Food choice of Antarctic soil arthropods clarified by stable isotope signatures (original) (raw)
Abstract
Antarctic soil ecosystems are amongst the most simplified on Earth and include only few soil arthropod species, generally believed to be opportunistic omnivorous feeders. Using stable isotopic analyses, we investigated the food choice of two common and widely distributed Antarctic soil arthropod species using natural abundances of 13C and 15N and an isotope labelling study. In the laboratory we fed the isotomid springtail Cryptopygus antarcticus six potential food sources (one algal species, two lichens and three mosses). Our results showed a clear preference for algae and lichens rather than mosses. These results were corroborated by field data comparing stable isotope signatures from the most dominant cryptogams and soil arthropods (C. antarcticus and the oribatid mite Alaskozetes antarcticus). Thus, for the first time in an Antarctic study, we present clear evidence that these soil arthropods show selectivity in their choice of food and have a preference for algae and lichens above mosses.
Similar content being viewed by others
Introduction
Soil arthropods are known to play an important role in decomposition processes and nutrient flows through the soil ecosystem (Brussaard 1998; Filser 2002). However, detailed knowledge of the specific role of individual species in the soil ecosystem is necessary to better understand ecosystem processes (Faber 1991). Food availability is likely to be an important determinant of the functional composition of the soil arthropod fauna (Hodkinson and Wookey 1999), while their specific role in any soil ecosystem can only be understood in relation to their diet. The functional role of soil arthropods is generally very difficult to investigate, due to the complexity of most soil food webs. However, in the typically species-poor Antarctic terrestrial ecosystems the soil food web is relatively simple (Convey 2001), which makes them very suitable for addressing this type of question. However, to date very few studies have addressed this subject in Antarctic soil ecosystems, see review by Hogg et al. (2006).
Within Antarctic soils and vegetated ecosystems there are only a small number of arthropod species (Davis 1981; Usher and Booth 1986; Convey et al. 1996, 1997; Convey 2001; Sinclair 2001). However, the abundance of some of these arthropods (e.g. the isotomid springtail Cryptopygus antarcticus) can be very high, up to 106 individuals m−2 (Convey and Smith 1997), while communities are often dominated by only one or two species (Goddard 1979; Block 1982; Richard et al. 1994). These large numbers suggest that there must be ample food available, and there is a generally accepted, but generally untested, assumption that food resources are not limiting for these arthropods (Convey 1996).
Maritime Antarctic vegetation is primarily composed of cryptogams (Longton 1967; Convey 2001) with mosses as the group that sequesters most of the carbon. As there are no large herbivores, almost all this carbon is transferred to the soil (Davis 1981). It has previously been suggested that most of the carbon and nutrients will flow directly into the decomposition pathway without passing through a consumer level (Block 1985). However, the contribution of the dominant groups of soil arthropods, springtails and mites, within these nutrient flows is not well understood.
Most existing data on feeding preferences of Antarctic soil arthropods is based on analysis of the gut content (Broady 1979; Burn 1981, 1984; Davidson and Broady 1996). This approach, while widely used, has the drawback that only the material that passes through the gut of an organism undamaged can be determined (Chamberlain et al. 2006a, b), while there is usually a large component of unidentifiable material remaining (Davis 1981). The use of stable isotopic analyses in soil food web studies has made it easier for ecologists to assess the role of soil organisms (Neilson et al. 1998; Briones et al. 1999; Scheu and Falca 2000; Schmidt et al. 2004; Staddon 2004). More importantly, assumptions about feeding preferences of species have been changed as a result of the use of this technique (Chamberlain et al. 2006a). Applying this technique on the components of Antarctic soil ecosystems will enable increased understanding of the feeding strategies of Antarctic soil arthropods, and of the specific roles these soil arthropods play in nutrient cycling in these ecosystems. Various studies have shown that the stable isotope signatures (δ 13C and δ 15N) of cryptogams differ considerably between species (Galimov 2000; Huiskes et al. 2006). These stable isotope differences in the producers should therefore lead to differences in consumer isotope ratios and assist in the determination of feeding preferences.
The aim of this study was to assess the food choice of the most dominant soil arthropods in maritime Antarctic soil ecosystems. We used two complementary approaches: (1) laboratory feeding-experiments with labelled and non-labelled food using C. antarcticus in order to determine which food sources are preferred by this species: and (2) a field survey on Anchorage Island (maritime Antarctic) in which we compared the isotopic signatures of the dominant springtail (C. antarcticus), mite (Alaskozetes antarcticus) and potential food sources.
Material and methods
Sampling site
Cryptogam and soil arthropod specimens were collected from Anchorage Island (maritime Antarctic). Anchorage Island is one of the Léonie Islands and lies in Marguerite Bay south of the Rothera Research Station of the British Antarctic Survey (67°61′S 68°22′W). The island is 2.5 km long and 500 m wide and is partly covered by semi-permanent snow and ice fields. The island includes several rocky ridges with a maximum height of 57 m a.s.l. On the slopes of these ridges, there are some patches of the moss Sanionia uncinata (Hedw.) Loeske, and the grass Deschampsia antarctica Desv. However, the vegetation consists mainly of lichens, with Usnea antarctica being most prominent.
Feeding experiments with the springtail Cryptopygus antarcticus
Cryptopygus antarcticus is by far the most abundant springtail in vegetated habitats of Anchorage Island (Convey and Smith 1997). Recent molecular phylogenetic research indicates that material currently referred to this species across its wide maritime and sub-Antarctic distribution is likely to represent several distinct lineages, with that from the Antarctic Peninsula referable to the subspecies C. antartcticus antarcticus (Stevens et al. 2006). Live specimens of Cryptopygus (henceforth referred to by generic name only) were transported to The Netherlands in plastic boxes with algae and mosses at 4°C. Two feeding experiments were performed. In the first experiment 20 individuals of Cryptopygus were placed in each of 30 polystyrene pots with a base of plaster of Paris. In all pots one of the following six food sources was placed: Prasiola crispa (alga), Umbilicaria decussata (lichen), U. antarctica (lichen), S. uncinata (moss), Pohlia nutans (moss) or Brachythecium austrosalebrosum (moss), each replicated five times. All these species occur naturally within local maritime Antarctic ecosystems. The pots were maintained in a climate chamber at 2°C for 6 weeks, the temperature being selected as it is the mean summer soil temperature at this location in the maritime Antarctic (S. Bokhorst et al. submitted). After this period, Cryptopygus and cryptogam samples were freeze-dried and analysed for their 13C and 15N content. Cryptopygus individuals were grouped together for each replicate in order to provide sufficient material for analyses (5 for 13C and 15 for 15N).
In the second experiment, the same six food sources were labelled with 13C and 15N. Labelling of the cryptogams and alga with 13C was achieved by placing moisturized parts of the above mentioned food sources in a desiccator with overhead lighting. Sodium bicarbonate (13C, 99%, Cambridge Isotope Laboratories Inc., Andover, MA, USA) and hydrochloric acid were placed in the desiccator for 3 h to produce the labelled 13CO2. The solution used for the 15N labelling was based on a standard medium for culturing algae (Schlosser 1997). The original solution contained sodium nitrate, which we replaced with potassium nitrate (0.18 g labelled K15NO3 and 0.71 g non-labelled KNO3) (15N, 98%, Cambridge Isotope Laboratories Inc.). The solution was then sprayed onto the cryptogams once a day over a two-week period. Cryptopygus (n = 20 individuals) were then maintained with the labelled food sources for a period of 6 weeks, as described above (n = 5 pots for each food source). After the experiment two replicate pots from each food source were sampled and the cryptogams and Cryptopygus (individuals within each replicate were grouped together to obtain sufficient material) were freeze-dried and analysed for 13C and 15N.
Natural abundance of 13C and 15N in food items and soil arthropods
For this part of the study, we focussed on the dominant soil arthropods (the isotomid springtail C. antarcticus and oribatid mite A. antarcticus) that were readily collectable from the field. At Anchorage Island both species were collected from lichen, moss and alga-dominated communities. Arthropods were either shaken from pieces of the vegetation or removed with a small brush. Individuals (n = 20) were placed in glass containers and frozen to −20°C as soon as possible after collection (typically on the day of collection, on return to the research station). Cryptogams were sampled (1–2 g per sample, n = 3) from the location at which the arthropods were collected and also stored in glass container. All samples were stored at −20°C and transported to The Netherlands. The cryptogam samples (n = 3 per species) were freeze-dried and milled before analysis of 13C and 15N. Due to their small size, soil arthropods were freeze-dried but not milled prior to analysis. Individuals were grouped to provide sufficient material for analyses: Cryptopygus, n ≈ 5 individuals, Alaskozetes, n ≈ 2 individuals for each analysis.
Chemical analyses
Analyses of 15N and 13C were carried out using a Fisons NA 1500 elemental analyzer coupled to a Finnigan conflo II interface, and a Finnigan MAT Delta S isotope ratio mass spectrometer (IRMS). Due to the low concentrations of these isotopes, the isotopic ratios (13C/12C or 15N/14N) were converted to delta units (δ) in parts per thousand, according to the formula:
delta=left(Rsamplemathordleft/vphantomRsampleRstandard−1right.kern−nulldelimiterspaceRstandard−1right)times1,000\delta = {\left( {{R_{{sample}} } \mathord{\left/ {\vphantom {{R_{{sample}} } {R_{{standard}} - 1}}} \right. \kern-\nulldelimiterspace} {R_{{standard}} - 1}} \right)} \times 1,000delta=left(Rsamplemathordleft/vphantomRsampleRstandard−1right.kern−nulldelimiterspaceRstandard−1right)times1,000
in which R is the molar ratio of heavy to light isotopes (13C/12C or 15N/14N). The standard ratio for carbon is V-PDB (13C/12C ≈ 1.124 × 10−3) and for nitrogen it is atmospheric air (15N/14N ≈ 3.67 × 10−3) (Dawson et al. 2002).
Statistical analysis
To control for non-homogeneity of variances, data were log-transformed prior to statistical analyses. Differences in stable isotope signatures between food types were analysed using a one-way ANOVA. A similar analysis was performed on the stable isotope signature of Cryptopygus obtained after feeding on different food types. In case of a significant main effect, differences among food items were a posteriori tested using Tukey HSD. Changes in stable isotope signatures of the food sources and of Cryptopygus as a result of labelling were tested with a factorial two-way ANOVA with species and labelling as independent factors. If there was a significant interaction between species and labelling the data were analysed for each species separately, using a one-way ANOVA. All analyses were completed using the program Statistica 7.0 (Statsoft Inc., Tulsa, OK, USA, http://www.statsoft.com).
Results
Feeding experiment
The variation in δ 13C values among the food items (±7‰) was considerably lower than the variation in δ 15N values (±25‰) (Fig. 1). Nevertheless, the food items differed significantly in δ 13C values (F = 14.3, P < 0.01). The three moss species had lower values than the two lichens, which were in turn lower than the alga, based on a Tukey HSD (Fig. 1a). The δ 15N of the food items also differed significantly (F = 159.0, P < 0.001). The lichens had the lowest values but there was considerable variation within this group. The mosses had higher values and were more closely grouped than the lichens. The alga had the highest value (17.0‰) based on Tukey HSD (Fig. 1b).
Fig. 1
Stable isotope contents (a δ 13C, b δ 15N) of food sources provided [mosses (m), lichens (l) and alga (a) indicated with the letters a, l and m between brackets], and of the springtail Cryptopygus antarcticus after having fed on the separate food sources for 6 weeks. Letters (a–c) between brackets indicate significant differences (P < 0.05) for food types and Cryptopygus separately, n = 2 for each bar, error bars indicated SE
The δ 13C values of Cryptopygus did not differ after feeding on any of the available food sources after 6 weeks (Fig. 1a). However, the δ 15N values of animals fed on the two lichen species were slightly lower (F = 12.2, P < 0.05) than those provided with moss or algae. Given the lower δ 15N values of the lichens compared to the alga and the mosses (Fig. 1b) this suggests that these lower values are reflected in the isotopic signature of Cryptopygus when fed with lichens. Overall, the isotopic signatures of all the Cryptopygus examined were very similar to the stable isotope signature of the alga P. crispa (Fig. 1).
Experiment with labelled food
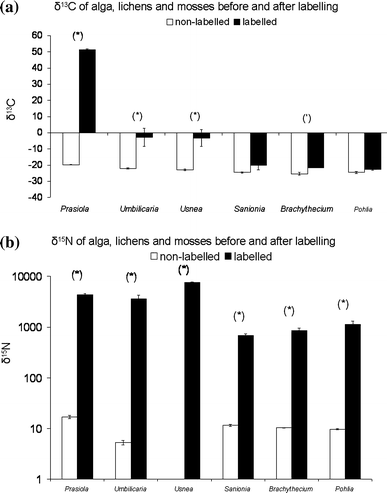
Labelling with 13C had a significant effect on the 13C signature (F = 212.1, P < 0.001) of the different food types presented. However, the increase in 13C was not significant for all the moss species (Fig. 2a) as is reflected by the significant (F = 60.8, P < 0.05) interaction between species and labelling. Labelling of food increased (F = 681.0, P < 0.001) the δ 15N for all food types. The increase in 15N was greater in the alga and lichens than the moss species (Fig. 2b), which was reflected by the significant (F = 92.1, P < 0.001) interaction between species and labelling.
Fig. 2
Stable isotope contents (a δ 13C; b δ 15N) of the alga Prasiola crispa, the lichens Umbilicaria decussata and Usnea antarctica and the mosses Brachythecium austrosalebrsoum, Sanionia uncinata and Pohlia nutans from experiment 1 (non-labelled) and experiment 2 (labelled). Note the log scale on the y-axis of b. (*) and (‘) indicate significant and marginal (P < 0.05 and 0.1 Tukey HSD) differences between labelled and non-labelled δ values. n = 2 for each bar, error bars indicate SE
For all food types, the δ 15N value of Cryptopygus increased (F = 51.2, P < 0.01) when provided with labelled food types. However when feeding on Prasiola, Umbilicaria and Usnea, the δ 15N increase was much greater than when feeding on the moss species (Fig. 3), indicating a preference for alga and lichens above moss. The δ 13C of Cryptopygus did not show a consistent change after eating labelled food.
Fig. 3
Stable isotope (δ 15N) contents of Cryptopygus antarcticus after feeding for 6 weeks on δ 15N labelled (experiment 2) and non-labelled (experiment 1) food. (*) and (‘) indicate significant (P < 0.05 and 0.1 Tukey HSD) differences. n = 2 for each bar, error bars indicate SE
The natural abundance of 13C and 15N of soil arthropods and cryptogams in the field
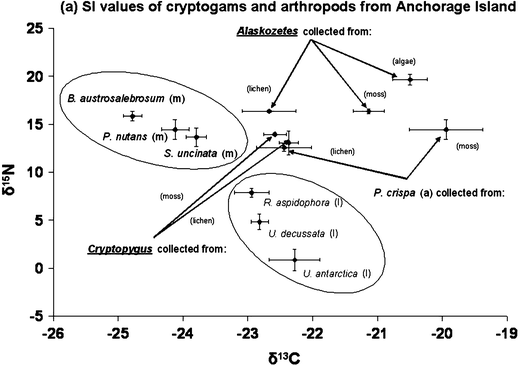
Moss and lichen species could be separated in two groups based on their δ 15N value (Fig. 4). Mosses had higher δ 15N values than lichen species, while the lichen species had large variability in their δ 15N value. The alga Prasiola had a similar δ 15N value to the moss species but the δ 13C value obtained from algae sampled amongst the lichen community was lower (P < 0.01) than in those samples obtained from the moss community (Fig. 4).
Fig. 4
Stable isotope (SI) (δ 13C and δ 15N) values of the dominant cryptogam species, Prasiola crispa (alga) and soil arthropods (Cryptopygus antarcticus and Alaskozetes antarcticus) from Anchorage Island. The circles group the lichen (l) and moss (m) species together based on SI values. The arrows indicate specific species collected from different community types. For all samples there was a minimum of three samples per species, error bars indicate SE
The δ 13C and δ 15N values obtained from field samples of Cryptopygus were similar to those of the alga Prasiola, indicating that this food type might be the main food source for this species. The other dominant soil arthropod A. antarcticus had higher δ 15N values than Cryptopygus. When sampled from a lichen community it had a lower (P < 0.05) δ 13C content than when sampled from the moss and alga community. As the alga Prasiola showed a similar separation in δ 13C it is highly likely that Alaskozetes also feeds primarily on alga. Overall these data indicate that algae are likely to be a dominant food resource for both these soil arthropod species.
Discussion
Food choice of antarctic soil arthropods
This study is the first to apply stable isotopic analyses to the study of the food webs of Antarctic soil ecosystems, although analogous studies have been successfully applied to Antarctic marine food webs (Kaehler et al. 2000; Gilpin et al. 2002; Jacob et al. 2006). This study had the primary aim of obtaining a better understanding of dietary selection in the most abundant isotomid springtail and oribatid mite of this region. When cultured on specific food types over a period of 6 weeks, the springtail Cryptopygus showed minimal changes in stable isotope signature except for a slight δ 15N decrease seen in those maintained on lichens. Analogous feeding experiments using temperate species maintained at higher temperatures have shown large changes in stable isotope composition of springtails after only 3–4 weeks (Briones et al. 1999). Therefore, the lack of response or very low response seen here could simply relate to the low temperature at which the experiment had to be maintained and/or the typically slow life cycle and development rates seen in Antarctic soil arthropods (Block and Convey 1995; Convey 1996).
Feeding with labelled food sources did result in a clear increase in δ15N content when Cryptopygus was provided with the labelled alga and lichen species. The increase in δ 15N after feeding on moss was much lower. The 15N labels of the food were not similar and may have affected the labelling of Cryptopygus. For instance, the 15N content of the lichen Usnea was twice as high as that of Prasiola. The value found in Cryptopygus was, however, higher when feeding on Prasiola than on Usnea, indicating that more of the alga was consumed than of the lichen. The same principle applies to the feeding on moss. There was an increase of δ 15N in Cryptopygus from about 15 to 30‰ when feeding on moss, but they also had a δ 15N content of about 900‰ while a higher value would be expected if the moss was readily fed from. This indicates that moss is less attractive to this springtail as a food source. A possible reason for this is that mosses contain inhibitory or refractory compounds and are difficult for soil arthropods to digest (Lawrey 1987; Davidson and Broady 1996).
The natural abundance signatures obtained from the field survey are more difficult to interpret. Literature suggests that consumers generally increase in δ 15N content (±3 to 4‰) with every trophic level. For δ 13C the increase per trophic level is about 0.1–1‰ (Peterson and Fry 1987; Robinson 2001; Dawson et al. 2002). If a species feeds on food types that differ in stable isotope composition, it normally obtains a stable isotope signature intermediate between the sources.
The similarity in stable isotope signature between Cryptopygus and Prasiola suggests that Cryptopygus mainly feeds on this source but lacks the expected 3–4‰ increase in δ 15N content. However, if Cryptopygus simultaneously feeds on lichens that have a lower δ 15N signature, the mixing of these two sources could result in an intermediate value. That we do not find an intermediate value might indicate a higher preference for Prasiola above the lichens. Feeding by Cryptopygus during the second experiment also suggests a preference for Prasiola as Cryptopygus obtained the highest δ 15N signature when provided with the alga as compared to the other food types. This suggestion is further supported by the observation that this occurred even when the δ 15N value of the labelled lichen (Usnea) was almost twice as high as that of the alga (Fig. 2). Cryptopygus had higher δ 13C values than the three moss species, but the difference was relatively high (>1.0), which exceeds the expected value for a δ 13C increase from producer to consumer. Our feeding study thus suggests that Cryptopygus generally does not feed on mosses.
Although we have not attempted similar feeding experiments with Alaskozetes the results from the field study also suggest a preference for Prasiola. This is supported by the comparable difference in δ 13C values to the alga Prasiola when the latter was sampled from the lichen or the moss vegetation (Fig. 4). The δ 13C difference of Prasiola when sampled from lichen or moss vegetation might be attributed to water availability. Very wet conditions can slow down the diffusion of CO2. This increases the internal CO2 pressure and consequently the δ 13C content of the photosynthetic products increases, with less negative δ 13C values as a result (Lange et al. 1988). Similar results for Prasiola have been reported by Huiskes et al. (2006).
Compared to Cryptopygus, Alaskozetes had a high δ 15N signature (13‰ vs. 16‰). In the absence of autoecological knowledge, such an increase might be used to suggest that Alaskozetes would be a predatory species, or at least forms part of a higher trophic level (Schneider et al. 2004). However, existing literature gives no support to the proposal that Alaskozetes would be a predator species (Block 1985; Block and Convey 1995; Worland and Lukesova 2000). Alaskozetes can however, be abundant on and near animal carcasses (Goddard 1982), and feeding on such food sources and excreta may lead to a higher δ 15N signature. Care must therefore be taken when using stable isotope signatures in understanding food web interactions and trophic levels.
The role of soil arthropod in antarctic soil ecosystems revised?
Our study has focussed on two arthropod species from Anchorage Island. These two study species are numerically the most abundant representatives of their respective groups on this island (Convey et al. 1996). Combined with the fact that there are no larger herbivores in the Maritime Antarctic region (Convey 2001), their feeding preferences are the most likely of any arthropod to play an important role in nutrient cycling in the food web.
Previous studies based on analyses of gut contents have suggested that Cryptopygus feeds on algae, dead organic material, and fungi and thus is an unselective feeder (Tilbrook 1970; Broady 1979; Burn 1984; Block 1985; Cannon 1986). Our results in part support these earlier suggestions, with the caveat that only a limited range of potential sources within each class of food was included in our feeding experiments. Although moss fragments have been reported in the gut contents of springtails in some studies (Broady 1979; Davidson and Broady 1996; Varga et al. 2002), our findings show a more active preference for algae and lichens. Feeding preference by springtails in general is far from uniform (Walter 1987; Lee and Widden 1996; Maraun et al. 2003). Moreover, a study by Worland and Lukesova (2000) has shown that Cryptopygus shows selectivity even between different alga species. Based on our results and the existing literature this might suggest that Cryptopygus is a selective feeder.
In conclusion, our findings suggest that Cryptopygus plays a larger role in the nutrient flows from the algae- and lichen-dominated components of maritime Antarctic terrestrial food webs as compared to the moss-dominated element. Alaskozetes clearly showed some preference for alga but further study is required to clarify this species’ role in nutrient flows. While clearly illustrating the potential of these techniques to be applied within Antarctic terrestrial ecosystems, more comprehensive studies of all the soil food web components and their autoecology is clearly necessary in order to understand the specific role played by each species in Antarctic soil ecosystems.
References
- Block W (1982) The Signy Island terrestrial reference sites: XIV. Population studies on the Collembola. Br Antarct Surv Bull 55:33–49
Google Scholar - Block W (1985) Arthropod interactions in an Antarctic Terrestrial community. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin Heidelberg New York
Google Scholar - Block W, Convey P (1995) The biology, life-cycle and ecophysiology of the Antarctic mite Alaskozetes antarcticus. J Zool 236:431–449
Article Google Scholar - Briones MJI, Ineson P, Sleep D (1999) Use of delta C-13 to determine food selection in collembolan species. Soil Biol Biochem 31:937–940
Article CAS Google Scholar - Broady PA (1979) Feeding studies on the collembolan Cryptopygus antarcticus Willem at Signy Island, South Orkney Islands. Br Antarct Surv Bull 48:37–46
Google Scholar - Brussaard L (1998) Soil fauna, guilds, functional groups and ecosystem processes. Appl Soil Ecol 9:123–135
Article Google Scholar - Burn AJ (1981) Feeding and growth in he Antarctic collembolan Cryptopygus antarcticus. Oikos 36:59–64
Article Google Scholar - Burn AJ (1984) Energy partitioning in the Antarctic collembolan Cryptopygus antarcticus. Ecol Entomol 9:11–21
Google Scholar - Cannon RJC (1986) Diet and acclimation on the cold tolerance and survival of an antarctic springtail. Br Antarct Surv Bull 71:19–30
Google Scholar - Chamberlain PM, Bull ID, Black HIJ, Ineson P, Evershed RP (2006a) Collembolan trophic preferences determined using fatty acid distributions and compound-specific stable carbon isotope values. Soil Biol Biochem 38:1275–1281
Article CAS Google Scholar - Chamberlain PM, Bull ID, Black HIJ, Ineson P, Evershed RP (2006b) The effect of diet on isotopic turnover in Collembola examined using the stable carbon isotopic compositions of lipids. Soil Biol Biochem 38:1146–1157
Article CAS Google Scholar - Convey P (1996) The influence of environmental characteristics on life history attributes of antarctic terrestrial biota. Biol Rev Camb Philos Soc 71:191–225
Google Scholar - Convey P (2001) Antarctic ecosystems. Encyclopedia Biodivers 1:171–184
Article Google Scholar - Convey P, Greenslade P, Richard KJ, Block W (1996) The terrestrial arthropod fauna of the Byers peninsula, Livingston Island, South Shetland Islands—Collembola. Polar Biol 16:257–259
Article Google Scholar - Convey P, Smith RIL (1997) The terrestrial arthropod fauna and its habitats in northern Marguerite Bay and Alexander Island, maritime Antarctic. Antarct Sci 9:12–26
Google Scholar - Davidson MM, Broady PA (1996) Analysis of gut contents of Gomphiocephalus hodgsoni carpenter (Collembola: hypogastruridae) at cape geology, Antarctica. Polar Biol 16:463–467
Google Scholar - Davis RC (1981) Structure and function of two Antarctic terrestrial moss communities. Ecol Monogr 51:125–143
Article Google Scholar - Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
Article Google Scholar - Faber JH (1991) Functional classification of soil fauna—a new approach. Oikos 62:110–117
Article Google Scholar - Filser J (2002) The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia 46:234–245
Google Scholar - Galimov EM (2000) Carbon isotope composition of Antarctic plants. Geochim Cosmochim Acta 64:1737–1739
Article CAS Google Scholar - Gilpin LC, Priddle J, Whitehouse MJ, Savidge G, Atkinson A (2002) Primary production and carbon uptake dynamics in the vicinity of South georgia-balancing carbon fixation and removal. Mar Ecol Prog Ser 242:51–62
Google Scholar - Goddard DG (1979) The Signy Island terrestrial reference sites: XI. Population studies on the Acari. Br Antarct Surv Bull 48:71–92
Google Scholar - Goddard DG (1982) Feeding biology of free-living acari at Signy Island, South Orkney Islands. Br Antarct Surv Bull 52:290–293
Google Scholar - Hodkinson ID, Wookey PA (1999) Functional ecology of soil organisms in tundra ecosystems: towards the future. Appl Soil Ecol 11:111–126
Article Google Scholar - Hogg ID, Craig Cary S, Convey P, Newsham KK, O’Donnell AG, Adams BJ, Aislabie J, Frati F, Stevens MI, Wall DH (2006) Biotic interactions in Antarctic terrestrial ecosystems: are they a factor? Soil Biol Biochem 38:3035–3040
Article CAS Google Scholar - Huiskes AHL, Boschker HTS, Lud D, Moerdijk-Poortvliet TCW (2006) Stable isotope ratios as a tool for assessing changes in carbon and nutrient sources in Antarctic terrestrial ecosystems. Plant Ecol 182:79–86
Article Google Scholar - Jacob U, Brey T, Fetzer I, Kaehler S, Mintenbeck K, Dunton K, Beyer K, Struck U, Pakhomov EA, Arntz WE (2006) Towards the trophic structure of the Bouvet Island marine ecosystem. Polar Biol 29:106–113
Article Google Scholar - Kaehler S, Pakhomov EA, McQuaid CD (2000) Trophic structure of the marine food web at the Prince Edward Islands (Southern Ocean) determined by delta C-13 and delta N-15 analysis. Mar Ecol Prog Ser 208:13–20
Google Scholar - Lange OL, Green TGA, Ziegler H (1988) Water status related photosynthesis and carbon isotope discrimination in species of the lichen genus pseudocyphellaria with green or blue-green photobionts and in photosymbiodemes. Oecologia 75:494–501
Article Google Scholar - Lawrey JD (1987) Nutritional ecology of lichen/moss arthropods. John, New York
Google Scholar - Lee Q, Widden P (1996) Folsomia candida, a “fungivorous” collembolan, feeds preferentially on nematodes rather than soil fungi. Soil Biol Biochem 28:689–690
Article CAS Google Scholar - Longton RE (1967) Vegetation in the maritime Antarctic. Philos Trans R Soc Lond B Biol Sci 252:213–235
Google Scholar - Maraun M, Martens H, Migge S, Theenhaus A, Scheu S (2003) Adding to ‘the enigma of soil animal diversity’: fungal feeders and saprophagous soil invertebrates prefer similar food substrates. Eur J Soil Biol 39:85–95
Article Google Scholar - Neilson R, Hamilton D, Wishart J, Marriott CA, Boag B, Handley L, Scrimgeour CM, McNicol JW, Robinson D (1998) Stable isotope natural abundances of soil, plants and soil invertebrates in an upland pasture. Soil Biol Biochem 30:1773–1782
Article CAS Google Scholar - Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Article Google Scholar - Richard KJ, Convey P, Block W (1994) The terrestrial arthropod fauna of the Byers-Peninsula, Livingston-Island, South-Shetland-Islands. Polar Biol 14:371–379
Article Google Scholar - Robinson D (2001) Delta N-15 as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Article PubMed Google Scholar - Scheu S, Falca M (2000) The soil food web of two beech forests (Fagus sylvatica) of contrasting humus type: stable isotope analysis of a macro- and a mesofauna-dominated community. Oecologia 123:285–296
Article Google Scholar - Schlosser UG (1997) Additions to the culture collection of Alga since 1994. Bot Acta 110:424–429
Google Scholar - Schmidt O, Curry JP, Dyckmans J, Rota E, Scrimgeour CM (2004) Dual stable isotope analysis (delta C-13 and delta N-15) of soil invertebrates and their food sources. Pedobiologia 48:171–180
Article Google Scholar - Schneider K, Migge S, Norton RA, Scheu S, Langel R, Reineking A, Maraun M (2004) Trophic niche differentiation in soil microarthropods (Oribatida, acari): evidence from stable isotope ratios (15N/14N). Soil Biol Biochem 36:1769–1774
Article CAS Google Scholar - Sinclair BJ (2001) On the distribution of terrestrial invertebrates at Cape Bird, Ross Island, Antarctica. Polar Biol 24:394–400
Article Google Scholar - Staddon PL (2004) Carbon isotopes in functional soil ecology. Trends Ecol Evol 19:148–154
Article PubMed Google Scholar - Stevens MI, Greenslade P, Hogg ID, Sunnucks P (2006) Examining Southern hemisphere springtails: could any have survived glaciation of Antarctica? Mol Biol Evol 23:874–882
Article PubMed CAS Google Scholar - Tilbrook PJ (1970) The biology of Cryptopygus antarcticus. In: Holdgate MW (ed) Antarctic ecology, vol 2. Academic, London
- Usher MB, Booth RG (1986) Arthropod communities in a maritime antarctic moss-turf habitat: multiple scales of pattern in the mites and Collembola. J Anim Ecol 55:155–170
Article Google Scholar - Varga J, Naar Z, Dobolyi C (2002) Selective feeding of collembolan species Tomocerus longicornis (Mull.) and Orchesella cincta (L.) on moss inhabiting fungi. Pedobiologia 46:526–538
Article Google Scholar - Walter DE (1987) Trophic behaviour of “Mycophagous” microarthropods. Ecology 68:226–229
Article Google Scholar - Worland MR, Lukesova A (2000) The effect of feeding on specific soil algae on the cold- hardiness of two Antarctic micro-arthropods (Alaskozetes antarcticus and Cryptopygus antarcticus). Polar Biol 23:766–774
Article Google Scholar
Acknowledgments
We are very grateful for the logistical support given by the British Antarctic Survey. This work was financially supported by The Netherlands Polar Programme (NPP-NWO 851.20.016), and forms an output of the SCAR “Evolution and Biodiversity in the Antarctic” program. The manuscript was improved by the comments of two anonymous reviewers.
Author information
Authors and Affiliations
- Centre for Estuarine and Marine Ecology, Netherlands Institute of Ecology, Korringaweg 7, 4401 NT, Yerseke, The Netherlands
S. Bokhorst, C. Ronfort & A. Huiskes - Natural Environmental Research Council, British Antarctic Survey, High Cross, Madingley Road, Cambridge, CB3 0ET, UK
P. Convey - Institute of Ecological Science, Department of Systems Ecology, Vrije Universiteit, De Boelelaan 1085, 1081 HV, Amsterdam, The Netherlands
R. Aerts
Authors
- S. Bokhorst
You can also search for this author inPubMed Google Scholar - C. Ronfort
You can also search for this author inPubMed Google Scholar - A. Huiskes
You can also search for this author inPubMed Google Scholar - P. Convey
You can also search for this author inPubMed Google Scholar - R. Aerts
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toS. Bokhorst.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bokhorst, S., Ronfort, C., Huiskes, A. et al. Food choice of Antarctic soil arthropods clarified by stable isotope signatures.Polar Biol 30, 983–990 (2007). https://doi.org/10.1007/s00300-007-0256-4
- Received: 14 November 2006
- Revised: 10 January 2007
- Accepted: 13 January 2007
- Published: 22 February 2007
- Issue Date: July 2007
- DOI: https://doi.org/10.1007/s00300-007-0256-4