Complicated intra-abdominal infections in a worldwide context: an observational prospective study (CIAOW Study) (original) (raw)
Abstract
Despite advances in diagnosis, surgery, and antimicrobial therapy, mortality rates associated with complicated intra-abdominal infections remain exceedingly high. The World Society of Emergency Surgery (WSES) has designed the CIAOW study in order to describe the clinical, microbiological, and management-related profiles of both community- and healthcare-acquired complicated intra-abdominal infections in a worldwide context. The CIAOW study (Complicated Intra-Abdominal infection Observational Worldwide Study) is a multicenter observational study currently underway in 57 medical institutions worldwide. The study includes patients undergoing surgery or interventional drainage to address complicated intra-abdominal infections. This preliminary report includes all data from almost the first two months of the six-month study period. Patients who met inclusion criteria with either community-acquired or healthcare-associated complicated intra-abdominal infections (IAIs) were included in the study. 702 patients with a mean age of 49.2 years (range 18–98) were enrolled in the study. 272 patients (38.7%) were women and 430 (62.3%) were men. Among these patients, 615 (87.6%) were affected by community-acquired IAIs while the remaining 87 (12.4%) suffered from healthcare-associated infections. Generalized peritonitis was observed in 304 patients (43.3%), whereas localized peritonitis or abscesses was registered in 398 (57.7%) patients.
The overall mortality rate was 10.1% (71/702). The final results of the CIAOW Study will be published following the conclusion of the study period in March 2013.
Similar content being viewed by others
Introduction
Intra-abdominal infections (IAIs) include a wide spectrum of pathological conditions, ranging from uncomplicated appendicitis to fecal peritonitis [1].
From a clinical perspective, IAIs are classified in two major categories: complicated and uncomplicated.
In uncomplicated IAIs, the infectious process only involves a single organ and does not spread to the peritoneum. Patients with such infections can be managed with either surgical resection or antibiotics. When the focus of infection is treated effectively by surgical excision, 24-hour perioperative prophylaxis is typically sufficient. Patients with less severe intra-abdominal infections, including acute diverticulitis and certain forms of acute appendicitis, may be treated non-operatively.
In complicated IAIs, the infectious process extends beyond a singularly affected organ, and causes either localized peritonitis or diffuse peritonitis. The treatment of patients with complicated intra-abdominal infections involves both source control and antibiotic therapy.
Intra-abdominal infections are further classified in two groups: community-acquired intra-abdominal infections (CA-IAIs) and healthcare-associated intra-abdominal infections (HA-IAIs). CA-IAIs are acquired directly in the community while HA-IAIs develop in hospitalized patients or residents of long-term healthcare facilities. HA-IAIs are associated with higher rates of mortality due to the patients’ poorer underlying health and an increased likelihood of infection by multi-drug resistant microorganisms.
Source control encompasses all measures undertaken to eliminate the source of infection and to control ongoing contamination.
The most common source of infection in community-acquired intra-abdominal infections is the appendix, followed by the colon, and then the stomach. Dehiscence complicates 5-10% of intra-abdominal bowel anastomoses and is associated with high rates of mortality [2].
Ultrasound- and CT-guided percutaneous drainage of abdominal and extra-peritoneal abscesses have proven to be safe and effective in select patients [3–10].
Surgery is the most important therapeutic recourse for controlling intra-abdominal infections.
Generally, the choice of the procedure depends on the anatomical source of infection, on the degree of peritoneal inflammation, on the generalized septic response and on the patient’s general conditions.
Patients suffering from severe peritonitis are prone to persisting intra-abdominal sepsis, even when the source of infection has been neutralized. Timely re-laparotomy is the only possible known surgical recourse, capable to significantly improve patient outcome in these cases.
In the event of secondary peritonitis, the decision and timing of re-laparotomy is largely subjective and is often based on a surgeon’s professional experience. Factors indicative of progressive or persistent organ failure during early postoperative follow-up analysis are the strongest indicators of ongoing infection and suggest positive findings upon re-laparotomy [11–13].
Three methods of localized, mechanical management of abdominal sepsis following the initial laparotomy, which was performed for purposes of source control, are currently debated within the medical community: open-abdomen, planned re-laparotomy and on-demand re-laparotomy
Antimicrobial therapy plays an integral role in the management of intra-abdominal infections, especially in critically ill patients requiring immediate empiric antibiotic therapy.
Empiric antibiotic therapy accounts for the most frequently isolated microorganisms as well as any local trends of antibiotic resistance.
The major pathogens involved in community-acquired intra-abdominal infections are Enterobacteriaceae and anaerobic microbes (especially B. fragilis).
An antimicrobial-based approach to treating intra-abdominal infections involves a delicate balance between the optimization of empirical therapy, which has been shown to improve clinical outcomes, and the reduction of excessive antimicrobial use, which has been proven to increase the rate of emergence of antimicrobial-resistant strains.
The threat of antimicrobial resistance is one of the major challenges associated with the antimicrobial management of complicated intra-abdominal infections.
The recent and rapid spread of serine carbapenemases in Klebsiella pneumoniae (KPC) has become an important concern when administering antimicrobial therapy in hospitals worldwide [14].
The growing emergence of multidrug-resistant bacteria and the limited availability of new antibiotics to counteract them has brought about an impending crisis with alarming implications (especially regarding gram-negative microorganisms).
Methods
Aim
The purpose of the study is to describe the clini-cal, microbiological, and treatment profiles of both community-associated and healthcare-acquired complicated intra-abdominal infections (IAIs) in a worldwide context.
Patients older than 18 years with both community-acquired and healthcare-associated intra-abdominal infections will be included in the database.
In Europe, the CIAO Study has recently ended, concluding a six-month, multicenter observational study across twenty European countries. The study’s findings have recently been published [15].
Given the promising results of the CIAO Study, the World Society of Emergency Surgery (WSES) has designed a prospective observational study investigating the management of complicated intra-abdominal infections in a worldwide context.
Study population
The CIAOW study (Complicated Intra-Abdominal infection Observational Worldwide Study) is a multicenter observational study currently underway in 57 medical institutions worldwide. The study includes patients undergoing surgery or interventional drainage to address complicated IAIs.
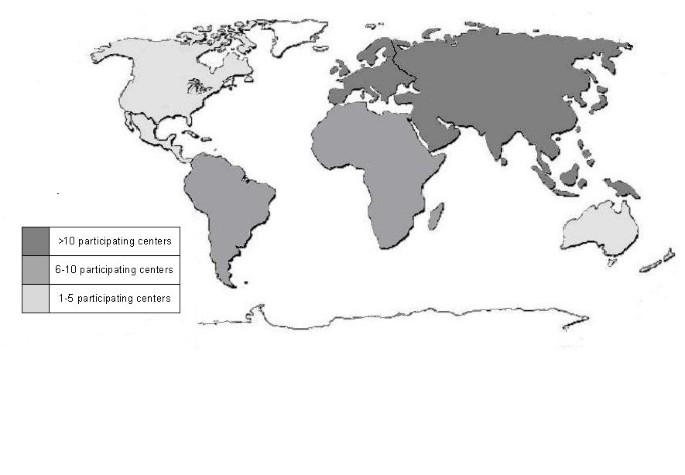
Medical institutions from each continent participate in the study. The geographical distribution of the participating centers is represented in Figure 1.
Figure 1
Participating centers for each continent.
Study design
The study does not attempt to change or modify the laboratory or clinical practices of the participating physicians, and neither informed consent nor formal approval by an Ethics Committee has been required.
The study meets the standards outlined in the Declaration of Helsinki and Good Epidemiological Practices.
The study is monitored by the coordination center, which investigates and verifies missing or unclear data submitted to the central database. It is performed under the direct supervision of the board of directors of WSES.
Data collection
In each center, the coordinator collects and compiles data in an online case report system.These data include the following: (i) patient and disease characteristics, i.e., demographic data, type of infection (community- or healthcare-acquired), severity criteria, previous curative antibiotic therapy administered in the 7 days preceding surgery; (ii) origin of infection and surgical procedures performed; and (iii) microbiological data, i.e., identification of bacteria and microbial pathogens within the peritoneal fluid, the presence of yeasts (if applicable), and the antibiotic susceptibilities of bacterial isolates.
The primary endpoints include the following:
- Clinical profiles of intra-abdominal infections
- Epidemiological profiles (epidemiology of the microorganisms isolated from intra-abdominal samples and these organisms’ resistance to antibiotics)
- Management profiles
Statistical analysis
At the end of the six-month study period statistical comparisons will be performed using the Student’s t-test, χ2 analysis, or the Kruskall–Wallis/Wilcoxon tests, as dictated by the natural parameters of the data. Statistical significance will be defined as a P-value less than 0.05 (P < 0.05). Multivariate analysis will be carried out by means of stepwise logistic regressions in order to assess the predictive factors of mortality during hospitalization. Adjusted odds ratios (OR) and their 95% confidence intervals (CI) will also be included.
Inclusion criteria
- Patients older than 18 years
- Community- and healthcare-acquired complicated intra-abdominal infections
Exclusion criteria
- Age under 18 years old
- Pancreatitis
- Primary peritonitis.
Preliminary results
Patients
This preliminary report includes all data from the first two months of the six-month study period.
702 patients with a mean age of 49.2 years (range 18–98) were enrolled in the study. 272 patients (38.7%) were women and 430 (62.3%) were men. Among these patients, 615 (87.6%) were affected by community-acquired IAIs while the remaining 87 (12.4%) suffered from healthcare-associated infections.
304 patients (43.3%) were affected by generalized peritonitis while 398 (57.7%) suffered from localized peritonitis or abscesses.
112 patients (15.9%) were admitted in critical condition (severe sepsis, septic shock).
Source control
The various sources of infection are outlined in Table 1. The most frequent source of infection was acute appendicitis. 243 cases were attributable to this condition.
Table 1 Source of infection
The most frequently performed procedure employed to address complicated appendicitis was the open appendectomy. 136 patients (55.9%) admitted for complicated appendicitis underwent open appendectomies: 95 patients (69.8%) for localized infection or abscesses and 41 patients (31.2%) for generalized peritonitis. A laparoscopic appendectomy was performed on 93 patients (39.4%) presenting with complicated acute appendicitis, 82 (88.2%) and 11 (11.8%) of whom underwent the procedure for localized peritonitis/abscesses and generalized peritonitis, respectively. Open colonic resection was performed on 1 patient to address complicated appendicitis. In the other cases of complicated appendicitis, conservative treatment (percutaneous drainage, surgical drainage, and non-operative treatment) was performed. 7 (3%) patients underwent percutaneous drainage to address appendicular abscesses.
For patients with complicated acute cholecystitis (104 cases), the most frequently performed procedure to address cholecystitis was the open cholecystectomy. 53 cholecystitis patients (51%) underwent this procedure. A laparoscopic cholecystectomy was performed on 27 patients (26%). In the remaining cases, conservative treatment methods (percutaneous drainage, non-operative treatment) were alternatively employed.
Among the patients with complicated diverticulitis (40) the Hartmann resection was the most frequently performed procedure. 12 patients (30%) underwent a Hartmann resection. All these resections were open procedures. 8 of these patients underwent a Hartmann resection for generalized peritonitis, while the remaining 4 underwent the same procedure for localized peritonitis or abscesses.
Colo-rectal resection was performed in 11 cases (27.5%) (4 with and 7 without stoma protection).
The other patients received conservative treatment (percutaneous drainage, non-operative treatment, surgical drainage and stoma). Only two (5%) underwent laparoscopic lavage and drainage.
Of the 100 patients with gastro-duodenal perforations, the most frequent surgical procedure was gastro-duodenal suture. It was performed in 91 patients (91%): 85 patients underwent open gastro-duodenal suture and 6 patients underwent laparoscopic gastro-duodenal suture. Four (4%) patients underwent gastro-duodenal resection. The remaining patients (5%) received conservative treatment (non-operative treatment, surgical drainage).
Among the 53 patients with small bowel perforations, 35 underwent open small bowel resection (79.5%) and one (4.5%) underwent laparoscopic small bowel resection. Fourteen patients were treated by stoma. Two patients were treated by open drainage
Among the 38 patients with colonic non-diverticular perforation, 15 patients (66%) underwent open Hartmann resection, 1 patient (2.6%) underwent laparoscopic Hartmann resection, 9 (25%) underwent open resection with anastomosis and without stoma protection, and 4 underwent open resection with stoma protection (10.5%).
Microbiology
Intraperitoneal specimens were collected from 415 (59.1%) patients.
Intraperitoneal specimens were isolated from 336 of the 615 patients with community-acquired intra-abdominal infections (54.6%). Among the remaining 87 patients with healthcare-associated intra-abdominal infections, intraperitoneal specimens were collected from 79 patients (90.9%).
The major pathogens involved in intra-abdominal infections were found to be Enterobacteriaceae.
The aerobic bacteria identified in samples of peritoneal fluid are reported in Table 2.
Table 2 Aerobic bacteria identified in peritoneal fluid
According to CIAOW Study data, ESBL producers were the most commonly identified drug-resistant microorganism involved in IAIs.
1 identified isolate of Klebsiella pneumoniae proved resistant to Carbapenems.
Among the identified aerobic gram-negative isolates, there were 25 isolates of Pseudomonas aeruginosa, comprising 5.5% of all identified aerobic bacteria isolates.
Among the identified aerobic gram-positive bacteria, Enterococci (E. faecalis and E. faecium) were the most prevalent, representing 10.5% of all aerobic isolates, 3 glycopeptide-resistant Enterococci were identified; 2 were glycopeptide-resistant Enterococcus faecalis isolates and 1 was glycopeptide-resistant Enterococcus faecium isolates.
Tests for anaerobes were conducted for 168 patients.
52 anaerobes were observed. The most frequently identified anaerobic pathogen was Bacteroides. 39 Bacteroides isolates were observed during the course of the study.
Additionally, 36 Candida isolates were collectively identified. 30 were Candida albicans and 6 were non-albicans Candida.
Outcome
The overall mortality rate was 10.1% (71/702).
68 patients (9.7%) were admitted to the intensive care unit in the early recovery phase immediately following surgery.
90 patients (12.8%) ultimately required additional surgeries; 54% of these underwent relaparotomies “on-demand”, 28.9% underwent open abdomen procedures.
According to univariate statistical analysis, a critical clinical condition (severe sepsis and septic shock) upon hospital admission was the most significant risk factor for death; indeed, the rate of patient mortality was 36.6% (41/112) among critically ill patients (patients presenting with septic shock and severe sepsis upon admission), but the mortality rate was only 5.1% (30/590) for clinically stable patients (p < 0.0001).
For patients with generalized peritonitis, the mortality rate was 18% (55/304) while patients with localized peritonitis or abscesses demonstrated a mortality rate of only 4% (16/398) (p < 0,001).
The immediate post-operative clinical course was a significant parameter for predicting mortality: the rate of patient mortality was 54.9% (51/93) among critically ill patients (patients presenting with septic shock and severe sepsis upon the immediate post-operative course), but the mortality rate was only 3.3% (20/609) for clinically stable patients (p < 0.0001).
Preliminary statistical analyses were performed using MedCalc® statistical software.
Conclusion
Complicated intra-abdominal infections remain an important cause of morbidity with poor clinical prognoses.
The purpose of the CIAOW Study is to describe the epidemiological, clinical, microbiological, and treatment profiles of both community-acquired and healthcare-acquired complicated intra-abdominal infections (IAIs) based on the data collected over a six-month period (October 2012 to March 2013) from 56 medical institutions Worldwide.
The final results of the CIAOW Study will be published following the conclusion of the study period in March 2013.
References
- Sartelli M, Viale P, Koike K, Pea F, Tumietto F, van Goor H, Guercioni G, Nespoli A, Tranà C, Catena F, Ansaloni L, Leppaniemi A, Biffl W, Moore FA, Poggetti R, Pinna AD, Moore EE, WSES consensus conference: Guidelines for first-line management of intra-abdominal infections. World J Emerg Surg. 2011, 6: 2.10.1186/1749-7922-6-2.
Article PubMed Central PubMed Google Scholar - Sartelli M: A focus on intra-abdominal infections. World J Emerg Surg. 2010, 5: 9-10.1186/1749-7922-5-9.
Article PubMed Central PubMed Google Scholar - Azzarello G, Lanteri R, Rapisarda C, Santangelo M, Racalbuto A, Minutolo V, Di Cataldo A, Licata A: Ultrasound-guided percutaneous treatment of abdominal collections. Chir Ital. 2009, 61: 337-340.
PubMed Google Scholar - Gazelle GS, Mueller PR: Abdominal abscess: Imaging and intervention. Radiol Clin North Am. 1994, 32: 913-932.
CAS PubMed Google Scholar - VanSonnenberg E, Ferrucci JT, Mueller PR, Wittenberg J, Simeone JF: Percutaneous drainage of abscesses and fluid collections: Technique, results, and applications. Radiology. 1982, 142: 1-10.
Article CAS PubMed Google Scholar - Bouali K, Magotteaux P, Jadot A, Saive C, Lombard R, Weerts J, Dallemagne B, Jehaes C, Delforge M, Fontaine F: Percutaneous catheter drainage of abdominal abscess after abdominal surgery: Results in 121 cases. J Belg Radiol. 1993, 76: 11-14.
CAS PubMed Google Scholar - VanSonnenberg E, Wing VW, Casola G, Coons HG, Nakamoto SK, Mueller PR, Ferrucci JT, Halasz NA, Simeone JF: Temporizing effect of percutaneous drainage of complicated abscesses in critically ill patients. Am J Roentgenol. 1984, 142: 821-826. 10.2214/ajr.142.4.821.
Article CAS Google Scholar - Bufalari A, Giustozzi G, Moggi L: Postoperative intra-abdominal abscesses: Percutaneous versus surgical treatment. Acta Chir Belg. 1996, 96 (5): 197-200.
CAS PubMed Google Scholar - VanSonnenberg E, Mueller PR, Ferrucci JT: Percutaneous drainage of 250 abdominal abscesses and fluid collections. I. Results, failures, and complications. Radiology. 1984, 151: 337-341.
Article CAS PubMed Google Scholar - Jaffe TA, Nelson RC, DeLong D, Paulson EK: Practice Patterns in Percutaneous Image-guided Intra-abdominal Abscess Drainage: Survey of Academic and Private Practice Centres. Radiology. 2004, 233: 750-756. 10.1148/radiol.2333032063.
Article PubMed Google Scholar - van Ruler O, Lamme B, Gouma DJ, Reitsma JB, Boermeester MA: Variables associated with positive findings at relaparotomy in patients with secondary peritonitis. Crit Care Med. 2007, 35 (2): 468-476. 10.1097/01.CCM.0000253399.03545.2D.
Article PubMed Google Scholar - Hutchins RR, Gunning MP, Lucas DN, Allen-Mersh TG, Soni NC: Relaparotomy for suspected intraperitoneal sepsis after abdominal surgery. World J Surg. 2004, 28 (2): 137-141. 10.1007/s00268-003-7067-8.
Article PubMed Google Scholar - Lamme B, Mahler CW, van Ruler O, Gouma DJ, Reitsma JB, Boermeester MA: Clinical predictors of ongoing infection in secondary peritonitis: systematic review. World J Surg. 2006, 30 (12): 2170-2181. 10.1007/s00268-005-0333-1.
Article PubMed Google Scholar - Hawser SP, Bouchillon SK, Lascols C, Hackel M, Hoban DJ, Badal RE, Woodford N, Livermore DM: Susceptibility of Klebsiella pneumoniae isolates from intra-abdominal infections and molecular characterization of ertapenem-resistant isolates. Antimicrob Agents Chemother. 2011, 55 (8): 3917-3921. 10.1128/AAC.00070-11.
Article PubMed Central CAS PubMed Google Scholar - Sartelli M, Catena F, Ansaloni L, Leppaniemi A, Taviloglu K, Goor H, Viale P, Lazzareschi DV, Coccolini F, Corbella D, Werra C, Marrelli D, Colizza S, Scibè R, Alis H, Torer N, Navarro S, Sakakushev B, Massalou D, Augustin G, Catani M, Kauhanen S, Pletinckx P, Kenig J, Saverio S, Jovine E, Guercioni G, Skrovina M, Diaz-Nieto R, Ferrero A: Complicated intra-abdominal infections in Europe: a comprehensive review of the CIAO study. World J Emerg Surg. 2012, 7 (1): 36-10.1186/1749-7922-7-36.
Article PubMed Central PubMed Google Scholar
Author information
Authors and Affiliations
- Department of Surgery, Macerata Hospital, Macerata, Italy
Massimo Sartelli - Emergency Surgery, Maggiore Parma Hospital, Parma, Italy
Fausto Catena - Department of General Surgery, Ospedali Riuniti, Bergamo, Italy
Luca Ansaloni, Elia Poiasina & Federico Coccolini - Department of Surgery, Denver Health Medical Center, Denver, CO, USA
Ernest Moore & Walter Biffl - American Board of Surgery, Philadelphia, PA, USA
Mark Malangoni - Harvard Medical School, Division of Trauma, Emergency Surgery and Surgical Critical Care Massachusetts General Hospital, Boston, MA, USA
George Velmahos & Miroslav P Peev - Department of Surgery, UC San Diego Health System, San Diego, CA, USA
Raul Coimbra - Department of Primary Care & Emergency Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan
Kaoru Koike & Norio Sato - Department of Abdominal Surgery, University Hospital Meilahti, Helsinki, Finland
Ari Leppaniemi - Department of Surgery, University of Newcastle, Newcastle, NSW, Australia
Zsolt Balogh & Cino Bendinelli - Department of Surgery, Govt Medical College and Hospital, Chandigarh, India
Sanjay Gupta, AK Attri & Rajeev Sharma - Department of General Surgery, Rambam Health Care Campus, Haifa, Israel
Yoram Kluger & Offir Ben-Ishay - Department of Surgery, Adria Hospital, Adria, Italy
Ferdinando Agresta - Trauma Surgery Unit, Maggiore Hospital, Bologna, Italy
Salomone Di Saverio & Gregorio Tugnoli - Department of Surgery, Maggiore Hospital, Bologna, Italy
Elio Jovine & Rita Barnabé - Department of Surgery, Universidad del Valle, Fundacion Valle del Lili, Cali, Colombia
Carlos Ordonez & Juan Sanjuan - Department of Surgery, Monte Sinai Hospital, Juiz de Fora, Brazil
Carlos Augusto Gomes - Emergency Unit, Department of Surgery, Ribeirão Preto, Brazil
Gerson Alves Pereira Junior - Department of Surgery, Chang Gung Memorial Hospital, Taoyuan, Taiwan
Kuo-Ching Yuan - Department of General Surgery, Hadassah Medical Center, Jerusalem, Israel
Miklosh Bala - Department of Surgery, Tianjin Nankai Hospital, Nankai Clinical School of Medicine, Tianjin Medical University, Tianjin, China
Yunfeng Cui - Department of Surgery, Pt BDS Post-graduate Institute of Medical Sciences, Rohtak, India
Sanjay Marwah - Department of Surgery, MOSC medical college, CochinCochin, India
Sanoop Zachariah - First Clinic of General Surgery, University Hospital /UMBAL/ St George Plovdiv, Plovdiv, Bulgaria
Boris Sakakushev - General Surgery Clinic, Medical University/University Hospital St.George, Plovdiv, Bulgaria
Boris Sakakushev - Department of Surgery, Edendale Hospital, Pietermaritzburg, Republic of South Africa
Victor Kong - Department of Surgery, Ahmadu Bello University Teaching Hospital Zaria, Kaduna, Nigeria
Adamu Ahmed - Department of Surgery, Mansoura University Hospital, Mansoura, Egypt
Ashraf Abbas - Department of Surgery, Faculdades Integradas Padre Albino, Catanduva, Brazil
Ricardo Alessandro Teixeira Gonsaga - Department of Surgery, Mazzoni Hospital, Ascoli Piceno, Italy
Gianluca Guercioni - Department of Surgery, Mellini Hospital, Chiari (BS), Italy
Nereo Vettoretto - Department of General and Digestive Surgery, Virgen de la Victoria, University Hospital, Malaga, Spain
Rafael Díaz-Nieto - Department of General Surgery and Surgical Oncology, Université de Nice Sophia-Antipolis, Universitary Hospital of Nice, Nice, France
Damien Massalou - Department of Surgery, Hospital and Oncological Centre, Novy Jicin, Czech Republic
Matej Skrovina - Department of General Surgery, Lviv Emergency Hospital, Lviv, Ukraine
Ihor Gerych - Department of Surgery, University Hospital Center Zagreb, Zagreb, Croatia
Goran Augustin - 3rd Department of General Surger Jagiellonian Univeristy, Narutowicz Hospital, Krakow, Poland
Jakub Kenig - Department of Surgery, Mozyr City Hospital, Mozyr, Belarus
Vladimir Khokha - Department of Surgery, Ancona University, Ancona, Italy
Cristian Tranà - Department of Surgery, Ripas Hospital, Bandar Seri Begawan, Brunei
Kenneth Yuh Yen Kok - Clinical Sciences, Regional Hospitals Limbe and Buea, Limbe, Cameroon
Alain Chichom Mefire - Department of Surgery, Severance Hospital, Seoul, Republic of Korea
Jae Gil Lee - Division of Trauma and Surgical Critical Care, Department of Surgery, University of Ulsan, Seoul, Republic of Korea
Suk-Kyung Hong - II Cátedra de Clínica Quirúrgica, Hospital de Clínicas, Asuncion, Paraguay
Helmut Alfredo Segovia Lohse - Department of Surgery, Khamis Mushayt General Hospital, Khamis Mushayt, Saudi Arabia
Wagih Ghnnam - Department of Surgery, Cutral Co Clinic, Neuquen, Argentina
Alfredo Verni - Department of Surgery, Faculty of Medicine Siriraj Hospital, Bangkok, Thailand
Varut Lohsiriwat - Department of Surgery, Thammasat University Hospital, Pathumthani, Thailand
Boonying Siribumrungwong - Department of Surgery, Hospital Regional de Alta Especialidad del Bajio, Leon, Mexico
Alberto Tavares - Department of Clinical and Experimental Sciences, Brescia University, Brescia, Italy
Gianluca Baiocchi - General Surgery, Adana Numune Training and Research Hospital, Adana, Turkey
Koray Das - Visceral Surgery, Military Hospital Desgenettes, Lyon, France
Julien Jarry - Visceral Surgery, Teaching Hospital Yalgado Ouedraogo, Ouagadougou, Burkina Faso
Maurice Zida - Department of Acute and Critical care medicine, Tokyo Medical and Dental University, Tokyo, Japan
Kiyoshi Murata - The Shock Trauma and Emergency Medical Center, Matsudo City Hospital, Chiba, Japan
Tomohisa Shoko - Emergency and Critical Care Center of Nippon Medical School, Tama-Nagayama Hospital, Tokyo, Japan
Takayuki Irahara - Department of Surgery, Our Lady of Lourdes Hospital, Drogheda, Ireland
Ahmed O Hamedelneel - Department of Surgery, Port Shepstone Hospital, Port Shepstone, South Africa
Noel Naidoo - Department of Surgery, College of Health Sciences, Obafemi Awolowo University Hospital, Ile-Ife, Nigeria
Abdul Rashid Kayode Adesunkanmi - Department of Emergency and Critical Care Medicine, Chiba University Hospital, Chiba, Japan
Yoshiro Kobe - Department of Surgery, Bahrain Defence Force Hospital, Manama, Bahrain
Tamer El Zalabany & Khalid Al Khalifa - Department of Emergency Medicine, Kyoto Second Red Cross Hospital, Kyoto, Japan
Wataru Ishii
Authors
- Massimo Sartelli
- Fausto Catena
- Luca Ansaloni
- Ernest Moore
- Mark Malangoni
- George Velmahos
- Raul Coimbra
- Kaoru Koike
- Ari Leppaniemi
- Walter Biffl
- Zsolt Balogh
- Cino Bendinelli
- Sanjay Gupta
- Yoram Kluger
- Ferdinando Agresta
- Salomone Di Saverio
- Gregorio Tugnoli
- Elio Jovine
- Carlos Ordonez
- Carlos Augusto Gomes
- Gerson Alves Pereira Junior
- Kuo-Ching Yuan
- Miklosh Bala
- Miroslav P Peev
- Yunfeng Cui
- Sanjay Marwah
- Sanoop Zachariah
- Boris Sakakushev
- Victor Kong
- Adamu Ahmed
- Ashraf Abbas
- Ricardo Alessandro Teixeira Gonsaga
- Gianluca Guercioni
- Nereo Vettoretto
- Elia Poiasina
- Offir Ben-Ishay
- Rafael Díaz-Nieto
- Damien Massalou
- Matej Skrovina
- Ihor Gerych
- Goran Augustin
- Jakub Kenig
- Vladimir Khokha
- Cristian Tranà
- Kenneth Yuh Yen Kok
- Alain Chichom Mefire
- Jae Gil Lee
- Suk-Kyung Hong
- Helmut Alfredo Segovia Lohse
- Wagih Ghnnam
- Alfredo Verni
- Varut Lohsiriwat
- Boonying Siribumrungwong
- Alberto Tavares
- Gianluca Baiocchi
- Koray Das
- Julien Jarry
- Maurice Zida
- Norio Sato
- Kiyoshi Murata
- Tomohisa Shoko
- Takayuki Irahara
- Ahmed O Hamedelneel
- Noel Naidoo
- Abdul Rashid Kayode Adesunkanmi
- Yoshiro Kobe
- AK Attri
- Rajeev Sharma
- Federico Coccolini
- Tamer El Zalabany
- Khalid Al Khalifa
- Juan Sanjuan
- Rita Barnabé
- Wataru Ishii
Corresponding author
Correspondence toMassimo Sartelli.
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MS designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sartelli, M., Catena, F., Ansaloni, L. et al. Complicated intra-abdominal infections in a worldwide context: an observational prospective study (CIAOW Study).World J Emerg Surg 8, 1 (2013). https://doi.org/10.1186/1749-7922-8-1
- Received: 28 December 2012
- Accepted: 02 January 2013
- Published: 03 January 2013
- DOI: https://doi.org/10.1186/1749-7922-8-1