OFLOXACIN (original) (raw)

OFLOXACIN
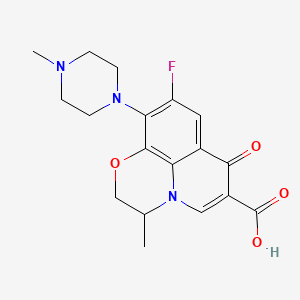
Molecular Formula: C18H20FN3O4; (Formula Weight: 361.37;
mp: 270-275°C;
Ofloxacin is one kind of white or almost powder or off-white solid.
The Systematic (IUPAC) name of this chemical is (RS)-7-fluoro-2-methyl-6-(4-methylpiperazin-1-yl)-10-oxo-4-oxa-1-azatricyclo[7.3.1.05,13]trideca-5(13),6,8,11-tetraene-11-carboxylic acid
Apazix; Bactocin; Exocin; Flobacin; Floxal; Floxil; Floxin; Girasid; Monoflocet; Ocuflox; Oflocet; Oflocin; Oxaldin; Tarivid; Urosin; Visiren; Zanocin
DL-8280; HOE-280; Ofloxacinum
OFLOXACIN was developed as a broader-spectrum analog of norfloxacin, the first fluoroquinolone antibiotic, Ofloxacin was first patented in 1982 (European Patent Daiichi) and received U.S. Food and Drug Administration (FDA) approval December 28, 1990. In the United States name branded ofloxacin is rarely used anymore, having been discontinued by the manufacturer (Ortho McNeil Janssen). Johnson and Johnson’s annual sales of Floxin in 2003 was approximately 30million,whereastheircombinedsalesofLevaquin/Floxinexceeded30 million, where as their combined sales of Levaquin/Floxin exceeded 30million,whereastheircombinedsalesofLevaquin/Floxinexceeded 1.15 billion in the same year. During the 2008 Johnson & Johnson shareholder’s meetings, the safety of both ofloxacin and levafloxacin were called into question. During the 2009 meeting, yet another shareholder who alleges to have been crippled by these drugs, John Fratti, raised these same issues having seen no significant changes in the warnings (regarding the issues raised during the 2008 meeting). Once again a public request for stronger warnings for both ofloxacin and levofloxacin was made.
Ofloxacin is a synthetic antibiotic of the fluoroquinolone drug class considered to be a second-generation fluoroquinolone.[1][2]
Ofloxacin was first patented in 1982 (European Patent Daiichi) and received approval from the U.S. Food and Drug Administration (FDA) on December 28, 1990. Ofloxacin is sold under a wide variety of brand names as well as generic drug equivalents, for oral and intravenous administration. Ofloxacin is also available for topical use, as eye drops and ear drops (marketed as Ocuflox and Floxin Otic respectively in the United States and marketed as Optiflox, eylox respectively in Jordan and Saudi Arabia[3]).
Ofloxacin is a racemic mixture, which consists of 50% levofloxacin (the biologically active component) and 50% of its “mirror image” or enantiomer dextrofloxacin.[4]
Ofloxacin has been associated with adverse drug reactions, such as tendon damage (including spontaneous tendon ruptures) and peripheral neuropathy (which may be irreversible); tendon damange may manifest long after therapy had been completed, and, in severe cases, may result in lifelong disabilities.[5]

History
Ofloxacin was developed as a broader-spectrum analog of norfloxacin, the first fluoroquinolone antibiotic,[6] Ofloxacin was first patented in 1982 (European Patent Daiichi) and received U.S. Food and Drug Administration (FDA) approval December 28, 1990.
In the United States name branded ofloxacin is rarely used anymore, having been discontinued by the manufacturer, Ortho-McNeil-Janssen, a subsidiary of Johnson & Johnson.[7] Johnson and Johnson’s annual sales of Floxin in 2003 was approximately 30million,whereastheircombinedsalesofLevaquin/Floxinexceeded30 million, whereas their combined sales of Levaquin/Floxin exceeded 30million,whereastheircombinedsalesofLevaquin/Floxinexceeded1.15 billion in the same year.[8][9] However generic use continues. The FDA website lists Floxin (Ortho McNeil Jannsen) as being discontinued, with just a few generic equivalents still in use. The otic solution continues to be listed as being available both as an original drug as well as a generic equivalent.
Medical uses
In the in the U.S. ofloxacin is approved for the treatment of bacterial infections such as:
- Acute bacterial exacerbations of chronic bronchitis
- Community-acquired pneumonia
- Uncomplicated skin and skin structure infections
- Nongonococcal urethritis and cervicitis
- Mixed Infections of the urethra and cervix
- Acute pelvic inflammatory disease
- Uncomplicated cystitis
- Complicated urinary tract infections
- Prostatitis
- Acute, uncomplicated urethral and cervical gonorrhea.
Ofloxacin has not been shown to be effective in the treatment of syphilis.[10] Floxin is no longer considered a first line treatment for gonnorrhea due to bacterial resistance.[11][12][13]
Available forms
Ofloxacin for systemic use is available as tablet (multiple strengths), oral solution (250 mg/mL), and injectable solution (multiple strengths). It is also used as eye drops and ear drops. It is also available in combination with ornidazole.
Mode of action
Ofloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV,[14] which is an enzyme necessary to separate (mostly in prokaryotes, in bacteria in particular) replicated DNA, thereby inhibiting bacterial cell division.
…………………………..
EP0047005
US4382892 Doi: 10.1248/cpb.34.4098
Doi: 10.1248/cpb.35.1896

………………………………
doi: 10.1248/cpb.34.4098

…………………………
http://www.google.com/patents/EP0271275A1?cl=en

Reference example
- By using 2,4,4-trimethylcyclopentyl acid as a starting material, ethyl 9,l0-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[l,2,3-de] [l,4]benzoxazine-6-carboxylate (IV) which is an important intermediate for synthesis of an antibacterial agent, ofloxacin (9-fluoro-3-methyl-l0-(4-methyl-l-piperazinyl)-7-oxo-2,3-dihydro-7H-pyrido[l,2,3-de][l,4]benzoxazine-6-carboxylic acid) was synthesized following the reaction schemes shown below.


[Step 1]
- To l2.6 g (0.066 mole) of 2,4,4-trimethylcyclopentyl acid was added 40 ml of acetic anhydride, and the mixture was stirred for l5 hours under reflux. The reaction mixture was poured into ice-cold water, and then extracted with chloroform. The chloroform layer was washed with water, condensed under reduced pressure and the residue was washed with n-hexane to give 6.l0 g of 3-acetoxy-2,4,5-trifluorobenzoic acid (V) as colorless powder.Mass (CI): m/e 235 (M⁺ + l), 2l7 (M⁺ – OH), l75 (M⁺ – CH₃COO)
[Steps 2, 3, 4, 5 and 6]
- In 200 ml of benzene was dissolved 6.l0 g (0.026 mole) of 3-acetoxy-2,4,5-trifluorobenzoic acid (V), and to the solution was added l5 ml of thionyl chloride and stirred for 4 hours under reflux. After completion of the reaction, benzene and excess thionyl chloride were completely distilled off under reduced pressure to give 3-acetoxy-2,4,5-trifluorobenzoyl chloride (VI).
- On the other hand, to l00 ml of anhydrous diethyl ether were added 3.l7 g (0.028 mole) of magnesium ethoxide and 4.30 g (0.027 mole) of diethyl malonate and refluxed for 3 hours to give a suspension of ethoxymagnesium malonic diethyl ester in diethylether. To the suspension was added dropwise a solution of the above acid chloride dissolved in 50 ml of anhyrous diethyl ether, and after completion of the dropwise addition, the mixture was further stirred for an hour at room temperature. After completion of the reaction, l N hydrochloric acid was added to the mixture to made it acidic, and the mixture was extracted with ethyl acetate. The organic layer was washed with water and dried, and then the solvent was distilled under reduced pressure to give l0.39 g of diethyl 3-acetoxy-2,4,5-trifluorobenzoylmalonate (VII) as yellowish oily product.
- Then, the yellowish oily product was dissolved in l20 ml of dioxane and 4.90 g (0.026 mole) of p-toluenesulfonic acid monohydrate was added to the mixture and refluxed for l5 hours. After completion of the reaction, dioxane was distilled under reduced pressure. To the residue were added l00 ml of water and 2.l5 g (0.026 mole) of sodium hydrogen carbonate and the mixture was extracted with chloroform. The chloroform layer was washed with water, dried and then distilled under reduced pressure to give 7.64 g of ethyl 3-acetoxy-2,4,5-trifluorobenzoylacetate (VIII) as reddish oily product.
- To 7.64 g (0.025 mole) of the ethyl 3-acetoxy-2,4,5-trifluorobenzoylacetate (VIII) thus obtained were added 20 ml of acetic anhydride and 6 ml of ortho-ethyl formate and the mixture was refluxed for 2 hours and then condensed under reduced pressure. The residue was dissolved in 50 ml of dichloromethane, added l.9l g (0.026 mole) of DL-2-aminopropanol and allowed to stand over night. Dichloromethane was distilled under reduced pressure and the residue was applied to silica gel column chromatography (solvent: mixture of toluene : ethyl acetate = l : l) to give 4.37 g of ethyl-2-(3-acetoxy-2,4,5-trifluorobenzoyl)-3-(2-hydroxy-l-methylethyl)aminoacrylate (X) as pale yellow oily product.Mass: m/e 389 (M⁺), 358 (M⁺ – CH₂OH), 43 (+

CH₃)
[Step 7]
- In 30 ml of dimethylformamide was dissolved 4.30 g of the ethyl-2-(3-acetoxy-2,4,5-trifluorobenzoyl)-3-(2-hydroxy-l-methylethyl)aminoacrylate (X) thus obtained and l.92 g (0.033 mole) of potassium fluoride was added to the mixture and the mixture was stirred at l40 to l50 °C for 2 hours. After completion of the reaction, the solvent was distilled under reduced pressure. To the residue was added water and the mixture was extracted with dichloromethane, and the organic layer was washed with water, dried and then condensed under reduced pressure. Then, the residue was washed with ethanol, and the residue was recrystallized from acetone to give l.40 g of ethyl-9,l0-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[l,2,3-de][l,4]benzoxazine-6-carboxylate (IV) as pale brown fine needle crystals.M.p.: 255 to 256 °C
Elemental analysis (%): as C₁₅H₁₃F₂NO₄

- According to the present invention, a novel compound 2,4,4-trimethylcyclopentyl acid useful as the synthetic intermediate for quinolone carboxylic acid derivatives which is useful as antibacterial agents can be provided, and the preparation steps of said quinolone carboxylic acid derivatives can be shortened to a great extent by use of said compound.
1H NMR PREDICT


13 C NMR PREDICT


OFLOXACIN COSY NMR
OFLOXACIN 13 C
OFLOXACIN
OFLOXACIN 1H NMR
OFLOXACIN HSQC NMR
OFLOXACIN MASS SPECTRUM
OFLOXACIN 13 C NMR
Production of Ofloxacin

The partial hydrolysis ot 2,3,4-trifluoronitrobenzene (I) with KOH in DMSO gives 2,3-difluoro-6-nitrophenol (II), which by condensation with chloroacetone (III) by means of K2CO3 – KI in refluxing acetone yields 2-acetonyloxy-3,4-difluoronitrobenzene (IV). The reductive cyclization of (IV) with H2 over Raney-Ni in ethanol affords 7,8-difluoro-2,3-dihydro-3-methyl-4H-benzoxazine (V), which is condensed with diethyl ethoxymethylenemalonate (VI) by heating at 145 C giving the malonic derivative (VII). The cyclization of (VII) by heating at 145 C with ethyl polyphosphate (PPE) yields ethyl 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate (VIII), which is hydrolyzed with HCl in refluxing acetic acid affording the corresponding free acid (IX). Finally, this compound is condensed with N-methylpiperazine (X) in DMSO at 110 C.
(1) 2,3,4-trifluoronitrobenzene as the starting material by selective alkaline hydrolysis, etherification, restore, and C2H5OCH=C(COOEt)2 or (CH3)2NCH=C (COOEt)2 condensation ringaggregate, after hydrolysis with acetic acid boron role, and then the introduction of N-methyl-piperazine-derived products.
(2) Phthalimide derivative as a raw material generated by fluorination tetrafluorophthalic phthalimide, hydrolysis, decarboxylation of 2,3,4,5-tetrafluoro-benzoic acid, and then chlorinated, acylatingdecarboxylated 2,3,4,5-tetrafluorobenzoyl ethyl acetate, and then the first and of triethyl orthoformate, and after 2-aminopropanol reaction, and then cyclization generated pyridine [1,2,3-de] [1,4] benzo Hey triazine derivatives, and finally reaction of ofloxacin and piperazine.

……………….
| Studies on NMR Behavior of Ofloxacin in Different pH Environment |
|---|
| QI Jian1, GAO Xiu-Xiang1, ZHAO Mei-Xian2, XIANG Jun-Feng3, LIN Chong-Xi1*, XU Yi-Zhuang1*, WU Jin-Guang1 |
| College of Chemical and Molecular Engineering, Peking University, Beijing 100871, China; Applied Chemistry Department, School of Science, Beijing University of Chemical Technology, Beijing 100029, China; Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, China |
http://www.cjcu.jlu.edu.cn/EN/Y2007/V28/I5/913#
Download: PDF (403 KB)
Systematic NMR spectroscopic investigation on ofloxacin in both acidic and alkaline solutions was carried out via 1H, 13C NMR, DEPT, COSY, HSQC spectra together with HMBC techniques. Complete assignment on 1H and 13C NMR of ofloxacin was obtained in different pH environments where the coupling constant between 13C and 19F was found to be very helpful for the assignment of aromatic 13C NMR signals. Additionally, the chemical shifts of 1H from the complex spin systems such as AA’BB’ were obtained using HSQC technique. Comparisons were made among the NMR spectra in acidic solution and those in alkaline solution, which demonstrate that: (1) deprivation of H+ from COOH in alkaline solution destroys the hydrogen bond between COOH and carbonyl group in ofloxacin. This brings about the redistribution of π elelctrons around the carboxyl and carbonyl groups so that significant variations of 13C NMR chemical shift and coupling constant JFC are observed. (2) In the alkaline solution, the removal of proton from N4 in piperazine ring induces considerable variation of chemical shift of methylene groups and causes remarkable changes of dynamic behavior of the piperazine ring.
References
- 1 Nelson, JM.; Chiller, TM.; Powers, JH.; Angulo, FJ. (Apr 2007). “Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story”. Clin Infect Dis 44 (7): 977–80. doi:10.1086/512369. PMID 17342653.
- 2
- Kawahara, S. (December 1998). “[Chemotherapeutic agents under study]”. Nippon Rinsho 56 (12): 3096–9. PMID 9883617.
- 3
- http://www.jfda.jo/
- 4
- http://www.wvnd.uscourts.gov/Opinions/orthoorder.pdf
- 5
- Clodagh Sheehy (August 2, 2008). “Warning over two types of antibiotic”. Republic of Ireland. Retrieved July 17, 2009.
- 6
- Mark B. Abelson, MD; Stephen J. Hallas (April 15, 2003). “The New Antibiotics: The Path of Least Resistance”. Review of Ophthalmology. Retrieved October 3, 2009.
- 7
- Douglas C. Throckmorton (May 19, 2009). “Novartis Pharmaceuticals Corp. et al.; Withdrawal of Approval of 92 New Drug Applications and 49 Abbreviated New Drug Applications”. Trading Markets. see also FDA docket number FDA-2009-N-0211
- 8
- Business Wire (September 2, 2003). “Teva Announces Approval of Ofloxacin Tablets, 200 mg, 300 mg, and 400 mg”. Business Wire.
- 9
- Johnson & Johnson (2003). “Building on a foundation of health” (PDF). Shareholder.
- 10
- Ortho-McNeil-Janssen Pharmaceuticals, Inc (2008). “FLOXIN Tablets (Ofloxacin Tablets)” (PDF). USA: FDA.
External links
- Ofloxacin: an overview – A site with its chemical properties and alternate brand names.
- U.S. National Library of Medicine: Drug Information Portal – Ofloxacin
Package insert links
 |
|---|
| Title: Ofloxacin CAS Registry Number: 82419-36-1 CAS Name: 9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7_H-_pyrido[1,2,3-de_]-1,4-benzoxazine-6-carboxylic acid Additional Names: ofloxacine Manufacturers’ Codes: DL-8280; HOE-280 Trademarks: Exocin (Allergan); Flobacin (Sigma-Tau); Floxil (Janssen-Cilag); Floxin (Ortho-McNeil); Monoflocet (Aventis); Ocuflox (Allergan); Oflocet (Aventis); Oflocin (GSK); Tarivid (Aventis) Molecular Formula: C18H20FN3O4 Molecular Weight: 361.37 Percent Composition: C 59.83%, H 5.58%, F 5.26%, N 11.63%, O 17.71% Literature References: Broad spectrum, fluorinated quinolone antibacterial. Prepn: I. Hayakawa et al., EP 47005; eidem, US 4382892 (1982, 1983 both to Daiichi). Total synthesis: H. Egawa et al., Chem. Pharm. Bull. 34, 4098 (1986). Synthesis and activity of optical isomers: S. Atarashi et al., ibid. 35, 1896 (1987). Antibacterial spectrum of racemate: K. Sato et al., Antimicrob. Agents Chemother. 22, 548 (1982). Mechanism of differential activity of enantiomers: I. Morrissey et al., ibid. 40, 1775 (1996). Toxicity data: H. Ohno et al., Chemotherapy (Tokyo) 32, Suppl. 1, 1084 (1984). Pharmacology and clinical efficacy: Infection **14,**Suppl. 1, S1-S109 (1986). Symposium on pharmacokinetics and therapeutic use: Scand. J. Infect. Dis. Suppl. 68, 1-69 (1990). Review of antibacterial spectrum, pharmacology, and clinical efficacy: J. P. Monk, D. M. Campoli-Richards, Drugs 33, 346-391 (1987); of mechanism of action: K. Drlica, Curr. Opin. Microbiol. 2, 504-508 (1999). Properties: Colorless needles from ethanol, mp 250-257° (dec). LD50 in male, female mice, male, female rats (mg/kg): 5450, 5290, 3590, 3750 orally; 208, 233, 273, 276 i.v.; >10000, >10000, 7070, 9000 s.c. (Ohno). Melting point: mp 250-257° (dec) Toxicity data: LD50 in male, female mice, male, female rats (mg/kg): 5450, 5290, 3590, 3750 orally; 208, 233, 273, 276 i.v.; >10000, >10000, 7070, 9000 s.c. (Ohno) . . . . Derivative Type: S-(-)-Form CAS Registry Number: 100986-85-4; 138199-71-0 (hemihydrate) Additional Names: Levofloxacin Manufacturers’ Codes: DR-3355 Trademarks: Cravit (Daiichi); Levaquin (Ortho-McNeil); Tavanic (Aventis); Quixin (Santen) Literature References: Toxicity study: M. Kato et al., Arzneim.-Forsch. 42, 365 (1992). Series of articles on pharmacology and toxicology: ibid., 368-418. Clinical study in bacterial conjunctivitis: D. G. Hwang et al., Br. J. Ophthalmol. 87, 1004 (2003).Review: D. S. North et al., Pharmacotherapy 18, 915-935 (1998). Properties: Prepd as the hemihydrate; needles from ethanol + ethyl ether, mp 225-227° (dec). [a]D23 -76.9° (c = 0.385 in 0.5_N_NaOH). Freely sol in glacial acetic acid, chloroform; sparingly sol in water. LD50 in male, female mice, male, female rats (mg/kg): 1881, 1803, 1478, 1507 orally (Kato). Melting point: mp 225-227° (dec) Optical Rotation: [a]D23 -76.9° (c = 0.385 in 0.5_N NaOH) Toxicity data: Freely sol in glacial acetic acid, chloroform; sparingly sol in water. LD50 in male, female mice, male, female rats (mg/kg): 1881, 1803, 1478, 1507 orally (Kato) Therap-Cat: Antibacterial. Keywords: Antibacterial (Synthetic); Quinolones and Analogs. |
Ofloxacin
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
| (RS)-7-fluoro-2-methyl-6-(4-methylpiperazin-1-yl)-10-oxo-4-oxa-1-azatricyclo[7.3.1.05,13]trideca-5(13),6,8,11-tetraene-11-carboxylic acid | |
| Clinical data | |
| Trade names | Floxin, Ocuflox |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a691005 |
| Pregnancy category | US: C |
| Legal status | US: ℞-only |
| Routes | Oral, IV, topical (eye drops and ear drops) |
| Pharmacokinetic data | |
| Bioavailability | 85% – 95% |
| Protein binding | 32% |
| Half-life | 8–9 hours |
| Identifiers | |
| CAS number | 82419-36-1  |
| ATC code | J01MA01 ,S01AE01, S02AA16 |
| PubChem | CID 4583 |
| DrugBank | DB01165 |
| ChemSpider | 4422  |
| UNII | A4P49JAZ9H  |
| KEGG | D00453  |
| ChEBI | CHEBI:7731  |
| ChEMBL | CHEMBL4  |
| Synonyms | (±)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7_H_-pyrido[1,2,3-_de_][1,4]benzoxazine-6-carboxylic acid |
| Chemical data | |
| Formula | C18H20FN3O4 |
| Molecular mass | 361.368 g/mol |