The COMET Handbook: version 1.0 (original) (raw)
2.1 Background
The development of a COS in health care involves working with key stakeholders to prioritise large numbers of outcomes and achieve consensus as to the core set. Various methods have been used to develop a COS and it is uncertain which are most suitable, accurate and efficient. Research to identify optimal methods of developing COS is ongoing and there is currently wide variation in the approaches used [14]. Methods include the Delphi technique [48, 49], nominal group technique [50, 51], consensus development conference [52] and semistructured group discussion [53]. Many studies have used a combination of methods to reach consensus’ for example, Ruperto et al. [54] used the Delphi approach followed by the nominal group technique at a face-to-face meeting, whilst Harman et al. [55], Potter et al. [56], van’t Hooft et al. [57] and Blazeby et al. [58] used the Delphi approach followed by face-to-face semistructured discussion.
One example where consensus work has been undertaken in two different ways is in paediatric asthma. The American Thoracic Society/European Respiratory Society employed an expert panel approach [59], whereas other researchers combined results from a Delphi survey with clinicians and interviews with parents and children [60]. The results were overlapping but not identical. Female sexual dysfunction is another disease area where different methods have been used to obtain consensus. In one study, a literature review was undertaken and critiqued by experts [61], whereas in another study, a modified Delphi method was used to develop consensus definitions and classifications [62]. Both studies resulted in the same primary outcome; however, secondary outcomes differed. Similarly, multiple COS have also been developed for systemic lupus erythematosus. OMERACT adopted a nominal group process to rank outcome domains [63], whereas EULAR adopted a consensus building approach [64]. The results from both studies were very similar, with EULAR recommending other additional outcomes.
COS developers have identified that methodological guidance for COS development would be helpful [[65](/article/10.1186/s13063-017-1978-4#ref-CR65 "Gargon EA. Developing the agenda for core outcome set development. PhD thesis, University of Liverpool. 2016. https://livrepository.liverpool.ac.uk/3001398/
.")\]. There is limited empirical evidence, however, regarding whether different methods lead to similar or different conclusions, and there is a need to develop evidence-based approaches to COS development.The OMERACT Handbook is a useful resource for those wishing to develop COS in the area of rheumatology under the umbrella of the OMERACT organisation [[46](/article/10.1186/s13063-017-1978-4#ref-CR46 "Boers M, et al. The OMERACT Handbook. 2015. Available from: https://www.omeract.org/pdf/OMERACT_Handbook.pdf
. Accessed 30 May 2017.")\]. We have previously identified issues to be considered in the development of COS more generally \[[21](/article/10.1186/s13063-017-1978-4#ref-CR21 "Williamson PR, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13(1):132.")\] and expand on those here, together with additional ones identified since this earlier publication. We present information about how COS developers have tackled these issues using data from our previous systematic reviews \[[14](/article/10.1186/s13063-017-1978-4#ref-CR14 "Gargon E, et al. Choosing important health outcomes for comparative effectiveness research: a systematic review. PLoS One. 2014;9(6):e99111."), [39](/article/10.1186/s13063-017-1978-4#ref-CR39 "Gorst SL, et al. Choosing Important Health Outcomes for Comparative Effectiveness Research: An Updated Review and User Survey. PLoS One. 2016;11(1):e0146444.")\] and describe results from methodological research studies where available.In the systematic review (227 COS identified), 63% of studies made recommendations about what to measure only. Some of the remaining studies also made recommendations about how to measure the outcomes that they included in their core set, with 35% of studies doing this as a single process, considering both what to measure and how to measure. The remaining 2% of studies in the systematic review of COS considered what to measure and how to measure outcomes included in the core set as a two-stage process, first considering what to measure and then considering how to measure. Thus, there appears to be consistency in that the first step in the process is typically to gain agreement about ‘what’ to measure, with decisions about ‘how’ and ‘when’ to measure these outcomes usually later in the process. This two-stage process has the advantage of being able to identify gaps where further research would be needed, e.g. if an outcome is deemed to be of core importance but no outcome measurement instrument exists with adequate psychometric properties.
This chapter provides guidance on developing consensus about what to measure, i.e. a COS, and provides recommendations for finding and selecting instruments for measuring the outcomes in the core set, i.e. the how.
2.2 Scope of a core outcome set
The scope of a COS refers to the specific area of health or health care of interest to which the COS is to be applied. The scope should be described in terms of the health condition, target population and interventions that the COS is to be applicable to, thus covering the first three elements of the PICO (Population, Intervention, Comparator, Outcomes) structure for a clinical trial.
This can be one of the most difficult aspects of the process, but clarity from the outset will likely reduce later problems of misinterpretation and ambiguity. This will help to focus the development of the COS and help potential users decide on its relevance to their work.
2.2.1 Health condition and target population
For example, in prostate cancer, a COS may be developed for all patients or it may focus on patients with localised disease.
2.2.2 Interventions
For example, a COS may be created for use in all trials of interventions to treat localised prostate cancer or just for surgery.
Of the 227 COS published up to the end of 2014, 53% did not specify whether the COS was intended for all interventions or a particular intervention type, 7% were for any intervention, and 40% were for a specific intervention type.
2.2.3 Setting
The focus of this Handbook is on the development of COS for effectiveness trials. A distinction is made between efficacy and effectiveness trials, since developing a COS to cover both designs may lead to difficulties with respect to particular domains such as health care resource use [48]. COS are equally applicable in other settings; for example, routine clinical practice (see ‘Chapter 4’).
2.3 Establishing the need for a core outcome set
2.3.1 Does a relevant core outcome set already exist?
The first thing to do is find out whether a relevant COS exists by reviewing the academic literature.
One of the difficulties in this area of research has been to identify whether studies have already been done, or are underway, to develop a COS. The COMET Initiative has developed an online searchable database, enabling researchers to check for existing or ongoing work before embarking on a new project, thus minimising unnecessary duplication of effort. A video of ‘How to search the COMET database’ can be found on the COMET website [[66](/article/10.1186/s13063-017-1978-4#ref-CR66 "COMET. How to search the COMET Initiative database. [cited 2016 April]. Available from: https://stream.liv.ac.uk/eqyg4t36
. Accessed 30 May 2017.")\].The COMET database is populated through an annual systematic review update of published studies, and by COS developers registering their new projects. To avoid missing any ongoing projects not yet registered in the COMET database, it is recommended that researchers contact other experts in the particular health condition, as well as the COMET project coordinator, to check whether any related work is ongoing. It may also be prudent to apply the COMET search strategy [14] with additional filter terms for the area of interest for the recent period since the last COMET annual update.
Although there may be no exact match for the scope of interest, it may be that a related COS exists, e.g. a COS for all interventions in the condition of interest has been developed but a COS for a specific intervention type is sought, or a COS was developed by relevant stakeholders in countries other than that of the team with the current interest, or a COS was developed with the same scope but did not involve obtaining patients’ views.
2.3.2 Is a core outcome set needed?
If a relevant COS does not exist, a review of previous trials [67] or systematic reviews [68] in the area can provide evidence of need for a COS. Systematic reviewers are starting to use the outcome matrix recommended by the ORBIT project [69] to display the outcomes reported in the eligible studies. This matrix may demonstrate inconsistency of outcomes measured to date in addition to potential outcome-reporting bias.
The rest of this chapter is written from the premise that the development of a new COS is warranted. If a COS already exists, but the quality could be improved by additional work related to particular stakeholder groups, countries, or alternative consensus methods, then certain sections below will also be of relevance. The issue of quality assessment is discussed in ‘Quality assessment/critical appraisal’ below and in ‘Chapter 4’.
2.3.3 Avoiding unnecessary duplication of effort
The COMET database is a useful resource for researchers to see what work has been done in their area of interest and for research funders wishing to avoid unnecessary duplication of effort when supporting new COS activities, as illustrated by the following two examples.
Example 1
In September 2014 Valerie Page (Watford General Hospital, UK) contacted COMET via the website to register the development of a COS in delirium. We followed up the request for additional information so that we could register this in the database, and in the meantime we logged this on the private non-database list that we use to keep track of work that we know about prior to inclusion in the database. Whilst waiting for this information to be returned, in May 2015 we received a second request for registration of COS development in the same clinical area by Louise Rose from the University of Toronto, Canada. The researchers were unaware of each other’s work. We got in touch with both researchers and asked for permission to share details of their work, as well as to pass on contact details. In September 2015 we received confirmation that Louise Rose and Valerie Page, with the European Delirium Association and American Delirium Society, are now working collaboratively on this. Details of this collaborative effort to develop a COS for delirium can be found in the database [[70](/article/10.1186/s13063-017-1978-4#ref-CR70 "Rose L, Page V. Developing a core outcome set for delirium prevention and/or treatment trials. [cited 2016 April ]. Available from: http://www.comet-initiative.org/studies/details/796
. Accessed 30 May 2017.")\].Example 2
Benjamin Allin (University of Oxford, UK) started planning a study to develop a COS for infants with gastroschisis in early 2015. He checked the COMET database to see if a COS existed, but nothing was registered at that time. He contacted COMET in September 2015 to register his project. On receiving this request, the COMET project coordinator checked the COMET database to find out if there was any relevant work in this area and identified an ongoing study registered in this same area of gastroschisis. This latter work had been registered by Nigel Hall (University of Southampton and Southampton Children’s Hospital, UK) in June 2015. Again, the two groups were put in touch, and they met up to discuss the proposed core sets, which resulted in a plan being drawn up for collaboration to work together to produce one COS rather than two. The existing gastroschisis COS entry in the database has been updated to reflect this collaborative effort [[71](/article/10.1186/s13063-017-1978-4#ref-CR71 "Allin B, et al. Developing a core outcome set (COS) for infants born with gastroschisis. [cited 2016 April]. Available from: http://www.comet-initiative.org/studies/details/746
. Accessed 30 May 2017.")\].2.4 Study protocol
There are potential sources of bias in the COS development process, and preparing a protocol in advance may help to reduce these biases, improve transparency and share methods with others. We recommend that a protocol be developed prior to the start of the study, and made publically available, either through a link on the COMET registration entry or a journal publication [72,73,74]. In a similar way to the development of the SPIRIT guidance for clinical trial protocols, there is a need to agree protocol content.
2.5 Project registration
One of the aims of the COMET Initiative is to provide a means of identifying existing, ongoing and planned COS studies. COS developers should be encouraged to register their project in a free-to-access, unrestricted public repository, such as the COMET database, which is the only such repository we are aware of.
The following information about the scope and methods used is recorded in the database for existing and ongoing work:
- Clinical areas for which the outcomes are being considered, identifying both primary disease and types of intervention
- Target population (age and sex), and any other details about the population within the health area
- Setting for intended use (e.g. research and/or practice)
- Method of development to be used for the COS
- People and organisations involved in identifying and selecting the outcomes, recording how the relative contributions will be used to define the COS
Details of any associated publications, including the protocol and the final report, can be recorded in the COMET database, added to the original COMET registration page.
2.6 Stakeholder involvement
It is important to consider which groups of people should be involved in deciding which outcomes are core to measure, and why. Bringing diverse stakeholders together to try to reach a consensus is seen to be the future of collaborative, influential research.
Key stakeholders may include health service users, health care practitioners, trialists, regulators, industry representatives, policy-makers, researchers and the public. Decisions regarding the stakeholder groups to be involved, how they are to be identified and approached, and the number from each group will be dependent upon the particular scope of the COS as well as upon existing knowledge, the methods of COS development to be used, and practical feasibility considerations. For example, a COS for an intervention that aims to improve body image, e.g. breast reconstruction following mastectomy, is likely to have predominantly patients as the key stakeholders [56].
The stages of involvement during the process should also be considered for each stakeholder group. For example, it may be considered appropriate to involve methodologists in determining how to measure particular outcomes, but not to be involved in determining what to measure. These decisions should be documented and explained in the study protocol.
Consideration should be given to the representativeness of the sample of stakeholders and the ability of people across the different groups to engage with the chosen consensus method (including online activities and face-to-face meetings).
Consideration should be given to potential conflicts of interest within the group developing the COS (for example, the developers of measurement instruments in the area of interest or those whose work is focussed on a specific outcome).
2.6.1 Patient and public involvement and participation
COMET recognises the expertise and crucial contribution of patients and carers in developing COS. COS need to include outcomes that are most relevant to patients and carers, and the best way to do this is to include them in COS development. Examples exist where patients have identified an outcome important to them as a group that might not have been considered if the COS had been developed by practitioners on their own [75, 76]. However, it is worth noting that examples also exist where health professionals have identified areas that patients were reluctant to talk about in focus groups; for example, sexual health [77].
2.6.1.1 Patient and public participation
We refer to patients taking part in the COS study as ‘research participants’ and the activity as research ‘participation’. People involved in a COS study as research participants give their views on the importance of outcomes and may also subsequently be asked their opinion on how those outcomes are to be measured.
Of the 227 COS that had been published up to the end of December 2014, 44 (19%) studies reported including patient participants in the COS development process. However, of these 44 COS, only 26 (59%) studies provided details of how patients had participated in the development process. The most commonly used methods to include patient participants were the Delphi technique and semistructured group discussion which were used in 38% and 35% of studies, respectively. Three of the 26 (12%) COS studies were developed with only patients as participants. Of the remaining 23 studies, patients participated alongside clinicians during the development process in 19 (83%) studies, as compared to two (9%) studies where patients and clinicians participated separately throughout the whole development process. In the two remaining studies, patients and clinicians participated separately in the initial stages, but then alongside side each other during the final stages of the development process. For the 21 studies where patients and clinicians did participate alongside each other for all or part of the COS development process, the percentage of patient participants included ranged from 4 to 50%.
Of ongoing COS studies (n = 127 as of 12 April 2016), 88% now include patients as participants. The question now is not whether patients should participate, but rather the nature of that participation. It is recommended that both health professionals and patients be included in the decision-making process concerning what to measure, as the minimum, unless there is good reason to do otherwise. ‘Qualitative methods in core outcome set development’ below discusses considerations to enhance patient participation in a COS.
2.6.1.2 Patient and public involvement
When planning a COS study that involves patients as research participants, it is important to also involve patients in designing the study. We refer to patients who are involved in designing and overseeing a COS study as ‘public research partners’ and this activity as ‘patient involvement’. PPI has been defined as where research is ‘being carried out “with” or “by” members of the public rather than “to”, “about” or “for” them’ [[78](/article/10.1186/s13063-017-1978-4#ref-CR78 "INVOLVE. What is public involvement in research? 2016. [cited 2016 19 March]. Available from: http://www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/
. Accessed 30 May 2017.")\].Involving public research partners in both the design and oversight of the COS development study may have the potential to:
- Provide advice on the best ways of identifying and accessing particular patient populations
- Inform discussions about ethical aspects of the study
- Facilitate the design of more appropriate study information
- Promote the development of more relevant materials to promote the study
- Enable ongoing troubleshooting opportunities for patient participation issues during the study, e.g. recruitment and retention issues of study participants
- Inform the development of a dissemination strategy of COS study results for patient participants and the wider patient population
- Ensure that your COS is relevant to patients and, crucially, that patients see it to be relevant and can trust that the development process has genuinely taken account of the patient perspective.
Involving public research partners in designing and overseeing the COS study requires that researchers plan for this involvement. They might choose different methods of doing this; for example, they might have one or two discussion groups in the planning stage and then ongoing involvement of one or two public contributors on the Study Advisory Group (SAG). For example, Morris et al. (2015) engaged parents at various stages of the research process and consulted with parents from their ‘Family Faculty’ in designing a plain language summary of the results of their COS [79]. Numerous resources now exist to help researchers to plan and budget for PPI in research; for example: in the UK, INVOLVE have numerous resources [[80](/article/10.1186/s13063-017-1978-4#ref-CR80 "INVOLVE. [cited 2016 April]. Available from: http://www.invo.org.uk/
. Accessed 30 May 2017.")\].COMET has also produced a checklist for COS developers to consider with public research partners when planning their COS study. These can be found on the COMET website.
2.7 Determining ‘what’ to measure – the outcomes in a core outcome set
2.7.1 Identifying existing knowledge about outcomes
It is recommended that potential relevant outcomes are identified from existing work to inform the consensus process. There are three data sources that should be considered: systematic reviews of published studies, reviews of published qualitative work, investigation into items collected in national audit data sets and interviews or focus groups with key stakeholders to understand their views of outcomes of importance. Depending on the resources available, protocols within clinical trial registries may also be a useful source of information.
2.7.1.1 Systematic review of outcomes in published studies
Systematic reviews are advantageous because they can efficiently identify an inclusive list of outcomes being reported by researchers in a given area. Nevertheless, it is important to note that systematic reviews of outcomes just aggregate the opinions of the previous researchers on what outcomes they deemed important to measure; hence the need for subsequent consensus development to agree with the wider community of stakeholders what outcomes should be included in a COS.
The scope of the systematic review should be carefully considered in the context of the COS to ensure that outcomes are included from all relevant studies without unnecessary data collection. The clinical area should be clearly defined and appropriate databases accessed accordingly. Commonly used databases include Medline, CINAHL, Embase, the Cochrane Database of Systematic Reviews and PsycINFO. In the systematic reviews of COS [14, 39], 57 (25%) studies carried out a review of outcomes [[65](/article/10.1186/s13063-017-1978-4#ref-CR65 "Gargon EA. Developing the agenda for core outcome set development. PhD thesis, University of Liverpool. 2016. https://livrepository.liverpool.ac.uk/3001398/
.")\]. The number of databases searched was not reported for 17 studies (30%), and two studies did not perform an electronic database search. Thirty-eight studies described which databases they searched (Table [1](/article/10.1186/s13063-017-1978-4#Tab1)).Table 1 Description of databases searched (n = 38)
There is no recommended time window to conduct systematic reviews. Some COS studies may examine all the available academic literature. This may be an enormous task in common disease areas. Scoping searches are useful to determine the number of identified studies for a specific area. Overly large reviews are resource intensive and may not yield important additional outcomes. One strategy is to perform the systematic review in stages to check if outcome saturation is reached. For example, a review of trials published over the last 5 years may be conducted initially and the outcomes extracted. The search may then extended, and the additional outcomes checked against the original list. If there are no further outcomes of importance then the systematic review may be considered complete. For most areas a recent search is recommended as a minimum (e.g. the past 24 months) to capture up-to-date developments and outcomes relevant to that COS. Seventeen studies in the systematic reviews of COS (30%) did not state the date range searched. Seven studies (12%) did not apply any date restrictions to their search. The number of years reported in the remaining 33 studies ranged between 2 and 59. Frequencies are provided in Table 2.
Table 2 Number of years searched (n = 33)
Data extraction should be considered in terms of:
- Study characteristics
- Outcomes
- Outcome measurement instruments and/or definitions provided by the authors for each outcome
In terms of outcome extraction from the academic literature, it is recommended that all are extracted verbatim from the source manuscript [81]. This transparency is important to allow external critical review of the COS right back to its inception. In addition, extraction of outcome definitions supplied by, and measurement instruments used by, the authors is recommended as this will inform the selection of the outcome measurement set which will occur at a later stage. This is necessary because outcome definitions may vary widely between investigators and it is often not clear as to what outcomes are measuring [67, 82,83,84].
2.7.1.2 How to extract outcomes from the academic literature to inform the questionnaire survey
It is likely that some outcomes will be the same but will have been defined or measured in different publications in various ways. For example, in a review of outcomes for colorectal cancer surgery some 17 different definitions were identified for ‘anastomotic leakage’ [85]. The first step is to group these different definitions together (extracting the wording description verbatim) under the same outcome name. Similarly, in a review of outcomes for weight loss surgery, it was apparent that different terminology is used for weight loss itself in the academic literature [84]. The 41 different outcome assessments referring to weight were all categorised into one item for a subsequent Delphi questionnaire survey.
The next step is to group these outcomes into outcome domains, constructs which can be used to classify broad aspects of the effects of interventions, e.g. functional status. Outcomes from multiple domains may be important to measure in trials, and several outcomes within a domain may be relevant or important. Initially researchers create outcome domains for each outcome to be grouped into (see ‘Ontologies for grouping individual outcomes into outcome domains’ below). The domains need discussion and to be agreed by the team for the list to be categorised. Each outcome will then be mapped to a domain (independently) and this will provide transparency. For example, in a systematic review of studies evaluating the management of otitis media with effusion in children with cleft palate, a total of 43 outcomes were listed under 13 domain headings (see Table 18 in [81]).
Categorisation of each verbatim outcome definition to an outcome name, and each outcome name to an outcome domain is recommended to be performed independently by two researchers from multiprofessional backgrounds. This may include expert health service researchers, clinicians (e.g. surgeons, dietician, nurses, health psychologists) and methodologists. Where two researchers work on this process a senior researcher will need to resolve differences and make final decisions.
2.7.1.3 Systematic review of studies to identify outcomes of importance to health service users
Similarly, it is necessary to systematically review the academic literature to identify Patient-reported Outcome Measures (PROMs) and then extract patient-reported outcome domains. These come from existing PROMs often at the level of the individual questionnaire item [86]. This is recommended because the scale name used in PROMs and the scores attributed to the combined items are often found to be inconsistent. Therefore, analyses at a granular level are recommended [86]. The full process for this is described in Fig. 1 of the paper by Macefield et al. At this stage it is worth extracting details of the patient-reported outcome development and validity which will be helpful when selecting measures with which to assess the core outcomes.
A PRO long list extracted from PROMs may be supplemented with additional domains derived from a review of qualitative research studies if time allows (e.g. [87, 88]). It is recommended that interpretation of data from qualitative papers is guided by experts in the field.
2.7.2 Identifying and filling the gaps in existing knowledge
It is important to identify which key stakeholder groups’ views are not encompassed by systematic reviews of outcomes in published studies or the existing academic literature more generally, and decide whether these are gaps that need to be filled. An initial list from published clinical studies may be supplemented by undertaking qualitative research with key stakeholders whose views are important yet unlikely to be represented within systematic reviews of outcomes in previous studies. Where resources are limited, consultation with an advisory group whose membership reflects the key stakeholders may be used as an alternative to qualitative research, but it should be noted that such consultation is not qualitative research and the information arising from it does not have the same standing as the knowledge generated by research.
Qualitative interviews or focus groups with key stakeholders, especially patients, are recommended, particularly if the PROMS have lacked detailed patient participation in their development. The following section outlines in more detail how qualitative work may contribute to COS development. Nevertheless, it is recommended that qualitative research is guided by researchers with expertise in these. Interviews should be performed with a purposeful sample and use a semistructured interview schedule to elicit outcomes of importance to that population. The interview schedule may be informed by the domain list generated from the academic literature or be more informed by a grounded theory approach and start with very open questions. Interviews are audio-recorded, transcribed and analysed for content. The information can then be used to create new outcome domains or supplement the long list [89, 90].
2.7.3 Ontologies for grouping individual outcomes into outcome domains
Outcome domain models or frameworks exist to attempt to provide essential structure to the conceptualisation of domains [91], and have been used to classify outcomes that have been measured in clinical trials in particular conditions. Despite their intended use to provide a framework, there is not always consistency between the different models. In a review of Health-related Quality of Life (HRQoL) models, Bakas et al. found that there were wide variations in terminology for analogous HRQoL concepts [91]. Outcome hierarchies have been proposed for specific conditions [92] and cancer [93].
There have been several frameworks to classify health, disease and outcomes to date. There are various conceptual frameworks relevant to outcomes in health and these cover somewhat different areas of outcomes, some of which are described below.
2.7.3.1 Outcome-related frameworks
**World Health Organisation (WHO)**The WHO definition of health, although strictly a definition of health, can be considered a framework as it includes three broad health domains [[94](/article/10.1186/s13063-017-1978-4#ref-CR94 "WHO. WHO definition of Health. Geneva 1948. [cited 28 Apr 2015]. Available from: http://www.who.int/about/mission/en/
. Accessed 30 May 2017.")\]: physical, mental and social wellbeing. This definition has not been amended since 1948 but is a useful starting place to study health. In a scoping review of conceptual frameworks, Idzerda et al. point out that although the three domains are clearly outlined, no further information about what should be included within each domain is provided \[[95](/article/10.1186/s13063-017-1978-4#ref-CR95 "Idzerda L, et al. Can we decide which outcomes should be measured in every clinical trial? A scoping review of the existing conceptual frameworks and processes to develop core outcome sets. J Rheumatol. 2014;41(5):986–93.")\].Patient-reported Outcomes Measurement Information System (PROMIS)
The PROMIS domain framework builds on the WHO definition of health to provide subordinate domains beneath the broad headings stated above [[41](/article/10.1186/s13063-017-1978-4#ref-CR41 "NIH. Patient-Reported Outcomes Measurement Information System–PROMIS. [cited 2015 28th April]. Available from: http://www.nihpromis.org/measures/domainframework
. Accessed 30 May 2017.")\]: physical (symptoms and functions), mental (affect, behaviour and cognition) and social wellbeing (relationships and function). It was developed for adult and paediatric measures as a way of organising outcome measurement tools.World Health Organisation International Classification of Functioning Disability and Health (WHO ICF)
The International classification of Functioning, Disability and Health (ICF) offers a framework to describe functioning, disability and health in a range of conditions. The ICF focuses on the assessment of an individual’s functioning in day-to-day life. It provides a framework for body functions, activity levels and participation levels in basic areas and roles of social life; providing domains of biological, psychological, social and environmental aspects of functioning [96]. In many clinical areas, ICF core sets have been developed. These core sets identify the most relevant ICF domains for a particular health condition.
5Ds
5Ds is presented as a systematic structure for representation of patient outcomes and includes five ‘dimensions’: death, discomfort, disability, drug or therapeutic toxicity, and dollar cost [97]. This representation of patient outcome was developed specifically for rheumatic diseases, and the authors claim that each dimension represents a patient outcome directly related to patient welfare; for example, they describe how a patient with arthritis may want to be alive, free of pain, functioning normally, experiencing minimal side effects and be financially solvent. This framework assumes that outcomes are multidimensional, and it is critical that the ‘concept of outcome’ is orientated to patient values.
Wilson and Cleary
Wilson and Cleary [98] propose a taxonomy or classification for different measures of health outcome. They suggest that one problem with other models is the lack of specification about how outcomes interrelate. They divide outcomes into five levels: biological and physiological factors, symptoms, functioning, general health perceptions, and overall quality of life. In addition to classifying these outcome measures, they propose specific causal relationships between them that link traditional clinical outcomes to measures of health-related quality of life. For example, ‘Characteristics of the environment’ are related to ‘Social and psychological supports’ which in turn relates to ‘Overall quality of life’. Ferrans et al. [99] revised the Wilson and Cleary model to further clarify and develop individual and environmental factors.
Outcome Measures in Rheumatology (OMERACT) Filter 2.0
The OMERACT Filter 2.0 [31] is a conceptual framework that encompasses ‘the complete content of what is measurable in a trial’. That is, a conceptual framework of measurement of health conditions in the setting of interventions. It comprises three core areas: death, life impact and pathophysiological manifestations; it also comprises one strongly recommended, resource use. These core areas are then further categorised into core domains. They liken the areas to ‘large containers’ for the concepts of interests (domains and subdomains). They recommend that the ICF domains are also considered under life impact (ICF domains: activity and participation) and pathophysiological manifestations (ICF domains: body function and structure). Although OMERACT recommends the inclusion in a COS of at least one outcome reflecting each core area, empirical evidence is emerging that this is not always considered appropriate [48].
Outcome Measures Framework (OMF)
The Outcome Measures Framework (OMF) project was funded by the Agency for Healthcare Research and Quality (a branch of the U.S. Department of Health and Human Services) to create a conceptual framework for development of standard outcome measures used in patient registries [100]. The OMF has three top-level broad domains: characteristics, treatments and outcomes. There are six subcategories within the outcomes domain: survival, disease response, events of interest, patient/caregiver-reported outcomes, clinician-reported outcomes and health system utilisation. The model was designed so that it can be used to define outcome measures in a standard way across medical conditions. Gliklich et al. conclude that ‘as the availability of health care data grows, opportunities to measure outcomes and to use these data to support clinical research and drive process improvement will increase’.
Survey of Cochrane reviews
Rather than attempting to define outcome domains as others have done, Smith et al. performed a review of outcomes from Cochrane reviews to see whether there were similar outcomes across different disease categories, in an attempt to manage and organise data [101]. Fifteen categories of outcomes emerged as being prominent across Cochrane Review Groups and encompassed person-level outcomes, resource-based outcomes, and research/study-related outcomes. The 15 categories are: adverse events or effects (AE), mortality/survival, infection, pain, other physiological or clinical, psychosocial, quality of life, activities of daily living (ADL), medication, economic, hospital, operative, compliance (with treatment), withdrawal (from treatment or study) and satisfaction (patient, clinician, or other health care provider). The authors recognise that these 15 categories might collapse further.
2.7.3.2 Use of outcome-related frameworks in core outcome set studies
In the systematic reviews of COS [14, 39], 17 studies provided some detail about how outcomes were grouped or classified (Table 3).
Table 3 Methods for classifying/grouping outcomes (n = 17)
2.7.3.3 Use of outcomes recommended in core outcome sets to inform an outcome taxonomy
Based on the classification of outcomes in two previous cohorts of Cochrane systematic reviews [101, 102] and the outcomes recommended in 198 COS [14], the following taxonomy has been proposed:
- Mortality
- Includes subsets all, cause-specific, quality of death, etc.
- Physiological (or Pathophysiological)
- Disease activity (e.g. cancer recurrence, asthma exacerbation, includes ‘physical consequence of disease’, etc.)
- Blood pressure, laboratory values, recanalisation
- Infection
- New, recurrent
- Pain
- Quality of life
- Includes Health-related Quality of Life (HRQoL)
- Mental health
- Psychosocial (includes behavioural)
- Function (or Functional status)
- Does this cover activities? Participation? (Read the Roberts and Counsell paper referenced in the review of stroke outcomes)
- Compliance with/withdrawal from treatment
- Satisfaction
- Reported by patient, health professional, etc.
- Resource use (or health resource utilisation)
- Includes subset hospital, community, additional treatment, etc.
- Adverse events (or side effects)
- Be clear that this could include things like death, pain, etc. when they are unanticipated harmful effects of an intervention
Pilot work is underway with selected Cochrane Review Groups to test the taxonomy for applicability. To date, one additional outcome domain, knowledge, has been identified as missing from the list.
2.7.4 Determining inclusion and wording of items to be considered in the initial round of the consensus exercise
It is important to spend time on this aspect of the process, in terms of the structure, content and wording of the list of items, to avoid imbalance in the granularity of item selection and description and ambiguity of language. Participants in the consensus process may identify such issues, necessitating revisions to the list during subsequent rounds [48]. A SAG (see ‘Achieve global consensus’ below) can provide valuable input at the design stage, prior to the start of the formal consensus process.
The review of existing knowledge, and research to fill gaps in that knowledge, has the potential to result in a long list of items. Consideration is needed regarding whether to retain the full list in the consensus exercise or whether to reduce the size of the list using explicit criteria. Preparatory work on how best to explain the importance of scoring all items on the list may help to improve levels of participation.
As noted in the section on qualitative research in COS development, because qualitative research involves patients and other stakeholders describing their views and experiences in their own terms, it gives COS developers access to the words, phrases and language that patients use to describe how conditions or interventions affect them. COS developers can, therefore, incorporate the words that patients use in interviews and focus groups to label and explain outcome items in a Delphi, thereby ensuring that the items are understandable and accessible for patients. Pilot or pretesting work involving cognitive or ‘think aloud’ interviews to examine how patients and other stakeholders interpret the draft items can help to refine the outcome labels and explanations [103, 104]. As the name suggests, this technique literally involves asking participants to think aloud as they work through the draft Delphi and provide a running commentary on what they are thinking as they read the items and consider their responses. This allows COS developers to understand the items from the perspective of participants. Cognitive interviews are widely used in questionnaire development to refine instruments and ensure they are understandable for the target groups.
Other methods previously used to determine the description of items include a reading-level assessment and amendment as necessary [55], and a review of terminology used in existing health frameworks such as ICF [96], PROMIS [[41](/article/10.1186/s13063-017-1978-4#ref-CR41 "NIH. Patient-Reported Outcomes Measurement Information System–PROMIS. [cited 2015 28th April]. Available from: http://www.nihpromis.org/measures/domainframework
. Accessed 30 May 2017.")\], the Wilson and Cleary model \[[98](/article/10.1186/s13063-017-1978-4#ref-CR98 "Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65.")\] as well as related COS \[[48](/article/10.1186/s13063-017-1978-4#ref-CR48 "Chiarotto A, et al. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J. 2015;24(6):1127–42.")\].2.7.5 Short- or longer-term outcome assessment
One issue to consider is whether, and how, to address the timing of outcome assessment. Many COS developers have identified an agreed set of outcomes to measure, leaving the timing of assessment as an issue for trialists to decide subsequently depending on their particular context of use. In an alternative approach, in the COS for rheumatoid arthritis [26] agreed at a face-to-face meeting, it is recommended that radiological damage is only measured in trials where the patients are to be followed up for longer than 1 year. It is recommended that the approach to handling this issue be made clear to participants from the outset in the subsequent consensus process, to avoid ambiguity later on.
2.7.6 Eliciting views about important outcomes
Having identified a list of potential outcomes, the next step is to assess the level of importance given to each. Considerations concerning the choice of assessment method include the need to build a consensus with methodological rigor, and to adopt strategies to ensure that a diverse range of opinions is heard.
Methods used in previous studies to elicit opinions and to develop consensus about important outcomes include expert panel meetings (sometimes using nominal group technique (NGT) methods) and Delphi surveys. A single, heterogeneous consensus panel comprising the various stakeholders may be deemed appropriate for particular areas of health care whereas separate panels for different stakeholder groups followed by work to integrate the multiple perspectives may be more appropriate for others.
If participants in a consensus process are shown a list of potential outcomes, we recommend that in general they should be given the opportunity to propose the inclusion of additional items, especially as the academic literature may not include outcomes associated with the most recent treatments available or the most pressing current concerns for stakeholders.
We consider a Delphi exercise to be a useful way of gaining information about opinion from a wide group of participants. Of 127 ongoing COS in the COMET database (as of 12 April 2016), 108 (85%) involve a Delphi survey, and hence we discuss this method in more detail below.
2.7.6.1 The Delphi technique
With the exception of the Delphi technique, all other methods for COS development described earlier involve face-to-face communication. The Delphi technique is advantageous in that it is anonymous, avoiding the effect of dominant individuals, and can be circulated to large numbers with wide geographic dispersion.
The Delphi technique was originally developed by Dalkey and Helmer (1963) at the Rand Corporation in the 1950s [[105](/article/10.1186/s13063-017-1978-4#ref-CR105 "RAND Corporation. Delphi Method. [cited 2016 April]. Available from: http://www.rand.org/topics/delphi-method.html
. Accessed 30 May 2017.")\]. In a COS framework, the method is used for achieving convergence of opinion from experts (stakeholders) on the importance of different outcomes in sequential questionnaires (or rounds) sent either by post or electronically. Responses for each outcome are summarised and fed back anonymously within the subsequent questionnaire. Participants are able to consider the views of others before re-rating each item and can, therefore, change their initial responses based on the feedback from the previous rounds. With no direct communication between participants this feedback provides a mechanism for reconciling different opinions of stakeholders and is, therefore, critical to achieving consensus.There remains, however, uncertainty as to the optimum way to use such methodology. Many issues need to be considered at the outset, all of which may have an impact on the final results. These include:
- Number of panels
- Group size
- Participant information
- Number of rounds
- Structure of the questionnaires
- Methods of scoring
- Nature of feedback presented between rounds
- Criteria for retaining outcomes between rounds
- Attrition (response bias) between rounds
- Consensus definitions
- How the degree of consensus will be assessed
In the following sections, we discuss each of the above issues in detail and offer guidance on different approaches.
Single or multiple panels for different stakeholders
The choice of stakeholder groups to be involved in the development of a COS has been discussed in ‘Stakeholder involvement’ in Chapter 2 above. There may be additional considerations regarding which groups should be involved in a Delphi survey. For example, in a COS for early stage dementia, interviews rather than Delphi survey participation may be considered to be the more appropriate way to include patient views.
What also requires consideration is how best to combine the views of different stakeholders within a Delphi survey. The issue of the impact of panel composition on Delphi performance has seldom been investigated in general [106]. Some COS studies have used a single panel of experts from one particular stakeholder group or combined a heterogeneous group of participants, representing multiple stakeholder groups, into a single panel (that is, ignoring stakeholder status when generating feedback and assessing consensus). Others have used multiple homogenous panels, each formed by a different stakeholder group. In rheumatology, Ruperto et al. [54] used a single panel of paediatric rheumatologists whilst developing a COS in systemic lupus erythematosus (JSLE) and juvenile dermatomyositis (JDM), whilst Taylor et al. [107] combined the views of rheumatologists and industry representatives into a single panel in the development of a COS in chronic gout. The MOMENT study (Management of Otitis Media with Effusion in Cleft Palate) considered eight separate stakeholder groups and treated them as multiple separate panels [55].
The single homogeneous panel approach will result in core outcomes deemed essential by only one stakeholder group. If a single panel is formed by combining heterogeneous stakeholder groups (such that feedback and criteria for consensus are based on the group overall and ignore stakeholder type), careful consideration and justification is needed of the panel mix. If the data are simply amalgamated with no consideration of the separate stakeholder groups, the resulting set may depend on the relative proportions of stakeholders participating or on weightings that may be used for different groups. As an example of imbalance in stakeholder representation, the Taylor et al. study [107] had only three industry representatives and the remaining 26 respondents were rheumatologists. The single-panel approach here is clearly in favour of the rheumatologists’ opinions.
In areas where differing stakeholder opinions are expected, a better approach would be to consider multiple panels, retaining distinct stakeholder groups when generating feedback and considering criteria for consensus (see later sections). The final core set, or outcomes taken forward to the next stage of COS development, may then consist of (1) outcomes deemed essential by all stakeholder groups or (2) outcomes deemed essential by any stakeholder group. The former option may, therefore, result in the most important outcomes for any particular group being excluded from the core set which may not be acceptable. At the same time, whilst including items deemed essential by any relevant stakeholder group ensures that outcomes essential to any group are included, the resulting set may be too extensive to be practical and it could be argued that in this scenario consensus has not been achieved since there will be items that not all groups agreed on. Alternative approaches will be described in a later section on defining consensus (see ‘Defining consensus’ below).
Group size
The decision regarding how many individuals to include in a Delphi process is not based on statistical power and is often a pragmatic choice. For example, the group size may be dependent on the number of experts or patients available within the scope of the COS being developed. These numbers may be particularly small if the condition is rare or the intervention of interest is not widely used. In their international Delphi study, Smith and Betts (2014) included only 12 acupuncturists working in pregnancy with at least 5 years’ experience in traditional Chinese medical techniques [108]. As a contrast, Ruperto et al. (2003) enrolled 174 paediatric rheumatologists from two professional organisations in an international Delphi survey [54]. Blazeby et al. (2015) recruited 185 patients and 126 consultants and specialist nurses in a UK-based study to identify a COS for surgery for oesophageal cancer [58], whilst van’t Hooft et al. (2015) involved 32 parents and 163 health professionals in an international Delphi survey as part of the development of a COS for the prevention of preterm birth [57].
Consideration should be given to the number of participants that are invited into the Delphi (allowing for attrition between rounds; see later). Dependent on the consensus definition, the results may be particularly sensitive with smaller numbers of participants. When potential numbers are small, stakeholder group members could be pooled, particularly if it is expected that opinions are unlikely to differ. Typically, such a decision should be done in consultation with the Steering Advisory Group to ensure the appropriateness of the grouping, and without knowledge of the results. Any revisions to the Delphi protocol should be documented with reason.
The key consideration with group size is that there should be good representation from key stakeholder groups with qualified experts who have a deep understanding of the issues. The more participants representing each stakeholder group the better, both in terms of the COS being generalisable to future patients and in convincing other stakeholders of its value.
Participant information
It is important for all participants to be fully aware of the purpose of the Delphi survey and what will be expected of them. This is crucial both in terms of enabling informed consent and equipping participants to be able to prioritise and score outcomes. The notion of a COS and even an outcome may not be clear to all. Participant Information Sheets may need to use different terminology for different stakeholder groups and should be piloted in advance. Plain language summaries for patients and carers, including a description of an outcome, a COS and a Delphi survey, are available on the COMET Initiative website [[109](/article/10.1186/s13063-017-1978-4#ref-CR109 "COMET. Plain Language Summary. [cited 2016 April]. Available from: http://www.comet-initiative.org/resources/PlainLanguageSummary
. Accessed 30 May 2017.")\].It is also advisable to ensure that the instructions provided within each round of the Delphi survey reiterate the overall aim of achieving consensus of a core set of outcomes.
Number of rounds
A Delphi survey must consider at least two rounds (that is, at least one round of feedback) to be considered a Delphi survey. The number of Delphi rounds varies across different COS development studies. Typically, COS studies contain two [56, 58, 60, 110] or three rounds [55, 107, 111]. One study reported six rounds [112]; however, this included several rounds of open-ended questions to generate debate in controversial areas in the field of infant spasms and West syndrome. Open-ended rounds may also be used to generate an initial list of outcomes prior to any outcome scoring [60, 113, 114], as an alternative to reviewing the academic literature for example.
Rather than pre-determining the number of rounds, the process can be dynamic with subsequent rounds incorporated if further prioritisation is warranted. Whilst we would not expect, nor require, consensus to be reached on all outcomes in the Delphi questionnaire, it is necessary that a reduced number of outcomes has been agreed (in terms of prespecified criteria) to be of most importance, in order to inform the COS. Outside COS development work, Custer et al. (1999) have recommended that three iterations are sufficient to collect the relevant information to reach a consensus in most cases [115].
From a practical perspective, the number of rounds may also be limited by time, cost or consideration of the burden on participants completing multiple rounds of Delphi. The time taken for participants to complete a round of Delphi is highly variable and will often depend on the number of outcomes being scored. It is advisable to pilot the questionnaires beforehand to ensure that it is practical. Typically, each round of Delphi will remain open for about 2 or 3 weeks, although latter rounds may be kept open longer if response rates are low to try to minimise the potential for attrition bias (see later). Following the closure of a Delphi round, an additional 2 or 3 weeks is required to analyse the data and set up the next round, although this will depend on the design and can be much shorter if using software developed specifically for online Delphi surveys; for example, the DelphiManager software developed by COMET [[116](/article/10.1186/s13063-017-1978-4#ref-CR116 "COMET. DelphiManager. [cited 2016 April]. Available from: http://www.comet-initiative.org/delphimanager/
. Accessed 30 May 2017.")\].Structure of the questionnaires
Careful consideration is needed when designing the Delphi questionnaire, as for any questionnaire. For example, outside of COS development Moser and Kalton [117] recommend that jargon and technical terms should be avoided in questionnaires; anecdotal evidence from the piloting of Delphi questionnaires for core sets for cancer surgery and OME with cleft palate suggest that lay terms are preferred to technical medical terms, even by health professionals. Stakeholder involvement in the design and piloting of the Delphi questionnaire is recommended to ensure that it is accessible, comprehensible and valid.
Order of questionnaire items
Previous research, outside of COS development, has demonstrated that the order in which questions are presented in a questionnaire could affect response rates and actual responses to question items [118]. The idea of the ‘consistency effect’, where items are answered in relation to responses to earlier items, has been researched for more than 50 years with recommendations that general questions should precede specific ones [119], and questions should be grouped into topics [120]. It has also been suggested that if there is evidence that respondents have stronger opinions on some items than others these should be placed first [118]. It has been argued that order effects will be greater for interview surveys and minimal for written surveys since participants have longer to respond to items and have the opportunity to look at all items before responding [121], but effects have been observed in written surveys [122].
Within the development of a COS we are only aware of one published abstract reporting the effects of question order. For surgery for oesophageal cancer recent methodological research considered the impact of the ordering of patient-reported and clinical outcomes in the Delphi survey. Participants were randomly allocated to receive a questionnaire with the patient-reported outcomes presented first and the clinical outcomes last, or vice versa. The study found that ordering of outcomes in a Delphi questionnaire may impact on both response rates and actual responses, hence subsequently impacting on the final core set [123]. Further research is needed to better understand potential order effects in the context of COS development. We are aware of an ongoing nested study in which participants are randomised to one of four orderings of the outcomes throughout the Delphi survey [[124](/article/10.1186/s13063-017-1978-4#ref-CR124 "Needham DM. Improving long-term outcomes research for acute respiratory failure [cited 2016 March]. Available from: www.comet-initiative.org/studies/details/360
. Accessed 30 May 2017.")\].Additional open questions
As described previously, there are different methods of identifying an initial long list of outcomes to inform the Delphi survey. Whatever method is employed the initial list may not be entirely exhaustive and there may be added value in including an open question in the round-1 questionnaire to identify additional outcomes. This open question could be placed at the beginning or the end of the questionnaire depending on the intended purpose.
If placed at the beginning of the questionnaire participants might be asked to identify a small number of outcomes that are of most importance to them before they see the outcomes included in the questionnaire. If placed at the end, participants might be asked to list any additional items that they do not feel have been considered in the questionnaire. The former approach will help to ensure that there are no key outcomes that have been omitted, whilst the latter approach will help to ensure that a more exhaustive list of outcomes. Whether included at the beginning or end of the questionnaire, criteria for including additional items in round 2 should be specified in a protocol; for example, any new outcome suggested may be included, alternatively only those new outcomes suggested by two or more respondents might be added.
Scoring system
Core outcome set studies have used a variety of different scoring systems to rate outcomes within a Delphi process, although the majority involve a Likert scale. Other methods include the ranking of outcomes [54, 114] and allocation of points (for example, division of 100 points across all outcomes) [110, 114, 125]. The 9-point Likert scoring system where outcomes are graded in accordance to their level of importance is a common method. Typically, 1 to 3 signifies an outcome is of limited importance, 4 to 6 important but not critical, and 7 to 9 critical [55, 126, 127]. This framework is recommended by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group for assessing the level of importance about research evidence [21, [128](/article/10.1186/s13063-017-1978-4#ref-CR128 "GRADE. GRADE working group. [cited 2016 April]. Available from: http://www.gradeworkinggroup.org
. Accessed 30 May 2017. ")\]. Others have used similar 9-point systems. For example Potter et al. \[[56](/article/10.1186/s13063-017-1978-4#ref-CR56 "Potter S, et al. Development of a core outcome set for research and audit studies in reconstructive breast surgery. Br J Surg. 2015;102(11):1360–71.")\] and Blazeby et al. \[[58](/article/10.1186/s13063-017-1978-4#ref-CR58 "Blazeby JM, et al. Core information set for oesophageal cancer surgery. Br J Surg. 2015;102(8):936–43.")\] asked participants to rate the importance of each outcome on a 1–9 scale where 1 was ‘not essential’ and 9 was ‘absolutely essential’. Some studies have also included an ‘unable to score’ category to allow for the fact that some stakeholder group members may not have the level of expertise to score certain outcomes. As an example, in the development of a COS for otitis media with effusion in children with cleft palate, some of the participants from the speech and language therapist stakeholder group chose not to score some of the outcomes related to the more clinical aspects of the condition \[[55](/article/10.1186/s13063-017-1978-4#ref-CR55 "Harman NL, et al. The importance of integration of stakeholder views in core outcome set development: otitis media with effusion in children with cleft palate. PLoS One. 2015;10(6):e0129514.")\]. Other studies have used four- \[[129](/article/10.1186/s13063-017-1978-4#ref-CR129 "Broder MS, et al. An agenda for research into uterine artery embolization: results of an expert panel conference. J Vasc Interv Radiol. 2000;11(4):509–15.")\], five- \[[111](/article/10.1186/s13063-017-1978-4#ref-CR111 "Smaïl-Faugeron V, et al. Development of a core set of outcomes for randomized controlled trials with multiple outcomes—Example of pulp treatments of primary teeth for extensive decay in children. PLoS ONE. 2013;8(1):e51908.")\] and seven- \[[107](/article/10.1186/s13063-017-1978-4#ref-CR107 "Taylor WJ, et al. A modified Delphi exercise to determine the extent of consensus with OMERACT outcome domains for studies of acute and chronic gout. [Erratum appears in Ann Rheum Dis. 2008 Nov;67(11):1652. Note: Mellado, J Vazquez [corrected to Vazquez-Mellado, J]. Ann Rheum Dis. 2008;67(6):888–91.")\] point Likert scales to score outcomes.Feedback between rounds
In order to increase the degree of consensus amongst participants, differing views need to be reconciled. The mechanism for this within a Delphi process is the feedback presented to participants in subsequent rounds, enabling different opinions to be considered before re-rating an outcome. At the end of each round the results for each outcome are aggregated across participants and descriptive statistics presented (see later in this section). Participants can be encouraged to provide a reason for their scores on individual outcomes, which can be summarised as part of the feedback.
The generation of these descriptive statistics will depend on whether a single panel or multiple panels have been used. In a single-panel study, feedback ignores any distinct stakeholder groups and summarises and presents scores for each outcome for all participants involved, hence hiding any disparate views between stakeholders. As described earlier, if there are disparate views the final COS will depend on the relative proportions of stakeholders. Calculation of summary scores could of course be weighted by stakeholder group, but it is difficult to ascertain what weightings should be given and there is no current guidance on this. In addition, recent evidence in the development of COS suggests that patients are more likely than health professionals to rate an outcome as essential; three studies found that the average score awarded to outcomes in the round-1 questionnaire was greater for patients than health professionals [130], so even in a study involving equal numbers of patients and health professionals, patients may be more likely to influence a core set if outcome scores are simply combined across stakeholder groups.
In a multiple panel study there are three possible approaches to providing feedback. Participants might receive an overall average across all stakeholder groups; however, this would be analogous with the single-panel approach. Alternatively, participants could receive feedback from their own stakeholder group only or from all stakeholder groups separately. If participants receive feedback from their own stakeholder group only, whilst this may enable consensus within stakeholder groups, it provides no opportunity for consensus across groups. Ongoing COS projects include nested randomised studies comparing these three approaches [131, 132].
Recent methodological work, including a before/after study [55] and nested randomised trials [130], examined the impact of providing feedback from all stakeholder groups separately compared to feedback from the participant’s own group only. Type of feedback presented did impact on the subsequent scoring of items [55, 130] and the items subsequently retained at the end of the Delphi process [130]. The research also demonstrated that providing feedback to participants from both stakeholder groups improved consensus between stakeholder groups in terms of reduced variability in responses and improved agreement in items to retain at the end of the Delphi process [130].
In some ongoing COS studies, participants are being asked for the reasons that they have changed scores between rounds particularly if the change in score is from critically important to a score of less importance or vice versa [133, 134]. This will enable us to better understand the impact of feedback and help optimise the Delphi.
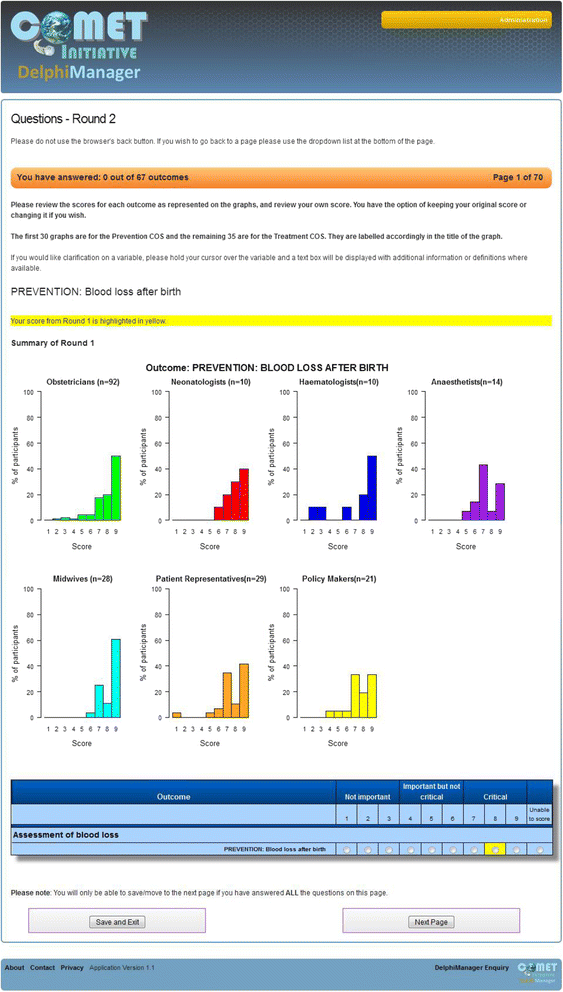
There are a number of ways that feedback can be presented. A summary statistic, such as a median or mean (if normally distributed), may be presented for each outcome [58]. Figure 2 demonstrates how feedback was presented in round 2 of a Delphi postal survey used within the development of a COS for surgery for colorectal cancer. Mean scores (rounded to the nearest integer) were presented for both stakeholder groups (patients and surgeons/nurses) included in the study. The participant’s individual score from round 1 is also presented. Single summary statistics are sometimes also presented with a measure of dispersion such as a standard deviation, interquartile range, range or other percentiles [107, 126, 127]. Alternatively, the percentage scoring above a prespecified threshold (for example, 7–9 on a 9-point Likert scale) may be presented [56]; or, the full distribution of scores may be provided graphically. Figure 3 provides a screenshot of an electronic round-2 Delphi questionnaire created within DelphiManager. In this instance a histogram of round-1 scores is presented for each stakeholder group. The participant’s round-1 score is this time highlighted in yellow.
Fig. 2
An outcome from a round-2 questionnaire for surgery for colorectal cancer presenting the mean score for round-1 for patients and health professionals separately
Fig. 3
An outcome from a round-2 questionnaire presenting the percentage distribution of scores across all stakeholder groups with options for participants to review their previous round score and re-score (taken from DelphiManager)
Further research is required to determine which presentation method is most useful and easily interpreted by participants; however, the optimum approach may differ depending on the setting and stakeholder groups involved. Preparatory work with a small group of participant representatives to ensure that the feedback is understood is advisable.
Retaining or dropping items between rounds
After the initial Delphi round, subsequent rounds might retain all outcomes [55, 57, 125], or some items may be dropped according to prespecified criteria [56, 58]. Whilst there are examples of both approaches in the academic literature, at present there is no empirical evidence of whether the decision impacts on the final core set. Retaining all items for all rounds may provide a more holistic approach, enabling participants to score and prioritise the list of outcomes as a whole. If items are dropped between rounds there may be items considered of most importance to some participants which are not present in later rounds and this may hinder their ability to prioritise the remaining items. This may be particularly pertinent when scoring systems require participants to allocate a certain number of points across all outcomes [125]. In addition, if items are dropped after the first round, participants will not get the opportunity to re-score those outcomes taking into account feedback on scores from other participants. Suppose that a particular outcome is rated highly by patients in round 1 but poorly by other stakeholder groups and that based on prespecified criteria the outcome is dropped. It is plausible that had participants seen that patients rated the outcome highly, other stakeholders would have increased their scores such that the outcome would have been retained at the end of round 2.
At the same time, if the initial list of outcomes is large, including them in each Delphi round may impose sufficient burden on participants to increase attrition from one round to another. If the decision is made to reduce the number of items from one round to the next, more inclusive criteria for retaining items in earlier rounds may be sensible. For example, in the recent development of a COS for surgery for oesophageal cancer, 67 outcomes were included in round 1. Criteria for inclusion in round 2 were that an item be rated 7 to 9 (on a 9-point Likert scale) by 50% or more participants and 1 to 3 by no more than 15% of participants in at least one stakeholder group [58]. Items were retained at the end of round 2 using stricter cut-off criteria; retained items were rated between 7 and 9 by over 70% of respondents and 1 to 3 by less than 15% by at least one stakeholder group. Using less stringent criteria in earlier rounds, and retaining items for which these criteria are met for any single stakeholder group, reduces the likelihood of dropping outcomes that may have been rated more highly in subsequent rounds had participants been given feedback on them.
In the absence of any empirical evidence to inform the optimum approach, the decision may be largely led by the initial number of outcomes. An intermediate approach, which may to some extent address the disadvantages of both methods, would be to retain all items between rounds 1 and 2, hence enabling participants to re-score in light of feedback for every item, and then drop items in subsequent rounds. Whatever design used, if any items are to be dropped from one round to the next, criteria need to be clearly defined in a protocol.
Attrition and attrition bias
The degree of non-response after the first round of the Delphi (attrition) may be highly variable between studies and may be dependent on the timing of Delphi rounds (for example, holiday season may increase attrition), the length of the Delphi (from previous knowledge of completing the previous round), the time elapsed between the first and final round (health care professionals may leave the service, or participants may become disinterested), and the method of recruitment of participants, as well as many other factors. For example, Bennett et al. (2012) observed 0% attrition in their small Delphi study (fewer than 10 participants) but their recruitment strategy was a targeted approach to known experts [126], whereas Smith and Betts (2014) observed higher attrition rates (17%) from 12 participants from inviting trial authors from the relevant academic literature [108]. Similar attrition rates to Smith and Betts were seen in a much larger study for oesophageal cancer surgery which recruited 126 surgeons and nurses identified through a meeting of the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, and by personal knowledge of surgeons, and 185 patients recruited from three clinical centres. Attrition rates between rounds 1 and 2 were 15% for professionals and 17% for patients.
If attrition rates are thought to be too high, either overall or for a particular stakeholder group, then strategies should be adopted to increase the response rates. Personalised reminder emails to participants (with details of current response rates), personalised emails from distinguished researchers in the field, direct telephone calls, and the offer of being acknowledged in the study publication have all been found to be helpful strategies in increasing response rates. Consideration should be given to keeping Delphi rounds open longer if it is thought that this may increase response rates. Whilst there is no guidance on what constitutes an acceptable response rate, typically around 80% for each stakeholder group would be deemed satisfactory in most situations.
Attrition bias will occur when the participants that do not respond in subsequent rounds have different views from their stakeholder group peers who continue to participate. For example, if the feedback a participant receives suggests that they are in a minority with regard to their scoring of importance about particular outcomes, then they may be more likely to drop out, leading to over-estimation of the degree of consensus in the final results [135].
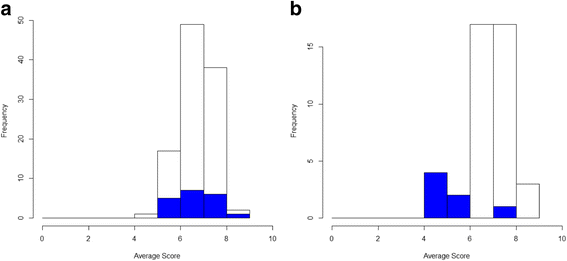
Only one study to date has examined whether attrition bias is present between Delphi rounds in a COS project [55] although many ongoing COS studies are now planning to consider this. In the Harman et al. study (2015), average round-1 scores were calculated for each participant then plotted according to whether participants completed round 2 or not. Figure 4 provides two hypothetical scenarios representing the responses from two different stakeholder groups. In stakeholder group A, we can see that the average scores for those completing only round-1 (blue bars) are well contained within those average scores of those completing round 1 and round 2 (white bars). On average, participants staying in have scored outcomes similarly to those leaving the study, suggesting that attrition bias is unlikely to affect the results. For stakeholder group B, we can see that the average round-1 scores of those who did not complete round 2 are lower. If too many participants drop out of the Delphi process with lower previous round scores than the majority opinion, this will overestimate the level of importance of outcomes and over-inflate the degree of consensus.
Fig. 4
Average scores in round 1 across all outcomes for (a) stakeholder group 1 and (b) stakeholder group 2. Shaded bars represent those who provided scores in round 1 only; open bars represent those scoring in both rounds 1 and 2
Inevitably, examining average scores between completers and non-completers has its limitations. For example, non-completers may score some outcomes much higher than completers and score other outcomes much lower than completers, but average scores may remain similar between the two groups. Another approach to examine potential attrition bias would be to look at average scores of individual outcomes amongst those who do and not complete later rounds. If formally comparing average scores through statistical hypotheses tests it should be remembered that there will be an issue of multiple significance testing and false positive findings. However, such testing may enable identification of obvious patterns or differences in scoring between the non-completers and completers; for example, if non-completers are scoring patient-reported outcomes more highly than clinical outcomes but the reverse is seen for completers.
COS developers should consider the potential nature and cause of likely attrition bias when deciding how best to examine its presence. The assessment of attrition bias should be repeated for further rounds of the Delphi, that is, average round-2 scores should be compared for those completing round 3 and those dropping out after round 2.
Defining consensus
As for the scoring system, there are numerous ways proposed to define the consensus criteria, although the choice of criteria is rarely justified [136]; commonly these relate to a mean or median value for each outcome or a percentage of participants scoring an outcome as ‘important’. For example, Bennett et al. (2012) defined ‘consensus in’ (outcomes to be included in the COS) to be those for which 75% or more of participants scored 7 to 9 on a 9-point Likert scale [126], whilst others have suggested lower or higher rates scoring the categories deemed to be important. Some studies have also defined specific conditions or combination criteria as their consensus definition. For example, Schmitt et al. (2011) defined outcomes to be important if at least 60% of the participants scored 7 to 9 in at least three out of the four stakeholder groups being considered [127]. Wylde et al. (2015) implemented a threshold for inclusion in the core set of 70% of participants scoring outcomes as 7 to 9 and 15% or less scoring 1 to 3 to be met by both the clinician and patient panels or 90% or more scoring 7 to 9 from any single panel [137].
Williamson et al. (2012), describe the rationale for the ‘70/15%’ consensus definition that was in part used by Wylde et al. (2015); this approach has been used by others (Harman (2015) [55], Potter (2015) [56], Blazeby (2015) [58]) [21]. The idea is that the majority regard that the outcome should be in the COS, with only a small minority considering it to have little or no importance. It can similarly be argued that an outcome should not be included in the COS if the majority (70%) have scored an outcome of little importance, with only the small minority (fewer than 15%) consider it to be critically important.
The choice of what consensus criteria to use is an important consideration in COS development. Too accommodating criteria may result in a long list of outcomes that are not considered to be minimal whilst too stringent criteria can potentially exclude key outcomes that may otherwise have been included in the COS. Regardless of the consensus criteria that it used, it is important to define the consensus criteria in a protocol) to avoid any potential bias from changing the criteria after the Delphi results have been analysed [138].
Assessing the degree of consensus
Some examination of the degree of consensus in each round is also advisable in order to ensure that the Delphi survey is working as a consensus method. As discussed earlier, the number of rounds in a Delphi can be dynamic but there will be a point beyond which a greater degree of consensus is unnecessary or unlikely to be achieved. One way to consider the degree of consensus is to examine the change in individuals’ scores between rounds. Brookes et al. (2015) [130] and Harman et al. (2015) [55] calculated the percentage of items for which a participant changed their score between rounds 1 and 2. Figure 5 presents the findings for the Harman et al. study. In this particular example, participants changed their opinions on only a small percentage of outcomes between rounds (indicated by the positive skew) which suggests that additional rounds would be unlikely to result in a much greater degree of consensus. Some ongoing COS studies are documenting the reasons for change between rounds [133, 134]; this may also help to determine whether additional rounds are useful.
Fig. 5
Percentage of scores changed between rounds 1 and 2 after viewing the results by stakeholder group
One metric that may also be useful for examining improved consensus is the reduction in variability of individual outcome scores between two adjacent rounds. In their examination of the impact of different feedback methods, Brookes et al. [130] calculated the standard deviation of round 1 and round 2 scores for each item in the Delphi survey. Reductions in the spread of scores were seen between rounds 1 and 2. Reductions in interquartile ranges have similarly been examined in this way [139].
Whilst an examination of the degree of consensus is important to help validate the Delphi survey, it should be reiterated that the aim of the Delphi survey in the development of a COS is to determine which outcomes are core as opposed to achieving consensus for every outcome. Hence, it is unnecessary to conduct numerous rounds until consensus has been reached for all outcomes.
Finally, all methodological decisions should be fully reported and explained in the main publication. Any revisions to the original protocol should be documented with reasons. This will be covered in more detail in ‘Reporting guidance’ below.
2.7.6.2 Face-to-face meeting
We recommend that representatives of key stakeholder groups have the opportunity for discussion of the results of the surveys to agree a final core set and undertake additional voting if required before a final COS is agreed.
Evidence for how such meetings are designed and conducted is lacking and published experience limited. A review identified just 10 examples of which nine included a face-to-face meeting [[65](/article/10.1186/s13063-017-1978-4#ref-CR65 "Gargon EA. Developing the agenda for core outcome set development. PhD thesis, University of Liverpool. 2016. https://livrepository.liverpool.ac.uk/3001398/
.")\]. Most held one meeting although a few studies undertook two or three. Some used a nominal group technique method to reach consensus although the majority had informal approaches. A nominal group technique allows all opinions to be considered initially, eliminates duplicate ideas and then asks participates to rank the importance of the remaining opinions \[[140](/article/10.1186/s13063-017-1978-4#ref-CR140 "Harvey N, Holmes CA. Nominal group technique: an effective method for obtaining group consensus. Int J Nurs Pract. 2012;18(2):188–94.")\]. This differs from traditional methods for decision-making which focus on the largest group initially supporting an idea.Most meetings lasted about half a day, included some sort of voting and included presentations and discussion. Details of who moderated the meetings were often lacking.
Involving patients in consensus meetings
Another challenge in undertaking a COS study with patients as participants is that of enabling their inclusion in consensus meetings. This may, in part, be influenced by whether or not all stakeholders are brought together in a consensus meeting or whether these are run separately for patient participants. There are issues of power when multiple stakeholders work together to seek agreement. Spoken language and non-verbal communication in such meetings can exclude or subtly undermine patient participants. Some COS developers recommend that face-to-face consensus meetings are held separately for patients and professionals to allow patients’ views to be heard without contamination from other parties [56]. Other groups have brought patients and professionals together to discuss their views alongside evidence arising from a Delphi survey [55] and to make recommendations about a COS. With such mixed views, there is a need for research into this aspect of the consensus process.
Good facilitation is crucial, regardless of whether separate or combined consensus meetings are held. The preparation and support of patient participants both before and during the meeting is also vital. Consideration needs to be given to the specific needs of the patient group as they may have particular requirements to enable them to fully participate. The common principles in ensuring an accessible venue obviously apply, but there may be other considerations; for example, fatigue or pain from prolonged sitting need to be considered in planning such meetings. De Witt et al. (2013) provide information on barriers to participation for patients with rheumatological conditions in a face-to-face consensus meeting and make recommendations for facilitating their participation [141].
Other issues to consider
A crucial decision to be made is who to invite to the face-to-face meeting. Some COS developers have invited only the Steering Group involved in the project, which may or may not be those involved in an early Delphi study. Others have invited a combination of Steering Group members and other Delphi participants. An approach that may be helpful is to decide on the total number attending (which may be limited by resource and/or timing), and then to consider the desirable number from each stakeholder group. Some COS developers have aimed to include an equal split of health professionals and patients [133]. If Delphi participants are to be invited, some COS developers have included a final question in the survey about willingness to participate in a face-to-face meeting [133]. Participants for the meeting are then randomly selected from Delphi completers who noted that they were interested in attending. The advantage of this approach is that one can check that the final Delphi round scores are comparable for those attending and not attending which may give some reassurance that due consideration will be given to the evidence from the Delphi study.
Based on experience of consensus meeting facilitation, we recommend that the following further issues are considered: whether the facilitator needs relevant clinical experience or methodological experience, or whether co-facilitators may be appropriate; the independence of the facilitator; the ability of the facilitator to bring everyone in to the discussion without pressure; that the time needed for the meeting will depend on the aims and the number of outcomes to be considered; the structure for the meeting, and whether any small group or breakout group discussions will be held. We recommend that attendees are sent a reminder of their personal Delphi scoring prior to the meeting. The objectives of the project and the meeting should be made clear at the start, in terms of the scope of the COS, the emphasis on identifying core outcomes, and the methods to be used on the day. Thought should be given to the order of presentation of outcome results since it may be better to take the outcomes in groups/domains. Time should be allowed for at the end of the meeting to review the recommended list of outcomes holistically and agree the next steps which may include determining how each outcome should be defined and measured.
If a consensus meeting is to form part of the COS study, teams may need to consider how to address language issues in the meeting for non-native language speakers. If interpreters are to be used it is important to ensure that they are qualified to undertake that role, and meeting participants are reminded to speak in plain language to reduce the likelihood of difficulties with interpretation. A member of the team should brief the interpreters prior to the meeting, as to the purpose of the meeting, to discuss issues of confidentiality and to emphasise that information should not be filtered as this may bias responses. In planning the project, it is important to ensure that interpreting services are appropriately budgeted for. Interpretation issues are complex and we recommend that research teams seek advice from their own organisations as we have only provided a few basic considerations in this text.
2.8 Determining the core outcome set
The development of a COS may involve several components. Consideration should be given in advance to the criteria that will be used to determine when consensus has been achieved. Specification of the decision-making process to determine the final COS should be given in the study protocol which should reduce the risk that the people leading the process will define consensus post-hoc in a way that would bias the conclusions toward their own beliefs. For example, a study advisory committee (see Achieve global consensus’ below) may oversee the process whereby a review of the academic literature combined with stakeholder interviews informs a Delphi survey, the results of which are presented at a face-to-face meeting of representative stakeholders, and either ratified (in the case of an outcome meeting a pre-defined consensus definition for the Delphi survey) or further discussed and a decision made.
It is important to ensure that views from all key stakeholder groups are considered when making the final decision regarding the COS, and that the process for reaching that decision is reported transparently.
Researchers should consider the potential impact of the following methodological decisions on the final results: group composition, questioning technique, the information that participants receive to inform their answers, whether or not responses are anonymous, how the group participants interacted with, or influenced, each other, the medium of the interaction, attrition bias, analysis which can miss or overstate the importance of certain outcomes, and the way in which consensus is reached.
All of the above require further investigation to develop transparent, reproducible and robust methods for decision-making during the COS development process. Until this is available it is recommended that reporting details of the process undertaken follows the recommended guidelines [142].
2.9 Qualitative methods in core outcome set development
As indicated throughout this handbook, COS development can involve several different stages as well as a mix of both research and consensus processes. Some COS developers have recently started to use qualitative methods as part of the wider COS development process [55, 74, 89, 133, 143, 144]. In this context qualitative methods may be useful for accessing the perspectives of groups, such as patients, carers, members of the public and health professionals, whose views may not be encompassed in systematic review of outcomes. Making COS development meaningful for these groups can be challenging and, as we outline below, qualitative methods can help COS developers to navigate this challenge. However, it is important to note that much remains to be learnt about the use of qualitative research in COS development and this section is based on experience of a limited number of projects.
2.9.1 Why use qualitative methods in core outcome set development?
2.9.1.1 To identify outcomes of relevance to the whole community of stakeholders
COS developers may conduct systematic reviews of the outcomes measured in published studies to develop ‘long lists’ of outcomes to go forward to consensus processes such as a Delphi survey. However, the opinions of clinical trial designers and researchers will inevitably have influenced the outcomes used in published studies and these same opinions will also be reflected in the findings of systematic reviews. Patients, carers and the public have historically had little say in what outcomes are measured in studies, so systematic reviews can overlook important outcomes. For example, the OMERACT group have pointed to how fatigue – an outcome of crucial importance to patients – was overlooked in rheumatology trials until relatively recently [28]. Qualitative studies with patients and other stakeholders can help to ensure that the long lists of outcomes that go forward to a consensus process are comprehensive from the perspective of the whole community of relevant stakeholders, not just the groups that have historically influenced what outcomes are measured in research.
2.9.1.2 To preserve the distinctive perspective of different stakeholders
Most people do not naturally think or talk about their experiences of health conditions, illness and treatments within an outcomes or research frame of reference [145]. In order for patients and other stakeholders to participate meaningfully in consensus processes, such as Delphi surveys, COS teams need to help them to understand what outcomes are, how these are used in trials and why COS are needed. This involves patients learning things about research which, by definition, involves influencing them. This learning could also potentially diminish the distinctiveness of their perspectives as patients. Qualitative studies can enable patients and other stakeholders to participate in COS development in ways that minimise such influences. For example, qualitative interviews involve asking participants open questions and allowing them to respond in their own words. Rather than asking patients to learn about outcomes or why COS are needed, the researcher adapts to the patients’ world and works within their existing capabilities. The overall purpose of the qualitative research must of course be clearly explained, but with a well-thought out qualitative study design and prompt guide (interview schedule) patients can describe their experiences of illness and treatment in ways that are both intrinsically meaningful to them and which simultaneously help the COS developer to identify what outcomes are important to patients.
2.9.1.3 To help make consensus processes accessible to patients
When patients and carers come to rate or vote on lists of outcomes during consensus processes, the outcomes need to make sense to them. Because qualitative research involves patients describing their views and experiences in their own terms, it gives researchers insight into how patients naturally conceptualise outcomes and the language they use. COS developers can use the findings from interviews and focus groups to make subsequent consensus processes accessible to patients. This might be by using the qualitative findings to take account of the patient perspective in deciding on the scope of a COS, and to ensure that the labelling and explanation of outcomes is understandable to patients.
2.9.1.4 To inform deliberations in the final stages of core outcome set development
Qualitative study findings can also illuminate why outcomes are important to patients, which may usefully inform the final stages of COS development if there is divergence between stakeholders about which outcomes are core and which are not.
2.9.1.5 To address gaps in existing core outcome sets
Where an existing COS has been developed without the perspective of patients or other key stakeholders, qualitative studies may help to address this omission. This would usually be as part of a wider review process as described in Chapter 3.
2.9.2 In what circumstances might core outcome set developers consider using qualitative methods?
Whilst qualitative methods can be helpful for the reasons described above, we recommend that they are usually used as part of wider COS development process. Qualitative studies are not designed to include large representative samples and so these designs are not suitable for estimating how many patients think that a particular outcome is important. Moreover, qualitative research studies are not consensus processes and analysis of interview or focus group data leaves considerable room for interpretation. Whilst this analysis can be documented and public research partners and other team members can be involved, to achieve a final COS that users will have confidence in COS developers will need to use processes that have been specially designed to reach consensus.
In deciding whether to incorporate qualitative methods as part of a wider COS development process, developers should consider the following:
- The specific purpose for which qualitative evidence is needed.
Whilst qualitative methods can be used to access the perspectives of patients, carers and other stakeholders [133, 146] developers will find it helpful to specifically consider what it is that they are hoping to achieve through the qualitative work, and whether a qualitative approach is the most suitable way to achieve these aims. For example:- о A COS developer may be concerned that not all potentially important outcomes have been identified to go forward to a consensus process and will use qualitative work to help ensure that no potentially important outcomes are missed
- о Retention of Delphi participants over several rounds can be problematic and qualitative studies may help to minimise the number of Delphi rounds needed. For example, qualitative research may enable developers to omit the initial ‘blank page’ or open-ended round of a Delphi survey
- о COS developers may be working with stakeholder groups, such as patients with dementia or learning disabilities, for whom other COS development activities are unsuitable
- о As we note above, qualitative research findings may also help developers to define the scope of a COS by informing the choice of population and interventions to be covered by a particular COS
- о As also noted above, qualitative research findings may help to ensure the consensus process (language, explanations, etc.) is accessible to patients
- о COS developers may need insights on why particular outcomes are important to patients; qualitative research is widely regarded as being useful in addressing ‘why’ questions
- Whether existing qualitative studies have been conducted that could address some of these aims.
Many qualitative studies have now been published describing patients’ and carers’ experiences of specific conditions and treatments. It may be possible to use this existing work to identify how well outcomes from systematic reviews of trials map to those of patients. Similarly, qualitative datasets may be available for secondary qualitative analysis that could serve the same aims. If previous qualitative evidence or data are available, COS developers will want to be clear about why additional primary qualitative research is needed. - Qualitative research requires specialist methodological expertise and COS developers will need to ensure that their team includes this expertise. Similarly, the collection and analysis of interview or focus group data requires time and resource and COS development teams will also need sufficient funds to support the qualitative work.
2.9.3 Issues to consider in designing primary qualitative research to inform core outcome set consensus processes
It is beyond the scope of this section to provide guidance on how to do qualitative research. This is already covered by an extensive literature. However, there are some specific issues in conducting qualitative research within COS development that may be particularly relevant for COS developers. Before turning to these we would like to emphasise that the issues we identify below are not exhaustive. We hope that COS developers will reflect critically on our suggestions, rather than regarding them prescriptively.
2.9.3.1 How the qualitative research and patient involvement complement one another
To an extent qualitative research and the involvement of public research partners may share similar overall goals. For example, both may aim to optimise the accessibility of the language used in the consensus process. Nevertheless, the contributions of public research partners, though crucial in COS development, cannot substitute for qualitative research.
2.9.3.2 Sampling
COS developers will usually aim to access participants who have direct experience of the illness, treatment or care process relevant to the COS as patients or service users. Additionally, the perspectives of carers, parents or professionals may also be valuable, for example where the capacity of patients to articulate their experiences is limited or where carers’ perspectives on outcomes are of interest in their own right. Sampling to qualitative studies is usually purposive (e.g. aiming for maximum diversity), rather than probabilistic (aiming for statistical representativeness [147]), but it should be noted that purposive sampling is not the same as convenience sampling. Whilst patient organisations or charities might offer a convenient route for accessing stakeholders, individuals who are contactable via such organisations may differ in important ways from the wider community of patients. If COS are to reflect the perspectives of this wider community, developers will want to access patients across a spectrum of sociodemographic and other characteristics. Sampling via different clinical, health and community settings will likely be best suited to this.
2.9.3.3 Eliciting participants’ perspectives
We have noted above that qualitative research may be particularly useful in COS development because it enables participants to talk about their priorities in their own terms. Even without an overt focus on outcomes or COS [148] – a frame of reference which may confuse patients and shape or colour what they say – much can be learnt from patients’ naturalistic accounts of their experiences and perspectives. A narrative style of interviewing can be helpful here, particularly at the outset of an interview. Beyond this, the questions and prompts can be more overtly focussed on outcomes although questions still need to be tailored to the participant group and topic, and be responsive to individual participants. As well as examining the qualitative academic literature relevant to COS development, in designing the interviews and prompt guides qualitative researchers should consult with patient research partners [149]. We offer the following further suggestions as tentative pointers and hope that COS developers will treat them only as starting points for developing their own questions and prompts:
- Interviews might begin by asking participants to talk about their actual experiences, including how the illness and treatment has affected their lives, about treatment decisions, how these decisions were made and what influenced them.
- Over the course of an interview, questions may become more focussed on outcomes and opinions; for example, asking patients what they had hoped for from their previous treatments or what they would want from a new treatment if one became available. Understandably, many patients with chronic conditions might hope for curative treatments and interviewers may need to be prepared to prompt patients to describe expectations that are more immediately achievable.
- Towards the end of interviewing, questions might become more research- or COS-specific. For example, the interviewer might summarise outcomes that patients have discussed in previous interviews and explore what words or phrases patients think researchers should use to label or explain outcomes for future patients.
- Where the qualitative work has a very specific focus; for example, informing an online Delphi survey for a particular stakeholder group, interviewing might focus on a prototype of the survey and use think-aloud techniques [103, 104] to explore how stakeholders interpret the outcomes and how the phrasing might be refined.
2.9.3.4 Data analysis
COS developers have drawn on a range of analytical orientations or approaches to qualitative analysis such as framework analysis [150], constant comparative method [146] thematic analysis, as well as interpretive phenomenological analysis [151]. Qualitative researchers will be best placed to identify an approach that best suits their aims. If interviews have been broad ranging or narrative, from an early point it will be important yet challenging to focus the analysis on those aims that are most pressing. For example, identifying what outcomes to go forward to the consensus process and how these are labelled may need to take precedence over identifying why certain outcomes are important to patients. As for any qualitative analysis, interpretation will need to contextualise the data and not just catalogue data extracts. This will mean considering what things patients might be reluctant to speak about as well as what might be taken for granted in the context of certain illnesses or treatments. In interviews stakeholders may not directly articulate some outcomes and identifying these may call for considerable interpretive work. For example, it took considerable qualitative work to identify empowerment as an outcome of genetic counselling [152, 153]. Finally, whilst qualitative researchers are likely to lead the analysis, they will want to closely involve other members of the COS development team, including the public research partners, to ensure the analysis is informed by a range of perspectives.
2.9.3.5 Writing up the findings
Guidance is available on writing up qualitative research [154], so here we focus on those aspects that may warrant particular consideration by COS developers. It should be clear what has been discovered from the qualitative work (i.e. how the findings add to what was previously known) and how the qualitative work has contributed to the COS development process. This might include a commentary on whether the qualitative work has identified potentially important outcomes beyond those already identified in systematic reviews of trial outcomes, how the qualitative work has informed the scope of the COS or how it has informed the language used in consensus process. COS developers will need to decide whether to publish the qualitative research separately from the other elements of the COS development process, or combine all elements in one article. Where the qualitative research is combined with other elements of the COS development process, authors may find it helpful to consult articles on writing up mixed-methods research [155]. Where the qualitative findings are published separately, it should be clear that the qualitative study is linked to the wider COS development process so that subsequent COS developers can learn from this work and unnecessary duplication can be avoided.
2.10 Considerations to enhance patient participation in a core outcome set
There are numerous challenges in facilitating patient participation in a COS study and these will depend on the patient group and the methods chosen. In the following discussion we explore some key challenges for patient participation in consensus processes such as Delphi studies. Patient participation through qualitative methods was discussed in the previous section. A checklist to support COS developers working with patient research partners in designing COS studies is available on the COMET website and may help with planning to address some of these challenges at the outset.
2.10.1 Accessing patients
Involving patients as participants in COS will require consideration of the need for ethical approval in each country where the study is taking place. The current situation in the UK is that ethical permission is required if the goal of COS development is to produce generalisable knowledge. Guidance regarding the need for ethical approval has been developed by the COMET PoPPIE (People and Patient Participation, Involvement and Engagement) Group [[156](/article/10.1186/s13063-017-1978-4#ref-CR156 "PoPPIE. COMET People and Patient Participation Involvement and Engagement group. [cited 2017 April]. Available from: http://www.comet-initiative.org/ppi/researchers
. Accessed 30 May 2017.")\].Like any study with this aim, accessing patients or other groups to participate in a COS study requires consideration of sampling strategies as appropriate to the research method being used. Patients may be sampled from primary, secondary and/or tertiary health care settings depending on the condition under study. The approaches for accessing patient participants may depend on whether participants are being sought for an online or face-to-face Delphi survey. Methods for accessing patients may also depend on whether the patient participants are being asked to join a consensus meeting with other stakeholders, where COS developers may specifically decide to select patients who can take on advocacy roles in this context and so help to ensure that the patient perspective gets heard.
Patient organisations may provide a route to access patients for certain conditions but their members may have special interests and may differ from the wider patient population (in terms of age, gender, socioeconomic status, ethnicity and other relevant characteristics). If patient organisations are to be used, purposive sampling techniques may help to ensure that a diverse sample is accessed. Patient communities also exist in social media, but COS developers need to be cautious about using them. There is evidence of poor response rates through social media and those who do respond may include a limited range of the patient population due to self-selection [[157](/article/10.1186/s13063-017-1978-4#ref-CR157 "Hamm M. Outcomes in child health: using social media to identify patient-centered outcomes. 2015 [cited 2016 April]. Available from: http://www.comet-initiative.org/assets/downloads/5th-meeting/MicheleHamm.pdf
. Accessed 30 May 2017.")\]. Links to guidance on the use of social media can be found on the COMET website. COS developers also need to ensure that patients or patient advocates from patient organisations have relevant experience of the condition and that this experience is relatively recent. Further discussions about sampling of patients to take part in a COS can be found in the previous section.In terms of promoting the COS study to potential participants, COS developers might consider a range of sources; for example, clinic waiting areas and through patient organisations and they might also plan for how to promote the study within hard-to-reach communities. Patient organisations and public research partners can help to advise on where studies might best be promoted for patients with a particular condition.
2.10.2 Information for patients
The way that COS studies are explained, finding the right language to do so and asking questions about outcomes with a range of stakeholders are other key challenges. COMET has developed two plain language documents with the involvement of patients: one explains what COS are and what the COMET Initiative is; the other describes what a Delphi study is. These resources can be found on the COMET website [[109](/article/10.1186/s13063-017-1978-4#ref-CR109 "COMET. Plain Language Summary. [cited 2016 April]. Available from: http://www.comet-initiative.org/resources/PlainLanguageSummary
. Accessed 30 May 2017.")\] and may be useful in developing information for COS studies.When communicating with patients and when developing written study information and questions about outcomes, it is important to use plain language. Free resources to help with writing in plain language are available online. Specific guidance on writing for people with particular needs, such as adults with learning disabilities, might also be sought where relevant, e.g. easy-read publications. Readability tools are also available to provide an indication of how readable study material is. For further information on relevant resources to help with writing and assessing readability see the COMET website.
Considering how to present written information can help with ensuring its accessibility. Any specific visual needs, such as colour blindness or sight problems, also need considering when designing participant materials.
For certain populations and if funding allows, it may be appropriate to provide information about the study through other media; for example, podcasts or video presentations. Consulting with patients and patient organisations in the design stage of the study will help to identify the most accessible means of providing information and ensuring its acceptability to the relevant groups.
2.10.3 What questions to ask when involving patients as participants in a core outcome set study?
Asking the overarching question about which outcomes are relevant and important to patients can be difficult as the word ‘outcomes’ may not commonly be understood. Providing examples of outcomes in conditions can be useful, but care needs to be taken in selecting an example that will not bias respondents. In a systematic review of COS, Gargon et al. (2014) found that the precise question used to ask about outcomes was not always reported [14]. It is important that we report on such aspects of study design so that we can develop best ways of engaging patients as participants in future COS studies.
Patient involvement is important in developing questions for use in a COS study. Examples that we know about are from the ACORN and the MoMENT studies. The Acne Core Outcome Research Network (ACORN) consulted with patients and clinicians when developing their question for a COS study on acne. They showed patients several questions and asked which would be the most appropriate to use in a survey to include patients. Following this consultation they decided on the question: ‘Please tell us in your own words how you decide if your treatment has been effective. Physicians do things like counting spots or using an improvement scale but how do you, or will you, do it?’ This question was incorporated into a James Lind Alliance Priority Setting Partnership international survey about research priorities for acne [158]. The MoMENT study (described below) developed their question through their study group with the involvement of the chief executive from a patient organisation and then piloted their question with patients.
Further examples of how researchers have previously asked patients/carers about outcomes of importance are presented below. Some questions focus on treatment and others focus on the patient experience of a condition [89]. The scope of the COS may influence the type of questions used; for example, particular question types might work best for COS where the scope relates to a particular type of intervention, whilst other studies may work better with more generic experiential questions:
- The MoMENT study [55] – this was a study to develop a COS for the management of otitis media with effusion in children with cleft palate. A consensus survey was used in this study. Parents were presented with a list of outcomes to score but could add in any missing outcomes that they considered relevant. The question used was: ‘Think about when your child had glue ear and how you might decide if their treatment for glue ear had worked. We would like you to look at the list below and tell us how important each thing on this list is in deciding if treatment has worked’ [55].
- An online Delphi consensus study was conducted with patients who suffered from migraine. The patients were asked two open-ended questions in round 1 of the Delphi survey and these were:
- о What do you find most bothersome about having a migraine attack?
- о If a new medicine was developed against migraine attacks, what would you wish the effect of this medication to be [159]
- A COS was developed for children with asthma [60]. In an online Delphi survey, parents, young people and clinicians were asked open-ended questions in round 1 to identify outcomes of importance. The following four open-ended questions were used with the parents and young people:
- о Over the last 12 months, have you generally felt that the regular preventer treatment that your child (you) takes has kept their asthma under control? Yes/No. If you ticked ‘Yes’, please tell us what aspects of your child’s (your) asthma, or their daily life, have made you feel happy that they are on the correct regular medication. If you ticked ‘No’, please leave this question blank
- о Over the last 12 months, have there been times when you felt that your child’s (your) regular preventer treatment should be increased or changed because their (your) asthma was not under control? Yes/No. If you ticked ‘Yes’, please tell us the reasons why you were not satisfied with the regular preventer treatment that they (you) were taking? If you ticked ‘No’, please leave this space blank
- о Does anything worry you about the fact that your child (you) has asthma? Yes/No. If you ticked ‘Yes’, please tell us the worries you have about the fact your child (you) has asthma. If you ticked ‘No’, please leave this space blank
- о Does anything worry you about the regular preventer treatment that your child (you) takes for their asthma? Yes/No. If you ticked ‘Yes’, please tell us what worries you have about the treatment your child takes for their asthma. Please be as specific as you can. If you ticked ‘No’, please leave this question blank
2.10.4 Maintaining patient involvement throughout a consensus process
Consensus processes, such as Delphi surveys, may run over several months and strategies to maintain the involvement of patients in this process are important. Involving patients and patient organisations in designing and overseeing the COS may help COS developers to plan for this at the outset. Regular communication with participants in COS may be important in maintaining their engagement – for example, updates on the progress of the study and forewarning of anticipated dates of subsequent survey rounds. Some researchers have considered incentives; for example, prize draws, as a mechanism for maintaining participation. Ethical guidance will need to be sought for this and patients involved in designing your COS study can help developers to identify the most appropriate incentives for particular patient groups; for example, young people may prefer a different incentive than those of other generations.
2.10.5 Disseminating survey results to patients/the patient population
Having taken the time to be involved in a COS study, patients should be offered access to the survey results in an accessible way and the results may also be of interest to the wider patient population; for example, through patient organisation newsletters. The rules of plain English again apply (see above) in developing such end-of-study information and patient research partners can help teams design accessible end-of-study information. Morris et al. (2015) involved public research partners throughout their research and in writing the end-of-study information for a ‘children with neuro-disability’ study (which included a COS) [79]. Links to this end-of-study information can be found on the COMET website.
2.11 Determining ‘how’ to define and measure an outcome in the core outcome set
The text above discusses the approach to establishing consensus about what outcomes are important to measure. Consensus is also needed on how selected outcomes should be defined and measured. Of 227 published COS, 84 (37%) considered both the what and the how to measure in the same study [14, 39]. A review of methods used in these studies to determine how to measure the chosen outcomes is currently underway.
Different outcomes may be measured by a single question, a questionnaire, a performance-based test, a physical examination, a laboratory measurement, an imaging technique, and so forth. A variety of either definitions, measurement instruments or devices is often found to be used for the same outcome. For example, in a review of outcomes for colorectal cancer surgery some 17 different definitions were identified for ‘anastomotic leakage’ [85]. In a review of PROMs from studies evaluating radical treatment for oesophageal cancer, searches identified 21 generic and disease-specific PROMs containing 116 scales and 32 single items with 94 different verbatim names [86].
2.11.1 Choice of measurement instrument for an outcome
Evidence synthesis is further hampered by incomparable scores from different instruments and variability in the quality (reliability and validity) of measures used [35]. Guidance is needed on how to select the best instrument for a given outcome.
A joint initiative between COMET and COSMIN aimed to address this gap by developing a guideline on how to select outcome measurement instruments for outcomes included in a COS [160].
Based on a Delphi study amongst a panel of international experts, consensus was reached on four main steps in the selection of outcome measurement instruments for a COS:
- Step 1. Conceptual considerations
- Step 2. Finding existing outcome measurement instruments, by means of a systematic review and/or a literature search
- Step 3. Quality assessment of outcome measurement instruments, by means of the evaluation of the measurement properties and feasibility aspects of outcome measurement instruments
- Step 4. Generic recommendations on the selection of outcome measurement instruments for outcomes included in a COS
This consensus-based guideline can be used in defining how to measure core outcomes for any disease or condition in health and social care [44].
It may be that several measurement scales exist for measuring a particular outcome, such that steps 2 and 3 may take some time. As an example, fatigue was identified by patients with rheumatoid arthritis to be an important outcome to be included in the COS. A systematic search for articles measuring fatigue discovered 23 scales. Applying the OMERACT Filter for truth, discrimination and feasibility, six were found to have sufficient evidence of validity to pass most criteria [30]. In 2006, fatigue was endorsed as an additional core outcome at the OMERACT 8 meeting following further work, undertaken to demonstrate responsiveness [30].
In general, it is recommended that once all definitions, tests, questionnaires, techniques, etc. for measuring a particular outcome have been identified, and their properties assessed, a further consensus process should be undertaken to agree how each should be measured.
2.12 Achieve global consensus
To compare and contrast all research in a topic area, a COS must be applicable and adopted across relevant settings and disciplines including internationally where appropriate.
Of the 227 COS studies that were identified in the systematic review, the majority have involved collaborators (n = 180, 79%) and participants (n = 154, 68%) from Europe and/or North America. In contrast, the remaining continents have been involved as collaborators in just over one fifth of studies (n = 47; 21%) and have participated in less than one third of studies (n = 73; 32%). The geographical locations of collaborators and participants who have been involved in developing COS are presented in Table 4.
Table 4 Geographical locations of collaborators and participants involved in core outcome set (COS) development
Health professionals from multiple countries have been engaged through both professional societies [59] and personal networks [161, 162]. The inclusion of patients from multiple countries is likely to be more difficult. Some groups have included patients from different countries but usually in small numbers based on personal contact [161]. A novel approach involves health professionals interviewing several patients in multiple countries following training and according to a common protocol [[163](/article/10.1186/s13063-017-1978-4#ref-CR163 "Olliaro P, et al. Cutaneous leishmaniasis (CL) clinical trial methodology using crowd sourcing to define core eligibility criteria and core outcome measures for cutaneous leishmaniasis clinical trials [cited 2016 April]. Available from: www.comet-initiative.org/studies/details/807
. Accessed 30 May 2017.")\].Interviews with COS developers have highlighted some of the considerations of undertaking this work internationally [[65](/article/10.1186/s13063-017-1978-4#ref-CR65 "Gargon EA. Developing the agenda for core outcome set development. PhD thesis, University of Liverpool. 2016. https://livrepository.liverpool.ac.uk/3001398/
.")\]. A prominent question amongst COS developers interviewed was whether a COS _should_ be developed internationally. This links to the intended reach of the COS recommendations. If the COS is intended to be used globally then this has implications for how COS are developed, who is involved in that process and the resources required. There was no consensus amongst those in the study as to whether COS should be developed internationally or not.Both published and ongoing developers talked about the challenges of undertaking COS development internationally, particularly the linguistic challenges that global participation entailed and the need to translate concepts and questionnaires. They spoke of the logistical and resource challenges of organising an international meeting, and the challenge of getting the balance between what is ideal and what is pragmatic. International ethical approval procedures were described as resource intensive and ‘bureaucratic hurdles’.
Considerations about efficiency and heterogeneity arise with global development, as well as generalisability. However, heterogeneity can be as great within countries as between so this should not serve as a barrier to internationally developed COS. In a letter to an editor in reference to the international HOME COS for eczema, it was pointed out that for a disease with global impact there was limited representation of non-western participants from countries where the societal burden of the disease is high [164]. If a COS is developed to have international applicability then there is an issue of inclusivity that needs to be addressed. The question of international representation in COS development is one that requires further research.
For practical and resource reasons, such as those described, stakeholders from a limited number of geographical areas may have been involved in the development of a COS. Consideration should be given to the generalisability of the results and the need for any further research involving additional stakeholders if the COS is to be used in settings other than the one in which it was developed.
2.13 Study team and study committees
There are a number of study committees, described below, that can be helpful in the development of a COS.
2.13.1 Study Management Group
The Study Management Group (SMG) is responsible for the day-to-day management of the study and should meet regularly (usually monthly but frequency may vary depending on study activities).
The SMG should represent a multidisciplinary skill set relevant to the study. For example:
- A clinical lead in the area covered by the COS
- A study coordinator with understanding, and ideally experience of systematic review and/or outcomes and/or Delphi studies
- A qualitative researcher – if undertaking qualitative components of the study and not covered by the experience of the study coordinator
- PPI research partners – ideally a minimum of two to provide support and cross cover
- Other clinical or methodological experts depending on the individual needs of the study
2.13.2 Study Advisory Group (SAG)
Where a COS is developed for a condition involving a large multidisciplinary health care team, representation of all disciplines on the SMG could make that group unmanageable. Instead a SAG could provide additional expertise for each discipline.
The timing and frequency of SAG meetings should be considered based on planned study activities. Generally, the SAG would meet less frequently than the SMG with meetings scheduled at critical points in the study that require multidisciplinary input. Such tasks may include review of the categorisation and description of outcomes, decisions regarding the structure and content of the list of items to be considered in a consensus process, and review of the final report following the consensus meeting.
2.13.3 Costing the project
There are a number of cost areas to consider. The list below describes some of the key costs but this is not exhaustive and each study will have particular considerations. The costs of a study will likely vary over the study duration, and a study GANTT chart that maps out when key activities will take place is a useful tool to help estimate the resources needed at each stage of the study.
2.13.3.1 Staff
The staff working on a study will vary depending on study complexity and should be considered on a case-by-case basis. Staff involvement may also vary over the duration of the time depending on planned activities.
Staff roles to consider:
- Co-applicants/members of the Study Management Group – who are the co-applicants of the study, what role will each have and how much time will they need to fulfil that role including contributions to the final report and study dissemination?
- Statistical support – consider statistical support for study design, analysis and reporting
- Information systems – will a study database or bespoke software be required? If so include time for an information systems developer to provide these resources
- Data management – consider any data management requirements of the study. For example, if using paper-based questionnaires, who will enter responses into the study database and who will populate and distribute follow-up questionnaires, if appropriate?
- Study coordination – the amount of coordination will depend on the activities of an individual study. Generally, study coordination time to schedule study meetings, distribute and follow-up survey responses and to schedule and prepare documentation for focus groups, consensus meetings, etc. should be considered. The coordinator may also take responsibility for systematic reviews and other methodological areas of the study depending on experience, financial management and communication with the funder, ethical or governance approvals needed for the study, monitoring of participant consent, etc. and preparation of the final study report
- Qualitative research – consider any qualitative aspects of the study and include time for data collection, analysis and write up
2.13.3.2 Software
The software chosen will depend on the needs of the study. Basic online survey software may be available at an institution level or might require an annual licence to be purchased
If in-house software is being developed then the time required by information systems developers, together with the cost of server space, should be considered (see ‘Staff’)
Delivering a Delphi survey on-line may benefit from a bespoke system that allows automated reminders and feedback of results. The cost of developing a bespoke system should be considered against the staff costs where manual population of data for each round and reminders are needed.
2.13.3.3 Websites
If the study and/or survey will be hosted on a study-specific website then the cost of domain name registration and annual server hosting costs should be included. These costs are usually on an annual basis and cannot be included pro-rata (particularly domain name registration).
2.13.3.4 Printing
In some circumstances information may need to be provided in hard copy. Examples include Participant Information Sheets, Consent Forms, postal surveys or Delphi questionnaires, study summaries, etc. If only a small number of participants are expected then costs might be included in a general consumables budget. However, where large numbers are needed these should be included separately. Where printing costs are included separately these should include an initial print run plus at least one amendment of materials.
2.13.3.5 Meetings
Meetings should be included where the meeting incurs costs. Face-to-face meetings should consider the cost of meeting rooms, refreshments and travel. Teleconference costs should consider the number of lines needed and the location of those joining (national or international). University or hospital telecoms may be able to provide teleconferencing facilities and pricing structures.
Types of meetings to consider are:
- Study Management Group and Study Advisory Group (SAG) meetings (viii) – consider frequency, whether the meeting is face to face requiring travel costs or by teleconference only. Take into account the location of participants, local, national, international
- Consensus meetings – you may need to include the cost of venue hire and subsistence; some locations will provide a per person cost that includes all facilities and subsistence. The location of the meeting and subsequent travel costs for participants should be included. If national and/or international participants are attending a location close to a main train station or airport would be most appropriate and the cost of venue hire can be considered against additional travel costs for attending an institutional venue
- Focus groups – take into account who the participants are. If health care professionals then consider where the meeting will take place, travel costs and subsistence. Focus groups for patients/parents should also include travel and subsistence for each person. Focus groups for patients/parents might take place in an alternative setting that also acts as an incentive for attendance. For example, holding the session at a zoo or an aquarium which allows participants to access the attraction free of charge after the meeting. Childcare/carer needs should be included for patient (see ‘PPI’ section below for cost calculator)
2.13.3.6 Travel
Travel for meetings has already been described. Applicants applying for funding should review their institutional policies on travel; these may include the purchase of standard class fares only and a limit on the mileage that can be incurred for a single trip without justification.
2.13.3.7 Transcription
Transcription costs for interviews, recorded meetings or focus groups may need to be included. Each institution will have a list of approved suppliers and quotations should be sought prior to applying for funding. Prices are usually provided per minute and will increase where there are multiple speakers who need to be identified in the transcript.
2.13.3.8 Translation
Translation costs should be included for each language appropriate to the trial. Translation would usually include forward and back translation of documents to ensure consistency between languages. Again institutions will usually have a list of approved translation services who can be approached to provide a cost per document.
2.13.3.9 Patient and Public Involvement (PPI) in the design and conduct of a study
In some countries, e.g. UK, payments are offered to public research partners for the time involved in undertaking their PPI activities. The acceptability of and approaches to payment for PPI may differ by country, hence advice should be sought locally. If patients are paid for PPI activities, researchers need to ensure that patients are aware of any implications of being paid for involvement, e.g. impact on any benefits that they are entitled to or tax implications.
Costings should be estimated for each type of involvement. For example:
- PPI membership of study committees/groups
- PPI co-applicants
- Other PPI activities. Examples include parent/patient facilitators of workshops or consensus meetings
- Training – consider whether PPI contributors require any training and who will provide this. This could include attendance at conferences, external training events or internal training. The time to attend the training together with travel, subsistence and childcare/carer costs should be included
- Childcare/carer time
Advice on costs for PPI in the UK is available via the INVOLVE website [[165](/article/10.1186/s13063-017-1978-4#ref-CR165 "INVOLVE. Payment, reward and recognition for involvement in research. [cited 2015 November]. Available from: http://www.invo.org.uk/payment/
. Accessed 30 May 2017.")\]. This site also includes resources for estimating PPI costs \[[166](/article/10.1186/s13063-017-1978-4#ref-CR166 "INVOLVE. Involvement Cost Calculator. [cited 2015 November]. Available from:
http://www.invo.org.uk/resource-centre/payment-and-recognition-for-public-involvement/involvement-cost-calculator/
. Accessed 30 May 2017. ")\].2.13.3.10 Equipment
Software and equipment is available to allow anonymous voting at face-to-face meetings. It might be possible to borrow this from an institution or it may need to be purchased.
If focus groups or consensus meetings need to be recorded then you may need to consider purchasing equipment to do this or to employ external companies with professional equipment, particularly if it is a large group.
Also consider laptops/PCs that are needed for study staff, particularly full-time members of staff employed specifically for the study.
2.13.3.11 Incentives
You may like to offer an incentive to study participants; this might be acknowledgment in the publications, a gift voucher for completing an interview or joining a focus group or a prize draw for those completing a survey. Examples of funded incentives include an entry into a prize draw for an iPad mini for those completing an online Delphi survey (one iPad mini for each stakeholder group, i.e. health care professionals and parents), a £20 gift voucher for parents attending a focus group, a £10 gift voucher for parents completing a qualitative interview [55].
2.13.3.12 Systematic review
If completing a systematic review as part of the study, costs, excluding staff costs, may include printing and costs for unsubscribed journals. Each institution should be able to provide a cost for an interlibrary loan and an estimated number of these included based on the estimated size of the academic literature, e.g. estimate approximately 10% of included papers.
2.13.3.13 Publication
Costs to publish the protocol and the final manuscript should be included, based on the current costs for the desired journal.
2.14 Reporting guidance
COS developers should provide a clear and transparent report of the methods they used. Reporting standards for a Delphi survey component of a COS study have been proposed previously by some authors of this Handbook [138]. A more general checklist of items to be reported for a COS study was then published [21].
The first comprehensive systematic review of COS highlighted the need for a more formal reporting guideline for COS development studies due to the amount of relevant information that is missing from journal articles [14]. A guideline for reporting COS studies has recently been developed [134].
2.15 Quality assessment/critical appraisal
It has previously been suggested that the potential impact of the following methodological decisions on the final results should be considered [21]: group composition, questioning technique, the information participants receive to inform their answers, whether or not responses are anonymous, how the group participants interacted with, or influenced, each other, the medium of the interaction, attrition bias, analysis which can miss or overstate the importance of certain outcomes, and the way in which consensus is reached.
However the definition of quality is a difficult one, and an area for further research may be to achieve consensus regarding minimum standards, as discussed in Chapter 4.