HBV Reactivation After Bariatric Surgery for HBV-Infected Obese Patients (original) (raw)
Abstract
Background
The association between non-alcoholic fatty liver disease and hepatitis B virus (HBV) infection is inconclusive. The aim of this study was to investigate the viral dynamic of HBV and its association with change of body mass index (BMI), aspartate transaminase (AST), and alanine transaminase (ALT) levels after bariatric surgery.
Methods
Patients who underwent bariatric surgery between June 2011 and May 2014 were selected in this retrospective study. BMI, AST, ALT, and HBV DNA levels were calculated pre-operatively and at 1st, 3rd, and 6th postoperative months.
Results
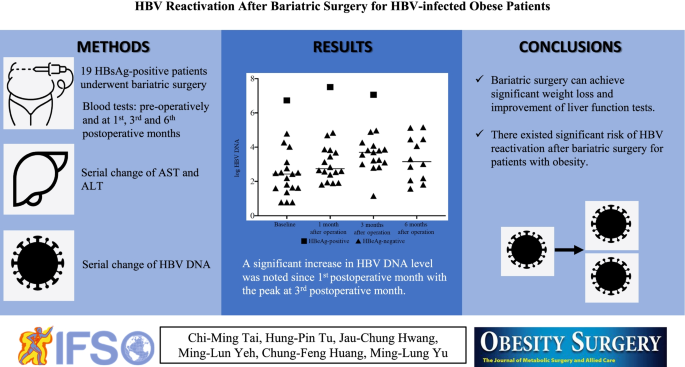
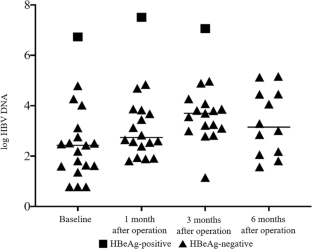
Two hundred and seventy-nine patients including 34 (12.2%) HBsAg-positive and 245 (87.8%) HBsAg-negative patients were enrolled. Eighteen HBsAg-positive and HBeAg-negative patients were matched with 36 HBsAg-negative patients. A significant decrease in BMI was found since 1st postoperative month in both groups. AST and ALT increased at 1st postoperative month, but decreased at 3rd and 6th postoperative months in both groups. However, a significant increase in HBV DNA level was observed in HBeAg-negative patients since 1st postoperative month with the highest peak at 3rd postoperative month. HBV reactivation occurred in 4 out of 17 (23.5%) patients, 8 out of 16 (50.0%) patients, and 4 out of 12 (33.3%) patients at 1st, 3rd, and 6th postoperative months, respectively. The change of HBV DNA was not associated with change of BMI, AST, or ALT after bariatric surgery.
Conclusion
Bariatric surgery can achieve significant weight loss and improvement of liver function tests. However, there existed significant risk of HBV reactivation after bariatric surgery for patients with obesity.
Graphical abstract
Access this article
Subscribe and save
- Starting from 10 chapters or articles per month
- Access and download chapters and articles from more than 300k books and 2,500 journals
- Cancel anytime View plans
Buy Now
Price excludes VAT (USA)
Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Fig. 1
Similar content being viewed by others
Abbreviations
HBV:
Hepatitis B virus
BMI:
Body mass index
AST:
Aspartate transaminase
ALT:
Alanine transaminase
NAFLD:
Nonalcoholic fatty liver disease
References
- Sarin SK, Kumar M, Eslam M, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5(2):167–228.
Article Google Scholar - Chen CL, Yang JY, Lin SF, et al. Slow decline of hepatitis B burden in general population: results from a population-based survey and longitudinal follow-up study in Taiwan. J Hepatol. 2015;63(2):354–63.
Article Google Scholar - Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
Article Google Scholar - Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57–73.
Article Google Scholar - Huang JF, Tsai PC, Yeh ML, et al. Risk stratification of non-alcoholic fatty liver disease across body mass index in a community basis. J Formos Med Assoc. 2020;119(1 Pt 1):89–96.
Article Google Scholar - Tai CM, Huang CK, Tu HP, Hwang JC, Chang CY, Yu ML. PNPLA3 genotype increases susceptibility of nonalcoholic steatohepatitis among obese patients with nonalcoholic fatty liver disease.Surg Obes Relat Dis 2015;11(4):888–94.
- Huang CK, Lee YC, Hung CM, Chen YS, Tai CM. Laparoscopic Roux-en-Y gastric bypass for morbidly obese Chinese patients: learning curve, advocacy and complications.Obes Surg 2008;18(7):776–81.
- Pedroso FE, Angriman F, Endo A, et al. Weight loss after bariatric surgery in obese adolescents: a systematic review and meta-analysis.Surg Obes Relat Dis 2018;14(3):413–22.
- Tai CM, Huang CK, Hwang JC, et al. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients.Obes Surg 2012;22(7):1016–21.
- Fakhry TK, Mhaskar R, Schwitalla T, et al. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis.Surg Obes Relat Dis 2019;15(3):502–11.
- Suliman I, Abdelgelil N, Kassamali F, Hassanein TI. The effects of hepatic steatosis on the natural history of HBV infection.Clin Liver Dis 2019;23(3):433–50.
- Xiong J, Zhang H, Wang Y, et al. Hepatitis B virus infection and the risk of nonalcoholic fatty liver disease: a meta-analysis.Oncotarget 2017;8(63):107295–302.
- Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients.J Gastroenterol Hepatol 2011;26(9):1361–7.
- Cheng YL, Wang YJ, Kao WY, et al. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up.PLoS One 2013;8(8):e72049.
- Wang MM, Wang GS, Shen F, Chen GY, Pan Q, Fan JG. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors.Dig Dis Sci 2014;59(10):2571–9.
- Hui RWH, Seto WK, Cheung KS, et al. Inverse relationship between hepatic steatosis and hepatitis B viremia: results of a large case-control study.J Viral Hepat 2018;25(1):97–104.
- Wang L, Wang Y, Liu S, et al. Nonalcoholic fatty liver disease is associated with lower hepatitis B viral load and antiviral response in pediatric population.J Gastroenterol 2019;54(12):1096–105.
- Lee WJ, Wang W, Lee YC, Huang MT. Clinical characteristics and outcome of morbidly obese bariatric patients with concurrent hepatitis B viral infection. Obes Surg. 2008;18(5):589–94.
Article Google Scholar - Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease.Hepatology 2005;41(6):1313–21.
- Yeh ML, Huang CF, Huang CI, et al. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection.J Hepatol 2020 Jul;73(1):62–71.
- Wong VW, Wong GL, Chu WC, et al. Hepatitis B virus infection and fatty liver in the general population.J Hepatol 2012;56(3):533–40.
- Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: a cohort study.Hepatology 2017;65(3):828–35.
- Zhu L, Jiang J, Zhai X, et al. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: a population-based cohort study. Liver Int. 2019;39(1):70–80.
Article CAS Google Scholar - Zhang Z, Pan Q, Duan XY, et al. Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model.J Gastroenterol Hepatol 2012;27(12):1858–64.
- Hu D, Wang H, Wang H, et al. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model.Hepatol Int 2018;12(5):438–46
- Liu Q, Mu M, Chen H, et al. Hepatocyte steatosis inhibits hepatitis B virus secretion via induction of endoplasmic reticulum stress. Mol Cell Biochem. 2021. https://doi.org/10.1007/s11010-021-04143-z.
Article PubMed PubMed Central Google Scholar - Shlomai A, Paran N, Shaul Y. PGC-1alpha controls hepatitis B virus through nutritional signals.Proc Natl Acad Sci U S A 2006;103(43):16003–8.
- Shlomai A, Shaul Y. The metabolic activator FOXO1 binds hepatitis B virus DNA and activates its transcription.Biochem Biophys Res Commun 2009;381(4):544–8.
- Mouler Rechtman M, Burdelova EO, Bar-Yishay I, et al. The metabolic regulator PGC-1alpha links anti-cancer cytotoxic chemotherapy to reactivation of hepatitis B virus. J Viral Hepat. 2013;20(1):34–41.
Article CAS Google Scholar - Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. 2021;15(5):1031–48.
Article Google Scholar - Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99.
Article Google Scholar - European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98.
Article Google Scholar - Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Article CAS Google Scholar
Funding
This study was supported by research grants from E-Da Hospital, Taiwan (EDAHP108003 and EDAHP109004).
Author information
Authors and Affiliations
- Department of Medicine, Division of Gastroenterology and Hepatology, I-Shou University, E-Da Hospital, Kaohsiung, Taiwan
Chi-Ming Tai - School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan
Chi-Ming Tai - Department of Public Health and Environmental Medicine, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Hung-Pin Tu - Department of Pathology, Lin Shin Hospital, Taichung, Taiwan
Jau-Chung Hwang - Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital, 100 Tz-You 1st road, Kaohsiung, Taiwan
Ming-Lun Yeh, Chung-Feng Huang & Ming-Lung Yu - School of Medicine and Hepatitis Research Center, College of Medicine, and Center for Liquid Biopsy and Cohort Research, Kaohsiung Medical University, Kaohsiung, Taiwan
Ming-Lun Yeh, Chung-Feng Huang & Ming-Lung Yu - National Pingtung University of Science and Technology, Pingtung, Taiwan
Ming-Lung Yu
Authors
- Chi-Ming Tai
- Hung-Pin Tu
- Jau-Chung Hwang
- Ming-Lun Yeh
- Chung-Feng Huang
- Ming-Lung Yu
Corresponding author
Correspondence toMing-Lung Yu.
Ethics declarations
Ethics Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Bariatric surgery can achieve significant weight loss and improvement of liver function tests.
• A significant increase in HBV DNA level was observed in HBeAg-negative patients after bariatric surgery.
• The change of HBV DNA was not associated with change of BMI, AST, or ALT after bariatric surgery.
Supplementary Information
Rights and permissions
About this article
Cite this article
Tai, CM., Tu, HP., Hwang, JC. et al. HBV Reactivation After Bariatric Surgery for HBV-Infected Obese Patients.OBES SURG 32, 3332–3339 (2022). https://doi.org/10.1007/s11695-022-05979-0
- Received: 29 November 2021
- Revised: 10 February 2022
- Accepted: 17 February 2022
- Published: 04 August 2022
- Version of record: 04 August 2022
- Issue date: October 2022
- DOI: https://doi.org/10.1007/s11695-022-05979-0
Keywords
Profiles
- Chi-Ming Tai View author profile
- Hung-Pin Tu View author profile
- Ming-Lung Yu View author profile