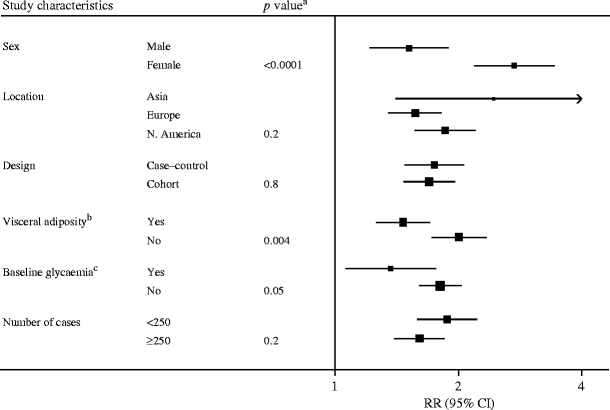
Sixteen studies (including the present study), comprising a total of 3,920 incident diabetes cases and 24,914 controls, were included in the meta-analysis. The combined risk ratio for diabetes comparing individuals in top vs bottom thirds of baseline CRP distribution was 1.72 (95% CI 1.54–1.92), with substantial heterogeneity between studies (I 2 = 52.8%, p = 0.007), as shown in Fig. 1. Subgroup analysis showed significant differences between subgroups defined by sex (p < 0.0001), whether or not the analyses were adjusted for central adiposity (p = 0.004) and whether or not the analyses were adjusted for baseline glycaemia (p = 0.05), as shown in Fig. 2.
Fig. 1
Prospective studies of the association between CRP levels and type 2 diabetes. The size of the squares is proportional to the inverse of the variance of the ORs. The diamond represents the summary estimates. ARIC, Atherosclerosis Risk in Communities; IRAS, Insulin Resistance Atherosclerosis Study; KIHD, Kuopio Ischaemic Heart Disease Risk Factor Study; MONICA/KORA, Monitoring of Trends and Determinants in Cardiovascular Disease/Cooperative Research in the Region of Augsburg; WOSCOPS, West of Scotland Coronary Prevention Study
Fig. 2
Subgroup analysis of study-level characteristics in 16 published studies. The size of the squares is proportional to the inverse of the variance of the ORs. a p value for heterogeneity between subgroups. bVisceral adiposity refers to whether studies have adjusted for indices of visceral adiposity. cBaseline glycaemia refers to whether studies have adjusted for indices of baseline glycaemia
Discussion
Our analysis of a nested case–control study within the prospective EPIC-Norfolk study provides evidence that elevated baseline CRP levels were not associated with increased risk of developing diabetes after controlling for risk factors for diabetes, WHR, GGT and adiponectin. An association was demonstrable when only a restricted list of possible confounding factors was considered, but was entirely attenuated when WHR, GGT and adiponectin were considered, suggesting that these factors are important confounders.
The possibility of GGT and adiponectin being confounders is strengthened by previous observations of the association of these factors with CRP [15, 25, 26] and also with diabetes risk [19–24]. CRP originates in the liver and is increased in people with liver steatosis [32], suggesting a link between chronic inflammation and non-alcoholic fatty-liver disease. The association of GGT with insulin resistance, fasting blood glucose and triacylglycerols [33–35] and type 2 diabetes [22–24] is well described.
Low levels of adiponectin have similarly been associated with the development of type 2 diabetes [19–21]. Plasma adiponectin levels are also inversely associated with CRP [25]. It is possible that adiponectin may act as an intermediate between CRP and diabetes. Recent in vitro evidence suggests that CRP inhibits adiponectin gene expression in adipocytes [36], and this may lead to reduced insulin sensitivity and diabetes. If true, adjustment for adiponectin in regression models would be overadjustment.
Although the meta-analysis of 16 prospective studies showed an overall association between CRP levels and incident diabetes, these studies vary in certain characteristics. Those studies which adjusted for visceral adiposity, as measured by WHR or waist circumference, showed weaker associations than those that did not. Visceral adipose tissue, compared with subcutaneous fat, is known to produce more pro-inflammatory cytokines [37] and is associated with CRP levels [38], suggesting that visceral adiposity could be an additional important confounding factor that was not considered in all studies.
Complex links exist between inflammation, adiposity and diabetes. If adiposity increases the risk of diabetes through both inflammatory and non-inflammatory processes, any significant association remaining after adjustment of measures of adiposity might be a result of residual confounding. However, if adiposity is caused in part by inflammatory processes, then any significant association might represent an overadjustment for adiposity. In our sensitivity analyses, the association between measures of adiposity (BMI and WHR) and the risk of diabetes did not change materially after adjustment for CRP, suggesting that CRP might not be an important mediator of adiposity. Adjusting for adiponectin and GGT attenuated the association of WHR on the risk of diabetes more significantly than adjusting for CPR, suggesting that GGT and adiponectin may mediate the effects of visceral adiposity.
Those studies which adjusted for baseline glycaemia, as measured by fasting blood glucose or HbA1c, had weaker associations than those that did not. Although the restriction in our meta-analysis to prospective studies strengthened our inferences about the direction of causality, since the observation of elevated CRP levels preceded the development of diabetes, these data do not completely rule out the possibility of reverse causation as an alternative explanation of our observation. The weaker association found after adjusting for baseline hyperglycaemia suggests that raised CRP levels could be associated with early alterations in glucose control. Thus, the elevated CRP levels could be a consequence of the diabetes process, rather than the cause of it.
Our data also show heterogeneity by sex, which is consistent with previous reports [16–18]. Whether this represents a fundamental biological process between sexes or more probably is a reflection of a different pattern of confounding between men and women is uncertain. In an attempt to examine the latter, we investigated whether hormone replacement therapy (HRT) could be one such sex-linked confounding factor, since HRT is associated with increased CRP levels in women [39]. However, the positive CRP–diabetes association in women could not be explained by use of oestrogen or HRT.
The strengths of our prospective analysis are the consideration of a wider range of potentially confounding factors than in previous studies, and the robust ascertainment of incident diabetes. In the meta-analysis, a particular strength relates to the conversion of different studies’ effect estimates into a common measure of the distribution of CRP level and an exploration of possible sources of heterogeneity.
Our study has potential limitations. The relatively wide CIs for the associations in the EPIC-Norfolk cohort suggested that more incident cases of diabetes would improve the precision of the effect estimates. We measured CRP on non-fasting blood samples, but a previous study has shown no postprandial increase in CRP levels [40]. Another potential weakness is the possibility of undiagnosed diabetes in the control population. However, because the highest HbA1c level in the control participants was 5.1%, this is unlikely. Finally, CRP level was measured in serum stored frozen for 7 years, but levels of CRP may remain stable for at least 12 years [41].
In conclusion, our primary analysis of the nested case–control study suggests that there is no association between CRP and risk of diabetes after taking into consideration a wide range of potentially confounding factors, including central adiposity, markers of liver dysfunction and adiponectin. Our meta-analysis differs from a previous report in that we have converted effect estimates into a common measure and have focused on exploring sources of heterogeneity between studies. The observation of substantial heterogeneity is probably explained by the varying consideration between studies of potential confounders. Taken together, our findings demonstrate that the associations between CRP and type 2 diabetes appear to be weaker than previously reported, and that CRP may not be an independent risk factor for type 2 diabetes. As a means to identify individuals at risk of diabetes, measuring CRP in a clinical setting currently has no usefulness beyond established risk factors. Our study also highlighted that the traditional approach of analysing the pattern of confounding in existing studies is a useful adjunct to alternative approaches for investigating causal inference through molecular epidemiological approaches.
Abbreviations
BMI:
Body mass index
CRP:
C-reactive protein
EPIC:
European Prospective Investigation of Cancer
GGT:
γ-Glutamyltransferase
References
- Pickup JC, Crook MA (1998) Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41:1241–1248
Article PubMed CAS Google Scholar - Pearson TA, Mensah GA, Alexander RW et al (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511
Article PubMed Google Scholar - Dehghan A, Kardys I, de Matt MPM et al (2007) Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 56:872–878
Article PubMed CAS Google Scholar - Doi Y, Kiyohara Y, Kubo M et al (2005) Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population. Diabetes Care 28:2497–2500
Article PubMed CAS Google Scholar - Laaksonen DE, Niskanen L, Nyyssonen K et al (2004) C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia 47:1403–1410
Article PubMed CAS Google Scholar - Hu FB, Meigs JB, Li TY, Rifai N, Manson JE (2004) Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 53:693–700
Article PubMed CAS Google Scholar - Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N (2003) Elevated C-reactive protein is risk factor for the development of type 2 diabetes in Japanese American. Diabetes Care 26:2754–2757
Article PubMed CAS Google Scholar - Spranger J, Kroke A, Mohlig M et al (2003) Inflammatory cytokines and the risk to develop type 2 diabetes. Diabetes 52:812–817
Article PubMed CAS Google Scholar - Freeman DJ, Norrie J, Caslake MJ et al (2002) C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 51:1596–1600
Article PubMed CAS Google Scholar - Barzilay JI, Abraham L, Heckbert SR et al (2001) The relation of markers of inflammation to the development of glucose disorders in the elderly. The Cardiovascular Health Study. Diabetes 50:2384–2389
Article PubMed CAS Google Scholar - Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Article PubMed CAS Google Scholar - Thorand B, Lowel H, Schneider A et al (2003) C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men. Arch Intern Med 163:93–99
Article PubMed CAS Google Scholar - Duncan BB, Schmidt MI, Pankow JS et al (2003) Low-grade systemic inflammation and the development of type 2 diabetes. The Atherosclerosis Risk in Communities Study. Diabetes 52:1799–1805
Article PubMed CAS Google Scholar - Festa A, D’Agostino R, Tracy RP, Haffner SM (2002) Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes. Diabetes 51:1131–1137
Article PubMed CAS Google Scholar - Krakoff J, Funahashi T, Stehouwer CDA et al (2003) Inflammatory markers, adiponectin and risk of type 2 diabetes in the Pima Indian. Diabetes Care 26:1745–1751
Article PubMed CAS Google Scholar - Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean MEJ, Haffner SM (2002) Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 25:2016–2021
Article PubMed CAS Google Scholar - Snijder M, Dekker JM, Visser M et al (2003) Prospective relation of C-reactive protein with type 2 diabetes. Diabetes Care 26:1656–1657
Article PubMed Google Scholar - Thorand B, Baumert J, Kolb H et al (2007) Sex differences in the prediction of type 2 diabetes by inflammatory markers. Result from the MONICA/KORA Augsburg case–cohort study, 1984–2002. Diabetes Care 30:854–860
Article PubMed CAS Google Scholar - Lindsay RS, Funahashi T, Hanson RL et al (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360:57–58
Article PubMed CAS Google Scholar - Spranger J, Kroke A, Mohlig M et al (2003) Adiponectin and protection against type 2 diabetes mellitus. Lancet 361:226–228
Article PubMed CAS Google Scholar - Duncan BB, Schmidt MI, Pankow JS et al (2004) Adiponectin and the development of type 2 diabetes. Diabetes 53:2473–2478
Article PubMed CAS Google Scholar - Lee DH, Silventoinen K, Jacobs DR Jr, Jousilahti P, Tuomileto J (2004) Gamma-glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 89:5410–5414
Article PubMed CAS Google Scholar - Nannipieri M, Gonzales C, Baldi S et al (2005) Liver enzymes, the metabolic syndrome and incident diabetes: the Mexico City Diabetes Study. Diabetes Care 28:1757–1762
Article PubMed CAS Google Scholar - Andre P, Balkau B, Born C, Charles MA, Eschwege E (2006) Three-year increase of gamma-glutamyltransferase level and development of type 2 diabetes in middle-aged men and women: the DESIR cohort. Diabetologia 49:2599–2603
Article PubMed CAS Google Scholar - Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB (2004) Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care 27:1680–1687
Article PubMed CAS Google Scholar - Lee DH, Jacobs DR Jr (2005) Association between serum gamma-glutamyltransferase and C-reactive protein. Atherosclerosis 178:327–330
Article PubMed CAS Google Scholar - Day N, Oakes S, Luben R et al (1999) EPIC-Norfolk: study design, characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 80(Suppl 1):95–103
PubMed Google Scholar - Harding AH, Day NE, Khaw KT et al (2004) Dietary fat and the risk of clinical type 2 diabetes: the European Prospective of Cancer—Norfolk Study. Am J Epidemiol 159:73–82
Article PubMed Google Scholar - Forouhi NG, Harding AH, Allison M et al (2007) Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia 50:949–956
Article PubMed CAS Google Scholar - Danesh J, Collins R, Appleby P, Peto R (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analysis of prospective studies. JAMA 279:1477–1482
Article PubMed CAS Google Scholar - Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analysis. BMJ 327:557–560
Article PubMed Google Scholar - Yoneda M, Mawatari H, Fujita K et al (2007) High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol 42:573–582
Article PubMed CAS Google Scholar - Rantala AO, Lilja M, Kauma H, Savolainen MJ, Reunanen A, Kesaniemi YA (2000) Gamma-glutamyl transpeptidase and the metabolic syndrome. J Intern Med 248:230–238
Article PubMed CAS Google Scholar - Kang YH, Min HK, Son SM, Kim IJ, Kim YK (2007) The association of serum gamma glutamyltransferase with components of the metabolic syndrome in the Korean adults. Diabetes Res Clin Pract 77:306–313
Article PubMed CAS Google Scholar - Sakugawa H, Nakayoshi T, Kobashigawa K et al (2004) Metabolic syndrome is directly associated with gamma glutamyl transpeptidase elevation in Japanese women. World J Gastroenterol 10:1052–1055
PubMed CAS Google Scholar - Yuan G, Chen X, Ma Q et al (2007) C-reactive protein inhibits adiponectin gene expression and secretion in 3T3-L1 adipocytes. J Endocrinol 194:275–281
Article PubMed CAS Google Scholar - Hamdy O, Porramatikul S, Al-Ozairi E (2006) Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2:367–373
PubMed Google Scholar - Greenfield JR, Samaras K, Jenkins AB et al (2004) Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation 109:3022–3028
Article PubMed CAS Google Scholar - Manning PJ, Sutherland WH, Allum AR, de Jong SA, Jones SD (2002) Effect of hormone replacement therapy on inflammation-sensitive proteins in post-menopausal women with type 2 diabetes. Diabet Med 19:847–852
Article PubMed CAS Google Scholar - Meier-Ewert HK, Ridker PM, Rifai N et al (2001) Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem 47:426–430
PubMed CAS Google Scholar - Danesh J, Wheeler JG, Hirschfield GM et al (2004) C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 350:1387–1397
Article PubMed CAS Google Scholar
Acknowledgements
The EPIC-Norfolk Study is funded by Cancer Research UK, the Medical Research Council, the British Heart Foundation, the Food Standards Agency, the Department of Health and the Academy of Medical Sciences. We are grateful to the participants and general practitioners who took part in the study.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
- MRC Epidemiology Unit, Institute of Metabolic Science, Box 285, Addenbrooke’s Hospital, Cambridge, CB2 0QQ, UK
C. C. Lee, A. I. Adler, M. S. Sandhu, S. J. Sharp, N. G. Forouhi & N. J. Wareham - Wolfson Diabetes and Endocrine Clinic, Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK
A. I. Adler - Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK
S. Erqou, R. Luben, S. Bingham & K. T. Khaw
Authors
- C. C. Lee
You can also search for this author inPubMed Google Scholar - A. I. Adler
You can also search for this author inPubMed Google Scholar - M. S. Sandhu
You can also search for this author inPubMed Google Scholar - S. J. Sharp
You can also search for this author inPubMed Google Scholar - N. G. Forouhi
You can also search for this author inPubMed Google Scholar - S. Erqou
You can also search for this author inPubMed Google Scholar - R. Luben
You can also search for this author inPubMed Google Scholar - S. Bingham
You can also search for this author inPubMed Google Scholar - K. T. Khaw
You can also search for this author inPubMed Google Scholar - N. J. Wareham
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA. I. Adler.
Rights and permissions
About this article
Cite this article
Lee, C.C., Adler, A.I., Sandhu, M.S. et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis.Diabetologia 52, 1040–1047 (2009). https://doi.org/10.1007/s00125-009-1338-3
- Received: 23 November 2008
- Accepted: 25 February 2009
- Published: 27 March 2009
- Issue Date: June 2009
- DOI: https://doi.org/10.1007/s00125-009-1338-3